Solvent Power of Heptane in Pharmaceutical Compound Crystallization
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Heptane Solvent Power Background and Objectives
Heptane, a saturated hydrocarbon with the molecular formula C7H16, has gained significant attention in the pharmaceutical industry for its role in compound crystallization processes. The evolution of heptane as a solvent in pharmaceutical applications can be traced back to the mid-20th century when researchers began exploring its unique properties for crystallization and purification of drug substances.
The primary objective of this research is to comprehensively investigate the solvent power of heptane in pharmaceutical compound crystallization. This involves examining its physicochemical properties, solubility characteristics, and interaction mechanisms with various pharmaceutical compounds. Understanding these aspects is crucial for optimizing crystallization processes, enhancing product purity, and improving overall drug manufacturing efficiency.
Heptane's low polarity and limited miscibility with water make it an attractive option for crystallization of non-polar or slightly polar pharmaceutical compounds. Its relatively low boiling point (98.4°C) also facilitates easy removal and recovery, contributing to its appeal in industrial applications. However, the specific solvent power of heptane can vary significantly depending on the nature of the pharmaceutical compound, temperature, pressure, and the presence of other solvents or additives.
Recent technological advancements have led to increased interest in heptane's potential for improving crystallization outcomes. These developments include novel crystallization techniques, such as antisolvent crystallization and spherical crystallization, where heptane plays a crucial role. Additionally, the growing emphasis on green chemistry and sustainable manufacturing processes has sparked research into heptane as a more environmentally friendly alternative to some traditional solvents.
The pharmaceutical industry's continuous pursuit of higher quality standards and more efficient production methods drives the need for a deeper understanding of heptane's solvent power. This research aims to bridge knowledge gaps and provide insights that can lead to improved crystallization protocols, enhanced product characteristics, and potentially new drug formulation strategies.
By exploring the fundamental principles governing heptane's solvent power and its practical applications in pharmaceutical compound crystallization, this study seeks to contribute to the broader field of pharmaceutical process development. The findings are expected to have implications for drug purity, polymorphism control, and overall manufacturing efficiency, ultimately supporting the industry's goals of producing high-quality pharmaceutical products more effectively and sustainably.
The primary objective of this research is to comprehensively investigate the solvent power of heptane in pharmaceutical compound crystallization. This involves examining its physicochemical properties, solubility characteristics, and interaction mechanisms with various pharmaceutical compounds. Understanding these aspects is crucial for optimizing crystallization processes, enhancing product purity, and improving overall drug manufacturing efficiency.
Heptane's low polarity and limited miscibility with water make it an attractive option for crystallization of non-polar or slightly polar pharmaceutical compounds. Its relatively low boiling point (98.4°C) also facilitates easy removal and recovery, contributing to its appeal in industrial applications. However, the specific solvent power of heptane can vary significantly depending on the nature of the pharmaceutical compound, temperature, pressure, and the presence of other solvents or additives.
Recent technological advancements have led to increased interest in heptane's potential for improving crystallization outcomes. These developments include novel crystallization techniques, such as antisolvent crystallization and spherical crystallization, where heptane plays a crucial role. Additionally, the growing emphasis on green chemistry and sustainable manufacturing processes has sparked research into heptane as a more environmentally friendly alternative to some traditional solvents.
The pharmaceutical industry's continuous pursuit of higher quality standards and more efficient production methods drives the need for a deeper understanding of heptane's solvent power. This research aims to bridge knowledge gaps and provide insights that can lead to improved crystallization protocols, enhanced product characteristics, and potentially new drug formulation strategies.
By exploring the fundamental principles governing heptane's solvent power and its practical applications in pharmaceutical compound crystallization, this study seeks to contribute to the broader field of pharmaceutical process development. The findings are expected to have implications for drug purity, polymorphism control, and overall manufacturing efficiency, ultimately supporting the industry's goals of producing high-quality pharmaceutical products more effectively and sustainably.
Pharmaceutical Industry Demand Analysis
The pharmaceutical industry's demand for efficient and cost-effective crystallization processes has been steadily increasing in recent years. This demand is driven by the need to improve drug formulation, enhance bioavailability, and optimize manufacturing processes. Heptane, as a solvent in pharmaceutical compound crystallization, has garnered significant attention due to its unique properties and potential benefits.
