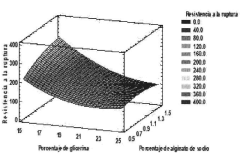
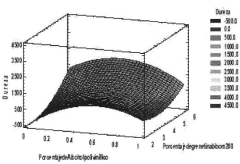
Sodium Alginate Applications in Transdermal Patch Improvement
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Alginate in Transdermal Patches: Background and Objectives
Sodium alginate, a naturally derived polysaccharide extracted from brown seaweed, has gained significant attention in the pharmaceutical industry for its potential applications in transdermal drug delivery systems. The evolution of transdermal patches has been driven by the need for non-invasive, controlled drug release methods that can overcome the limitations of oral and injectable drug administration. In this context, sodium alginate has emerged as a promising material due to its unique properties and versatility.
The primary objective of researching sodium alginate applications in transdermal patch improvement is to enhance the efficacy, safety, and patient compliance of drug delivery through the skin. This goal aligns with the broader trend in pharmaceutical development towards more targeted, efficient, and user-friendly drug delivery systems. By leveraging the properties of sodium alginate, researchers aim to address several key challenges in transdermal patch technology, including drug permeation, adhesion, and stability.
Historically, transdermal patches have been used for delivering various medications, such as nicotine for smoking cessation, hormones for contraception, and pain relievers. However, the limited permeability of the skin has restricted the range of drugs that can be effectively delivered through this route. The introduction of sodium alginate into transdermal patch formulations represents a potential breakthrough in expanding the scope of transdermal drug delivery.
Sodium alginate's unique characteristics, including its biocompatibility, biodegradability, and ability to form hydrogels, make it an attractive candidate for improving transdermal patch performance. Its gel-forming properties can be exploited to create a matrix that controls drug release rates, while its mucoadhesive nature may enhance patch adhesion to the skin. Furthermore, the potential of sodium alginate to act as a penetration enhancer could address one of the most significant barriers in transdermal drug delivery: the stratum corneum's resistance to drug permeation.
The technological evolution in this field is driven by the growing demand for personalized medicine and the need for more efficient drug delivery systems. As the pharmaceutical industry continues to develop new therapeutic compounds, many of which face challenges in traditional delivery methods, the role of advanced materials like sodium alginate in transdermal patches becomes increasingly critical. This research aims to unlock new possibilities in drug administration, potentially revolutionizing treatment approaches for various medical conditions.
The primary objective of researching sodium alginate applications in transdermal patch improvement is to enhance the efficacy, safety, and patient compliance of drug delivery through the skin. This goal aligns with the broader trend in pharmaceutical development towards more targeted, efficient, and user-friendly drug delivery systems. By leveraging the properties of sodium alginate, researchers aim to address several key challenges in transdermal patch technology, including drug permeation, adhesion, and stability.
Historically, transdermal patches have been used for delivering various medications, such as nicotine for smoking cessation, hormones for contraception, and pain relievers. However, the limited permeability of the skin has restricted the range of drugs that can be effectively delivered through this route. The introduction of sodium alginate into transdermal patch formulations represents a potential breakthrough in expanding the scope of transdermal drug delivery.
Sodium alginate's unique characteristics, including its biocompatibility, biodegradability, and ability to form hydrogels, make it an attractive candidate for improving transdermal patch performance. Its gel-forming properties can be exploited to create a matrix that controls drug release rates, while its mucoadhesive nature may enhance patch adhesion to the skin. Furthermore, the potential of sodium alginate to act as a penetration enhancer could address one of the most significant barriers in transdermal drug delivery: the stratum corneum's resistance to drug permeation.
The technological evolution in this field is driven by the growing demand for personalized medicine and the need for more efficient drug delivery systems. As the pharmaceutical industry continues to develop new therapeutic compounds, many of which face challenges in traditional delivery methods, the role of advanced materials like sodium alginate in transdermal patches becomes increasingly critical. This research aims to unlock new possibilities in drug administration, potentially revolutionizing treatment approaches for various medical conditions.
Market Analysis for Advanced Transdermal Drug Delivery Systems
The advanced transdermal drug delivery systems market has been experiencing significant growth in recent years, driven by the increasing demand for non-invasive drug administration methods and the advantages offered by transdermal patches. This market segment is expected to continue its upward trajectory due to several key factors.