The global pharmaceutical market is projected to reach $1.5 trillion by 2023, with a compound annual growth rate of 3-6%. This growth is accompanied by an increased focus on developing novel drug delivery systems and improving existing formulations. Crystallization plays a crucial role in this process, as it directly impacts the physicochemical properties of pharmaceutical compounds, including solubility, stability, and bioavailability.
The use of heptane in pharmaceutical compound crystallization addresses several key industry needs. Firstly, it offers a non-polar solvent option, which is particularly valuable for crystallizing hydrophobic compounds. This characteristic is essential for many modern drugs, as an estimated 40% of approved drugs and nearly 90% of developmental pipeline drugs are poorly water-soluble.
Furthermore, the pharmaceutical industry is under constant pressure to reduce production costs and improve process efficiency. Heptane's relatively low boiling point (98.4°C) allows for easier solvent removal and recovery, potentially leading to significant cost savings in large-scale manufacturing processes. This aligns with the industry's push towards more sustainable and economical production methods.
The demand for heptane in pharmaceutical crystallization is also driven by its compatibility with continuous manufacturing processes. As the industry shifts towards continuous production to improve efficiency and reduce costs, solvents that can facilitate this transition are highly sought after. Heptane's properties make it suitable for use in continuous crystallization systems, which are expected to become increasingly prevalent in pharmaceutical manufacturing.
Additionally, regulatory considerations play a significant role in solvent selection for pharmaceutical processes. Heptane is classified as a Class 3 solvent by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), indicating lower toxicity concerns compared to many other organic solvents. This classification makes heptane an attractive option for pharmaceutical companies seeking to comply with stringent regulatory requirements while optimizing their crystallization processes.
In conclusion, the pharmaceutical industry's demand for research on the solvent power of heptane in compound crystallization is driven by the need for improved drug formulations, cost-effective manufacturing processes, and compliance with regulatory standards. As the industry continues to evolve and face new challenges, the exploration of heptane's potential in crystallization processes remains a key area of interest for pharmaceutical companies and researchers alike.
The global pharmaceutical market is projected to reach $1.5 trillion by 2023, with a compound annual growth rate of 3-6%. This growth is accompanied by an increased focus on developing novel drug delivery systems and improving existing formulations. Crystallization plays a crucial role in this process, as it directly impacts the physicochemical properties of pharmaceutical compounds, including solubility, stability, and bioavailability.
The use of heptane in pharmaceutical compound crystallization addresses several key industry needs. Firstly, it offers a non-polar solvent option, which is particularly valuable for crystallizing hydrophobic compounds. This characteristic is essential for many modern drugs, as an estimated 40% of approved drugs and nearly 90% of developmental pipeline drugs are poorly water-soluble.
Furthermore, the pharmaceutical industry is under constant pressure to reduce production costs and improve process efficiency. Heptane's relatively low boiling point (98.4°C) allows for easier solvent removal and recovery, potentially leading to significant cost savings in large-scale manufacturing processes. This aligns with the industry's push towards more sustainable and economical production methods.
The demand for heptane in pharmaceutical crystallization is also driven by its compatibility with continuous manufacturing processes. As the industry shifts towards continuous production to improve efficiency and reduce costs, solvents that can facilitate this transition are highly sought after. Heptane's properties make it suitable for use in continuous crystallization systems, which are expected to become increasingly prevalent in pharmaceutical manufacturing.
Additionally, regulatory considerations play a significant role in solvent selection for pharmaceutical processes. Heptane is classified as a Class 3 solvent by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), indicating lower toxicity concerns compared to many other organic solvents. This classification makes heptane an attractive option for pharmaceutical companies seeking to comply with stringent regulatory requirements while optimizing their crystallization processes.
In conclusion, the pharmaceutical industry's demand for research on the solvent power of heptane in compound crystallization is driven by the need for improved drug formulations, cost-effective manufacturing processes, and compliance with regulatory standards. As the industry continues to evolve and face new challenges, the exploration of heptane's potential in crystallization processes remains a key area of interest for pharmaceutical companies and researchers alike.