Firstly, the global aging population and the rising prevalence of chronic diseases have created a substantial need for more convenient and efficient drug delivery methods. Transdermal patches offer a patient-friendly alternative to traditional oral medications and injections, particularly for elderly patients who may have difficulty swallowing pills or maintaining complex medication regimens.
The market for advanced transdermal drug delivery systems is also benefiting from technological advancements in patch design and formulation. Innovations such as microneedle patches, iontophoresis, and nanocarrier-based systems are expanding the range of drugs that can be delivered transdermally, including larger molecules like proteins and peptides. This broadening of applicable drug types is opening up new therapeutic areas and market opportunities.
Geographically, North America currently holds the largest market share, followed by Europe. This dominance is attributed to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years, driven by improving healthcare access, rising disposable incomes, and increasing awareness of advanced drug delivery methods.
The market is characterized by intense competition among key players, including 3M Company, Boehringer Ingelheim, Johnson & Johnson, Novartis AG, and GlaxoSmithKline plc. These companies are investing heavily in research and development to improve patch technologies and expand their product portfolios.
One of the most promising areas within this market is the development of smart transdermal patches. These patches incorporate sensors and wireless technology to monitor drug release and patient response in real-time, allowing for personalized and optimized treatment regimens. This trend aligns with the broader movement towards digital health and personalized medicine, potentially revolutionizing chronic disease management.
Despite the positive outlook, the market faces challenges such as stringent regulatory requirements and the high cost of developing new transdermal drug delivery systems. Additionally, there are limitations in the types of drugs that can be effectively delivered through the skin, which may constrain market growth in certain therapeutic areas.
Firstly, the global aging population and the rising prevalence of chronic diseases have created a substantial need for more convenient and efficient drug delivery methods. Transdermal patches offer a patient-friendly alternative to traditional oral medications and injections, particularly for elderly patients who may have difficulty swallowing pills or maintaining complex medication regimens.
The market for advanced transdermal drug delivery systems is also benefiting from technological advancements in patch design and formulation. Innovations such as microneedle patches, iontophoresis, and nanocarrier-based systems are expanding the range of drugs that can be delivered transdermally, including larger molecules like proteins and peptides. This broadening of applicable drug types is opening up new therapeutic areas and market opportunities.
Geographically, North America currently holds the largest market share, followed by Europe. This dominance is attributed to the presence of major pharmaceutical companies, advanced healthcare infrastructure, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth rate in the coming years, driven by improving healthcare access, rising disposable incomes, and increasing awareness of advanced drug delivery methods.
The market is characterized by intense competition among key players, including 3M Company, Boehringer Ingelheim, Johnson & Johnson, Novartis AG, and GlaxoSmithKline plc. These companies are investing heavily in research and development to improve patch technologies and expand their product portfolios.
One of the most promising areas within this market is the development of smart transdermal patches. These patches incorporate sensors and wireless technology to monitor drug release and patient response in real-time, allowing for personalized and optimized treatment regimens. This trend aligns with the broader movement towards digital health and personalized medicine, potentially revolutionizing chronic disease management.
Despite the positive outlook, the market faces challenges such as stringent regulatory requirements and the high cost of developing new transdermal drug delivery systems. Additionally, there are limitations in the types of drugs that can be effectively delivered through the skin, which may constrain market growth in certain therapeutic areas.
Current Challenges in Transdermal Patch Technology
Transdermal patch technology has made significant strides in drug delivery systems, yet several challenges persist in its development and application. One of the primary obstacles is achieving consistent and controlled drug release rates. The variability in skin permeability among individuals and across different body sites can lead to unpredictable drug absorption, potentially compromising therapeutic efficacy.
Another critical challenge lies in the limited range of drugs suitable for transdermal delivery. Many pharmaceutical compounds have molecular weights too large to penetrate the skin barrier effectively or lack the necessary physicochemical properties for transdermal absorption. This restriction significantly narrows the scope of medications that can be administered via this route.
The adhesion and wear time of transdermal patches also present ongoing challenges. Ensuring patches remain firmly attached to the skin for the intended duration of treatment, while simultaneously allowing for easy and painless removal, requires a delicate balance of adhesive properties. Environmental factors such as humidity, temperature, and physical activity can further complicate this issue.
Skin irritation and allergic reactions remain significant concerns in transdermal patch technology. The prolonged contact between the patch components and the skin can lead to local adverse effects, ranging from mild irritation to severe allergic responses. Developing biocompatible materials that minimize these reactions while maintaining drug delivery efficiency is an ongoing challenge.
The stability of drugs within the patch matrix over extended periods is another area of concern. Ensuring that the active pharmaceutical ingredients remain stable and potent throughout the shelf life of the product and during the entire wear time is crucial for maintaining therapeutic effectiveness.
Furthermore, the development of transdermal patches for macromolecules, such as proteins and peptides, presents unique challenges. These large molecules typically cannot penetrate the skin barrier without enhancement techniques, which may compromise skin integrity or cause discomfort.
Lastly, the cost-effectiveness of transdermal patch production remains a challenge, particularly for complex formulations or those incorporating advanced technologies. Balancing manufacturing costs with therapeutic benefits is essential for the widespread adoption of this drug delivery method.
Another critical challenge lies in the limited range of drugs suitable for transdermal delivery. Many pharmaceutical compounds have molecular weights too large to penetrate the skin barrier effectively or lack the necessary physicochemical properties for transdermal absorption. This restriction significantly narrows the scope of medications that can be administered via this route.
The adhesion and wear time of transdermal patches also present ongoing challenges. Ensuring patches remain firmly attached to the skin for the intended duration of treatment, while simultaneously allowing for easy and painless removal, requires a delicate balance of adhesive properties. Environmental factors such as humidity, temperature, and physical activity can further complicate this issue.
Skin irritation and allergic reactions remain significant concerns in transdermal patch technology. The prolonged contact between the patch components and the skin can lead to local adverse effects, ranging from mild irritation to severe allergic responses. Developing biocompatible materials that minimize these reactions while maintaining drug delivery efficiency is an ongoing challenge.
The stability of drugs within the patch matrix over extended periods is another area of concern. Ensuring that the active pharmaceutical ingredients remain stable and potent throughout the shelf life of the product and during the entire wear time is crucial for maintaining therapeutic effectiveness.
Furthermore, the development of transdermal patches for macromolecules, such as proteins and peptides, presents unique challenges. These large molecules typically cannot penetrate the skin barrier without enhancement techniques, which may compromise skin integrity or cause discomfort.
Lastly, the cost-effectiveness of transdermal patch production remains a challenge, particularly for complex formulations or those incorporating advanced technologies. Balancing manufacturing costs with therapeutic benefits is essential for the widespread adoption of this drug delivery method.
Existing Sodium Alginate Applications in Transdermal Patches
01 Composition of sodium alginate transdermal patches
Sodium alginate is used as a key component in transdermal patch formulations. These patches often include additional polymers, adhesives, and active ingredients to enhance drug delivery through the skin. The composition may be optimized for specific drug molecules and desired release profiles.- Composition of sodium alginate transdermal patches: Sodium alginate is used as a key component in transdermal patch formulations. These patches often include other ingredients such as polymers, plasticizers, and active pharmaceutical ingredients to create a stable and effective drug delivery system. The composition is designed to enhance skin permeation and control drug release.
- Manufacturing methods for sodium alginate patches: Various manufacturing techniques are employed to produce sodium alginate transdermal patches. These may include solvent casting, electrospinning, or 3D printing methods. The process often involves preparing a homogeneous mixture of sodium alginate with other components, forming it into a film or matrix, and incorporating the active drug.
- Drug release mechanisms and kinetics: Sodium alginate patches are designed to control drug release through various mechanisms. These may include diffusion, erosion, or swelling of the alginate matrix. The release kinetics can be tailored by adjusting the patch composition, crosslinking density, or incorporating release-modifying agents to achieve desired therapeutic effects.
- Enhancing skin permeation and bioavailability: Techniques to improve skin permeation and bioavailability of drugs from sodium alginate patches are explored. These may include the use of penetration enhancers, nanocarriers, or physical methods like iontophoresis. The goal is to increase the amount of drug that can pass through the skin barrier and reach systemic circulation.