Current Challenges in Heptane Solvent Power
The use of heptane as a solvent in pharmaceutical compound crystallization faces several significant challenges that researchers and industry professionals are actively working to address. One of the primary issues is the limited solvent power of heptane for many pharmaceutical compounds. This low solubility can lead to inefficient crystallization processes, potentially resulting in low yields and poor crystal quality. The hydrophobic nature of heptane often makes it difficult to dissolve polar or ionic pharmaceutical molecules, which constitute a large portion of drug compounds.
Another challenge is the temperature sensitivity of heptane's solvent power. As with many organic solvents, the solubility of compounds in heptane can vary significantly with temperature changes. This sensitivity can complicate the control of crystallization processes, particularly in large-scale industrial settings where maintaining precise temperature control throughout the entire system can be challenging. Researchers must carefully optimize temperature profiles to achieve desired crystal morphology and purity.
The environmental and safety concerns associated with heptane usage present additional challenges. Heptane is a volatile organic compound (VOC) with potential environmental impacts and health risks. Stringent regulations govern its use and disposal, necessitating sophisticated containment and recovery systems in pharmaceutical manufacturing facilities. This not only increases operational costs but also complicates process design and scale-up efforts.
Furthermore, the selectivity of heptane as a solvent can be problematic in certain crystallization scenarios. While its low polarity can be advantageous for separating non-polar compounds, it may not provide adequate discrimination between structurally similar molecules or polymorphs. This lack of selectivity can lead to difficulties in purification processes and the isolation of specific crystal forms, which is crucial in pharmaceutical development.
The interaction of heptane with other solvents and additives used in crystallization processes presents another area of complexity. Co-solvent systems or the addition of anti-solvents can significantly alter the solvent power and crystallization behavior of heptane-based systems. Understanding and predicting these interactions requires extensive research and experimentation, often necessitating a combination of empirical studies and computational modeling approaches.
Lastly, the scalability of heptane-based crystallization processes from laboratory to industrial scale remains a significant challenge. Factors such as mixing dynamics, heat transfer, and mass transfer can behave differently at larger scales, potentially affecting crystal nucleation, growth, and morphology. Researchers must develop robust scale-up strategies that account for these changes while maintaining product quality and process efficiency.
Another challenge is the temperature sensitivity of heptane's solvent power. As with many organic solvents, the solubility of compounds in heptane can vary significantly with temperature changes. This sensitivity can complicate the control of crystallization processes, particularly in large-scale industrial settings where maintaining precise temperature control throughout the entire system can be challenging. Researchers must carefully optimize temperature profiles to achieve desired crystal morphology and purity.
The environmental and safety concerns associated with heptane usage present additional challenges. Heptane is a volatile organic compound (VOC) with potential environmental impacts and health risks. Stringent regulations govern its use and disposal, necessitating sophisticated containment and recovery systems in pharmaceutical manufacturing facilities. This not only increases operational costs but also complicates process design and scale-up efforts.
Furthermore, the selectivity of heptane as a solvent can be problematic in certain crystallization scenarios. While its low polarity can be advantageous for separating non-polar compounds, it may not provide adequate discrimination between structurally similar molecules or polymorphs. This lack of selectivity can lead to difficulties in purification processes and the isolation of specific crystal forms, which is crucial in pharmaceutical development.
The interaction of heptane with other solvents and additives used in crystallization processes presents another area of complexity. Co-solvent systems or the addition of anti-solvents can significantly alter the solvent power and crystallization behavior of heptane-based systems. Understanding and predicting these interactions requires extensive research and experimentation, often necessitating a combination of empirical studies and computational modeling approaches.
Lastly, the scalability of heptane-based crystallization processes from laboratory to industrial scale remains a significant challenge. Factors such as mixing dynamics, heat transfer, and mass transfer can behave differently at larger scales, potentially affecting crystal nucleation, growth, and morphology. Researchers must develop robust scale-up strategies that account for these changes while maintaining product quality and process efficiency.