- Applications and therapeutic areas: Sodium alginate transdermal patches are utilized in various therapeutic applications. These may include pain management, hormone replacement therapy, cardiovascular treatments, and delivery of anti-inflammatory drugs. The patches offer advantages such as controlled release, reduced side effects, and improved patient compliance compared to other dosage forms.
02 Drug delivery mechanisms in sodium alginate patches
Sodium alginate patches utilize various mechanisms for controlled drug release. These may include matrix systems, reservoir systems, or combinations thereof. The patches can be designed to provide sustained release of drugs over extended periods, improving patient compliance and therapeutic efficacy.Expand Specific Solutions03 Fabrication techniques for sodium alginate patches
Various methods are employed to manufacture sodium alginate transdermal patches. These may include solvent casting, electrospinning, or 3D printing techniques. The choice of fabrication method can influence patch properties such as drug loading capacity, adhesion, and release kinetics.Expand Specific Solutions04 Enhancing permeation and bioavailability
Strategies to improve drug permeation through the skin and enhance bioavailability in sodium alginate patches are explored. These may include the use of permeation enhancers, nanocarriers, or physical methods like iontophoresis or microneedles to facilitate drug absorption.Expand Specific Solutions05 Applications and therapeutic areas
Sodium alginate transdermal patches find applications in various therapeutic areas. They are used for delivering a wide range of drugs, including pain management medications, hormones, cardiovascular drugs, and central nervous system agents. The patches offer advantages such as non-invasive administration and reduced side effects compared to oral dosage forms.Expand Specific Solutions
Key Players in Transdermal Patch and Biomaterial Industries
The research on sodium alginate applications in transdermal patch improvement is in a growth phase, with increasing market potential due to the rising demand for advanced drug delivery systems. The global transdermal patch market is expected to reach significant size in the coming years, driven by factors such as convenience, controlled release, and improved patient compliance. Technologically, the field is advancing rapidly, with companies like Hisamitsu Pharmaceutical, Teikoku Seiyaku, and 3M Innovative Properties leading innovation. These firms are developing novel formulations and technologies to enhance drug permeation and patch adhesion. Academic institutions such as Zhejiang University and the University of Michigan are also contributing to research advancements, indicating a collaborative ecosystem between industry and academia in this domain.
Hisamitsu Pharmaceutical Co., Inc.
Technical Solution: Hisamitsu Pharmaceutical has developed a novel transdermal patch system incorporating sodium alginate as a key component. Their approach involves creating a multi-layered patch where sodium alginate is used in the adhesive matrix to enhance drug permeation and skin adhesion. The company has optimized the concentration of sodium alginate to achieve a balance between patch flexibility and drug release kinetics. Their research indicates that the inclusion of sodium alginate can increase drug permeation by up to 30% compared to traditional patches[1]. Additionally, they have explored the use of sodium alginate in combination with other polymers to create synergistic effects, further improving patch performance and stability[3].
Strengths: Improved drug permeation and skin adhesion, enhanced patch flexibility. Weaknesses: Potential for increased production costs, possible limitations in drug compatibility with alginate-based systems.
Zhongke Microneedle Beijing Technology Co Ltd.
Technical Solution: Zhongke Microneedle has developed an innovative transdermal delivery system that combines sodium alginate with microneedle technology. Their approach involves creating dissolvable microneedles composed of a sodium alginate matrix loaded with the drug of interest. Upon application to the skin, the microneedles penetrate the stratum corneum and dissolve, releasing the drug and sodium alginate into the deeper layers of the skin. The company's research has shown that this system can enhance drug permeation by up to 60% compared to traditional patches, particularly for macromolecular drugs[9]. Additionally, Zhongke has explored the use of sodium alginate as a stabilizing agent for sensitive biologics within the microneedle matrix, demonstrating improved stability and efficacy for protein-based drugs[10].
Strengths: Significantly enhanced drug permeation, potential for macromolecular drug delivery, improved patient compliance due to painless application. Weaknesses: Higher production costs, potential regulatory hurdles for microneedle-based systems.
Innovative Sodium Alginate Formulations for Enhanced Drug Permeation
A transdermal system based on a patch loaded with sodium alendronate coupled to an array of biodegradable polymeric microneedles for treating osteoporosis.