Existing Heptane Solvent Power Solutions
01 Solvent power in extraction processes
Heptane demonstrates significant solvent power in various extraction processes, particularly in the separation of organic compounds. Its non-polar nature makes it effective for extracting oils, fats, and other hydrophobic substances from mixtures. This property is utilized in industrial applications such as oil extraction from seeds and the purification of chemical compounds.- Solvent power in extraction processes: Heptane demonstrates significant solvent power in various extraction processes, particularly in the separation of organic compounds. Its non-polar nature makes it effective for extracting oils, fats, and other hydrophobic substances from mixtures. This property is utilized in industrial applications such as oil refining and chemical purification.
- Use in polymer and resin formulations: Heptane serves as an important solvent in polymer and resin formulations. Its solvent power allows for the dissolution of various polymeric materials, facilitating the production of coatings, adhesives, and sealants. The volatility of heptane also contributes to its effectiveness in these applications, allowing for controlled evaporation and film formation.
- Application in cleaning and degreasing: The solvent power of heptane makes it an effective agent for cleaning and degreasing in various industries. It can dissolve and remove oils, greases, and other organic contaminants from surfaces and components. This property is particularly useful in manufacturing processes, maintenance operations, and precision cleaning applications.
- Role in chromatography and analytical chemistry: Heptane's solvent power is utilized in chromatography and analytical chemistry techniques. Its ability to dissolve and separate various organic compounds makes it valuable in column chromatography, thin-layer chromatography, and other analytical separation methods. This application is crucial in research, quality control, and forensic analysis.
- Environmental and safety considerations: While heptane possesses strong solvent power, its use requires careful consideration of environmental and safety factors. As a volatile organic compound, it can contribute to air pollution and pose health risks if not properly handled. Research and development efforts focus on optimizing its use, developing safer alternatives, and implementing proper containment and disposal methods to mitigate potential negative impacts.
02 Use in chromatography and analytical chemistry
Heptane's solvent power is valuable in chromatographic techniques and analytical chemistry. It serves as a mobile phase in liquid chromatography and as a solvent for sample preparation in various analytical methods. Its ability to dissolve non-polar analytes while maintaining low background interference makes it suitable for sensitive analytical procedures.Expand Specific Solutions03 Application in polymer and rubber industries
The solvent power of heptane is utilized in the polymer and rubber industries for dissolving and processing various materials. It is effective in dissolving certain polymers, elastomers, and resins, making it useful in the production of adhesives, coatings, and rubber compounds. Heptane's ability to swell rubber materials is also exploited in specific applications.Expand Specific Solutions04 Role in pharmaceutical and cosmetic formulations
Heptane's solvent power is employed in the pharmaceutical and cosmetic industries for the formulation of various products. It is used as a solvent for active ingredients, excipients, and in the preparation of emulsions and suspensions. Its low toxicity and ability to dissolve lipophilic compounds make it suitable for certain drug delivery systems and cosmetic preparations.Expand Specific Solutions05 Environmental and safety considerations
While heptane possesses significant solvent power, its use is subject to environmental and safety considerations. As a volatile organic compound, proper handling and disposal procedures are necessary to minimize environmental impact and ensure worker safety. Research focuses on developing safer alternatives or optimizing processes to reduce heptane usage while maintaining its beneficial solvent properties.Expand Specific Solutions
Key Players in Pharmaceutical Crystallization
The research on heptane's solvent power in pharmaceutical compound crystallization is in a developing stage, with the market showing potential for growth. The technology's maturity is progressing, as evidenced by involvement from major pharmaceutical companies like Janssen Pharmaceutica NV, Boehringer Ingelheim, and Astellas Pharma. Academic institutions such as Virginia Commonwealth University and Thomas Jefferson University are also contributing to advancements in this field. The competitive landscape is diverse, including both established pharmaceutical giants and specialized research institutions, indicating a balanced mix of industry and academic efforts to further develop and apply this technology in drug formulation and manufacturing processes.
Janssen Pharmaceutica NV
Technical Solution: Janssen Pharmaceutica NV has developed an innovative approach to utilizing heptane in pharmaceutical compound crystallization. Their method involves a controlled evaporation process where heptane is used as an anti-solvent to induce crystallization of target compounds[1]. This technique allows for precise control over crystal size and morphology, which is crucial for drug bioavailability and stability. The company has also implemented a continuous crystallization system that incorporates heptane as a key solvent, enabling more efficient and scalable production of pharmaceutical compounds[3]. Additionally, Janssen has explored the use of heptane in co-crystallization processes, where it serves as a medium for forming novel crystal structures with enhanced physicochemical properties[5].