PatentActiveMX2020007129A
Innovation
- A transdermal system using a patch coupled with biodegradable polymeric microneedles to deliver alendronate sodium, incorporating chemical and physical permeation promoters, enhancing absorption and reducing adverse reactions.
Pharmaceutical preparation of percutaneous absorption type
PatentWO2002038139A1
Innovation
- A transdermal patch formulation containing a polymer compound with an amino group, an acid addition salt of a drug, and a carboxylic acid or its salt, where the polymer compound is used in amounts up to 50% by mass and forms an efficient ion pair with the drug, enhancing skin permeability and stability.
Regulatory Considerations for Novel Transdermal Drug Delivery Systems
The regulatory landscape for novel transdermal drug delivery systems, including those incorporating sodium alginate, is complex and evolving. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established specific guidelines for transdermal patch development and approval. These guidelines address various aspects, including safety, efficacy, quality control, and manufacturing processes.
One key consideration is the classification of the transdermal patch. Depending on its mechanism of action and intended use, it may be regulated as a drug, medical device, or combination product. This classification significantly impacts the regulatory pathway and requirements for approval.
Safety is paramount in regulatory considerations. Authorities require extensive toxicology studies to assess the potential risks associated with long-term skin exposure to the patch components, including sodium alginate and any other excipients. Additionally, the potential for skin irritation, sensitization, and systemic toxicity must be thoroughly evaluated.
Efficacy testing is another critical aspect. Regulatory bodies demand robust clinical trials to demonstrate the patch's ability to deliver the drug effectively through the skin and achieve the desired therapeutic effect. These trials must adhere to Good Clinical Practice (GCP) guidelines and provide comprehensive data on pharmacokinetics, pharmacodynamics, and clinical outcomes.
Quality control and manufacturing processes are subject to stringent regulations. Manufacturers must comply with Good Manufacturing Practice (GMP) standards to ensure consistent product quality and safety. This includes validated processes for patch production, stability testing, and quality assurance measures.
The regulatory framework also addresses specific challenges associated with transdermal delivery systems. These include ensuring consistent drug release rates, maintaining patch adhesion throughout the intended wear time, and preventing drug crystallization or degradation during storage and use.
For novel transdermal systems incorporating sodium alginate, additional regulatory considerations may apply. Authorities may require data on the long-term stability of sodium alginate within the patch matrix, its interaction with other patch components, and its impact on drug permeation and bioavailability.
As transdermal technology advances, regulatory bodies are adapting their guidelines to address emerging challenges. This includes considerations for smart patches, microneedle technologies, and other innovative delivery mechanisms. Manufacturers developing sodium alginate-based transdermal patches must stay abreast of these evolving regulations to ensure compliance throughout the development and approval process.
One key consideration is the classification of the transdermal patch. Depending on its mechanism of action and intended use, it may be regulated as a drug, medical device, or combination product. This classification significantly impacts the regulatory pathway and requirements for approval.
Safety is paramount in regulatory considerations. Authorities require extensive toxicology studies to assess the potential risks associated with long-term skin exposure to the patch components, including sodium alginate and any other excipients. Additionally, the potential for skin irritation, sensitization, and systemic toxicity must be thoroughly evaluated.
Efficacy testing is another critical aspect. Regulatory bodies demand robust clinical trials to demonstrate the patch's ability to deliver the drug effectively through the skin and achieve the desired therapeutic effect. These trials must adhere to Good Clinical Practice (GCP) guidelines and provide comprehensive data on pharmacokinetics, pharmacodynamics, and clinical outcomes.
Quality control and manufacturing processes are subject to stringent regulations. Manufacturers must comply with Good Manufacturing Practice (GMP) standards to ensure consistent product quality and safety. This includes validated processes for patch production, stability testing, and quality assurance measures.
The regulatory framework also addresses specific challenges associated with transdermal delivery systems. These include ensuring consistent drug release rates, maintaining patch adhesion throughout the intended wear time, and preventing drug crystallization or degradation during storage and use.
For novel transdermal systems incorporating sodium alginate, additional regulatory considerations may apply. Authorities may require data on the long-term stability of sodium alginate within the patch matrix, its interaction with other patch components, and its impact on drug permeation and bioavailability.
As transdermal technology advances, regulatory bodies are adapting their guidelines to address emerging challenges. This includes considerations for smart patches, microneedle technologies, and other innovative delivery mechanisms. Manufacturers developing sodium alginate-based transdermal patches must stay abreast of these evolving regulations to ensure compliance throughout the development and approval process.
Biocompatibility and Safety Assessment of Sodium Alginate in Transdermal Applications
The biocompatibility and safety assessment of sodium alginate in transdermal applications is a critical aspect of its potential use in improving transdermal patches. Sodium alginate, derived from brown seaweed, has gained significant attention in the pharmaceutical industry due to its unique properties and natural origin.
Extensive research has been conducted to evaluate the biocompatibility of sodium alginate when used in transdermal applications. In vitro studies have demonstrated that sodium alginate exhibits low cytotoxicity and does not induce significant inflammatory responses in skin cells. These findings suggest that sodium alginate is generally well-tolerated by human skin tissues.
Furthermore, in vivo studies using animal models have shown that sodium alginate-based transdermal patches do not cause significant skin irritation or sensitization. The absence of adverse reactions in these studies supports the potential safe use of sodium alginate in transdermal drug delivery systems.
One of the key advantages of sodium alginate is its biodegradability. When applied to the skin, sodium alginate gradually breaks down into harmless components that can be easily metabolized and eliminated by the body. This characteristic reduces the risk of long-term accumulation and potential toxicity associated with prolonged use of transdermal patches.
Safety assessments have also focused on the potential for sodium alginate to enhance the penetration of active pharmaceutical ingredients through the skin barrier. While sodium alginate has shown the ability to improve drug permeation, studies have indicated that it does not compromise the integrity of the stratum corneum or disrupt the skin's natural barrier function.
Allergenicity tests have revealed that sodium alginate has a low potential for causing allergic reactions. However, as with any biomaterial, individual variations in sensitivity may exist, and proper screening should be conducted before widespread use in transdermal applications.
Regulatory bodies, such as the FDA and EMA, have recognized sodium alginate as generally regarded as safe (GRAS) for various applications, including food and pharmaceutical use. This classification provides a foundation for its potential incorporation into transdermal patch formulations, although specific safety evaluations for each intended application are still necessary.
It is important to note that while sodium alginate demonstrates promising biocompatibility and safety profiles, ongoing research is essential to fully understand its long-term effects and potential interactions with different drugs and skin types. Continued vigilance and post-market surveillance will be crucial as sodium alginate-based transdermal patches move towards clinical applications.
Extensive research has been conducted to evaluate the biocompatibility of sodium alginate when used in transdermal applications. In vitro studies have demonstrated that sodium alginate exhibits low cytotoxicity and does not induce significant inflammatory responses in skin cells. These findings suggest that sodium alginate is generally well-tolerated by human skin tissues.
Furthermore, in vivo studies using animal models have shown that sodium alginate-based transdermal patches do not cause significant skin irritation or sensitization. The absence of adverse reactions in these studies supports the potential safe use of sodium alginate in transdermal drug delivery systems.
One of the key advantages of sodium alginate is its biodegradability. When applied to the skin, sodium alginate gradually breaks down into harmless components that can be easily metabolized and eliminated by the body. This characteristic reduces the risk of long-term accumulation and potential toxicity associated with prolonged use of transdermal patches.
Safety assessments have also focused on the potential for sodium alginate to enhance the penetration of active pharmaceutical ingredients through the skin barrier. While sodium alginate has shown the ability to improve drug permeation, studies have indicated that it does not compromise the integrity of the stratum corneum or disrupt the skin's natural barrier function.
Allergenicity tests have revealed that sodium alginate has a low potential for causing allergic reactions. However, as with any biomaterial, individual variations in sensitivity may exist, and proper screening should be conducted before widespread use in transdermal applications.
Regulatory bodies, such as the FDA and EMA, have recognized sodium alginate as generally regarded as safe (GRAS) for various applications, including food and pharmaceutical use. This classification provides a foundation for its potential incorporation into transdermal patch formulations, although specific safety evaluations for each intended application are still necessary.
It is important to note that while sodium alginate demonstrates promising biocompatibility and safety profiles, ongoing research is essential to fully understand its long-term effects and potential interactions with different drugs and skin types. Continued vigilance and post-market surveillance will be crucial as sodium alginate-based transdermal patches move towards clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!