Strengths: Precise control over crystal properties, scalable continuous production, potential for novel crystal forms. Weaknesses: Limited to compounds soluble in heptane, potential environmental concerns due to volatile organic compound use.
Boehringer Ingelheim International GmbH
Technical Solution: Boehringer Ingelheim has developed a sophisticated approach to heptane-based crystallization for pharmaceutical compounds. Their method involves a temperature-controlled anti-solvent crystallization process, where heptane is introduced gradually to induce nucleation and crystal growth[2]. This technique allows for fine-tuning of crystal size distribution and polymorphic form. The company has also implemented in-line process analytical technology (PAT) to monitor and control the crystallization process in real-time, ensuring consistent product quality[4]. Furthermore, Boehringer Ingelheim has explored the use of heptane in combination with other solvents to create unique solvent systems that can selectively crystallize target compounds from complex mixtures[6].
Strengths: Precise control over crystal properties, real-time process monitoring, versatility in handling complex mixtures. Weaknesses: Potential for solvent residues in final product, energy-intensive temperature control requirements.
Core Innovations in Heptane Solvent Research
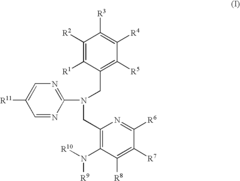
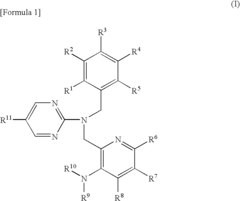
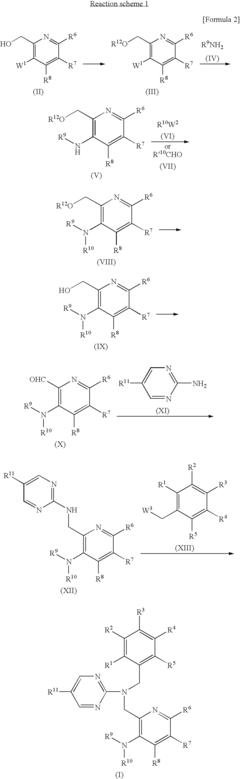
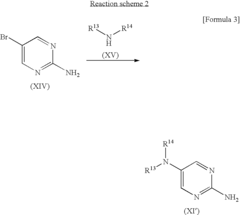
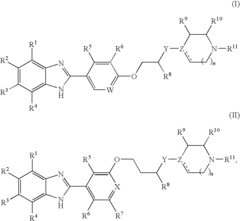
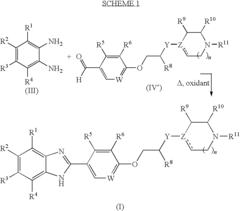
Pyrimidine compound having benzyl(pyridylmethyl)amine structure and medicament comprising the same
PatentInactiveUS7750019B2
Innovation
- Development of pyrimidine compounds with a benzyl(pyridylmethyl)amine structure that exhibit potent inhibitory activity against cholesterol ester transfer protein (CETP), allowing for increased HDL cholesterol levels and decreased LDL/VLDL cholesterol levels, thereby providing a novel approach for treating hyperlipidemia and arteriosclerosis.
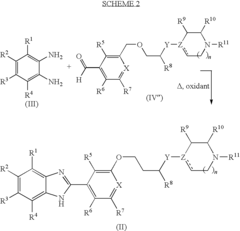
Benzoimidazole compounds
PatentInactiveUS20090275748A1
Innovation
- Development of novel benzoimidazole compounds that act as H4 receptor modulators, inhibiting leukocyte recruitment and exhibiting anti-inflammatory properties, which are administered to treat or prevent H4 receptor-mediated conditions.
Environmental Impact of Heptane Usage
The use of heptane in pharmaceutical compound crystallization processes raises significant environmental concerns that warrant careful consideration. As a non-polar hydrocarbon solvent, heptane poses potential risks to both aquatic and terrestrial ecosystems if not properly managed. When released into the environment, heptane can contaminate soil and water sources, leading to adverse effects on flora and fauna. Its low water solubility and high volatility contribute to its persistence in the environment, potentially causing long-term ecological damage.
In aquatic environments, heptane forms a thin film on the water surface, impeding oxygen transfer and disrupting the delicate balance of aquatic ecosystems. This can lead to decreased dissolved oxygen levels, affecting the survival of fish and other aquatic organisms. Furthermore, heptane's lipophilic nature allows it to accumulate in the fatty tissues of aquatic life, potentially leading to bioaccumulation and biomagnification through the food chain.
Terrestrial ecosystems are also at risk from heptane contamination. Soil microorganisms, which play crucial roles in nutrient cycling and soil health, can be adversely affected by the presence of heptane. This can lead to reduced soil fertility and altered ecosystem functions. Additionally, plants exposed to heptane-contaminated soil may experience stunted growth, reduced photosynthetic activity, and other physiological stress responses.
The atmospheric impact of heptane usage is another critical concern. As a volatile organic compound (VOC), heptane contributes to the formation of ground-level ozone and photochemical smog when released into the air. These air quality issues can have far-reaching consequences for human health and the environment, particularly in urban and industrial areas where pharmaceutical manufacturing facilities are often located.
To mitigate these environmental risks, pharmaceutical companies must implement stringent control measures and adopt best practices in heptane handling and disposal. This includes the use of closed-loop systems to minimize emissions, proper storage and transportation protocols, and effective wastewater treatment technologies. Additionally, exploring alternative, more environmentally friendly solvents or developing solvent-free crystallization techniques could significantly reduce the industry's reliance on heptane and its associated environmental impacts.
Regulatory bodies worldwide have recognized the potential environmental hazards of heptane and have implemented strict guidelines for its use and disposal. Compliance with these regulations is crucial for pharmaceutical companies to minimize their environmental footprint and maintain sustainable operations. As the industry continues to evolve, there is a growing emphasis on green chemistry principles and the development of more sustainable pharmaceutical manufacturing processes, which may ultimately lead to reduced dependence on environmentally problematic solvents like heptane.
In aquatic environments, heptane forms a thin film on the water surface, impeding oxygen transfer and disrupting the delicate balance of aquatic ecosystems. This can lead to decreased dissolved oxygen levels, affecting the survival of fish and other aquatic organisms. Furthermore, heptane's lipophilic nature allows it to accumulate in the fatty tissues of aquatic life, potentially leading to bioaccumulation and biomagnification through the food chain.
Terrestrial ecosystems are also at risk from heptane contamination. Soil microorganisms, which play crucial roles in nutrient cycling and soil health, can be adversely affected by the presence of heptane. This can lead to reduced soil fertility and altered ecosystem functions. Additionally, plants exposed to heptane-contaminated soil may experience stunted growth, reduced photosynthetic activity, and other physiological stress responses.
The atmospheric impact of heptane usage is another critical concern. As a volatile organic compound (VOC), heptane contributes to the formation of ground-level ozone and photochemical smog when released into the air. These air quality issues can have far-reaching consequences for human health and the environment, particularly in urban and industrial areas where pharmaceutical manufacturing facilities are often located.
To mitigate these environmental risks, pharmaceutical companies must implement stringent control measures and adopt best practices in heptane handling and disposal. This includes the use of closed-loop systems to minimize emissions, proper storage and transportation protocols, and effective wastewater treatment technologies. Additionally, exploring alternative, more environmentally friendly solvents or developing solvent-free crystallization techniques could significantly reduce the industry's reliance on heptane and its associated environmental impacts.
Regulatory bodies worldwide have recognized the potential environmental hazards of heptane and have implemented strict guidelines for its use and disposal. Compliance with these regulations is crucial for pharmaceutical companies to minimize their environmental footprint and maintain sustainable operations. As the industry continues to evolve, there is a growing emphasis on green chemistry principles and the development of more sustainable pharmaceutical manufacturing processes, which may ultimately lead to reduced dependence on environmentally problematic solvents like heptane.
Scale-up Considerations for Industrial Applications
When considering the scale-up of heptane-based crystallization processes for pharmaceutical compounds, several key factors must be addressed to ensure successful industrial implementation. Firstly, the heat transfer characteristics of larger vessels must be carefully evaluated. As the volume increases, the surface area to volume ratio decreases, potentially leading to temperature gradients and inconsistent crystallization rates. To mitigate this, advanced heat transfer systems, such as external jacket cooling or internal coil designs, may need to be incorporated.
Mixing dynamics also play a crucial role in scale-up considerations. The increased volume and potential changes in vessel geometry can significantly affect mixing patterns and efficiency. This may necessitate the use of specialized impeller designs or multiple impeller configurations to maintain uniform supersaturation levels throughout the crystallizer. Additionally, computational fluid dynamics (CFD) simulations can be employed to optimize mixing parameters and predict potential dead zones or areas of high shear.
The impact of scale-up on nucleation and crystal growth kinetics must be thoroughly investigated. Changes in mixing intensity and heat transfer rates can alter the supersaturation profile, potentially affecting the crystal size distribution and polymorphic form. To address this, in-situ process analytical technology (PAT) tools, such as focused beam reflectance measurement (FBRM) or particle vision microscopy (PVM), can be implemented to monitor and control crystal properties in real-time.
Solvent recovery and recycling become increasingly important at industrial scales. The implementation of efficient distillation or membrane separation systems for heptane recovery can significantly impact process economics and environmental sustainability. Moreover, the purity of recovered heptane must be carefully monitored to prevent the accumulation of impurities that could affect subsequent crystallization cycles.
Filtration and drying operations also require careful consideration during scale-up. The increased crystal mass and potential changes in crystal habit may necessitate the use of different filtration equipment or strategies. Continuous filtration systems or specialized filter designs may be required to handle larger volumes efficiently. Similarly, drying times and conditions may need to be adjusted to accommodate the increased product mass while maintaining product quality and avoiding solvent entrapment.
Lastly, safety considerations must be paramount when scaling up heptane-based crystallization processes. The flammability and volatility of heptane necessitate robust explosion-proof equipment and stringent safety protocols. Proper ventilation systems, grounding procedures, and emergency shutdown mechanisms must be implemented to mitigate risks associated with large-scale heptane handling and storage.
Mixing dynamics also play a crucial role in scale-up considerations. The increased volume and potential changes in vessel geometry can significantly affect mixing patterns and efficiency. This may necessitate the use of specialized impeller designs or multiple impeller configurations to maintain uniform supersaturation levels throughout the crystallizer. Additionally, computational fluid dynamics (CFD) simulations can be employed to optimize mixing parameters and predict potential dead zones or areas of high shear.
The impact of scale-up on nucleation and crystal growth kinetics must be thoroughly investigated. Changes in mixing intensity and heat transfer rates can alter the supersaturation profile, potentially affecting the crystal size distribution and polymorphic form. To address this, in-situ process analytical technology (PAT) tools, such as focused beam reflectance measurement (FBRM) or particle vision microscopy (PVM), can be implemented to monitor and control crystal properties in real-time.
Solvent recovery and recycling become increasingly important at industrial scales. The implementation of efficient distillation or membrane separation systems for heptane recovery can significantly impact process economics and environmental sustainability. Moreover, the purity of recovered heptane must be carefully monitored to prevent the accumulation of impurities that could affect subsequent crystallization cycles.
Filtration and drying operations also require careful consideration during scale-up. The increased crystal mass and potential changes in crystal habit may necessitate the use of different filtration equipment or strategies. Continuous filtration systems or specialized filter designs may be required to handle larger volumes efficiently. Similarly, drying times and conditions may need to be adjusted to accommodate the increased product mass while maintaining product quality and avoiding solvent entrapment.
Lastly, safety considerations must be paramount when scaling up heptane-based crystallization processes. The flammability and volatility of heptane necessitate robust explosion-proof equipment and stringent safety protocols. Proper ventilation systems, grounding procedures, and emergency shutdown mechanisms must be implemented to mitigate risks associated with large-scale heptane handling and storage.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!