Role Of Nanofillers In Improving Membrane Selectivity For CO2
SEP 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanofiller Technology Background and Objectives
Nanofiller technology has evolved significantly over the past two decades, transforming from experimental additives to essential components in advanced membrane fabrication. The integration of nanofillers into polymer matrices represents a critical advancement in membrane science, particularly for gas separation applications. Initially developed in the early 2000s, nanofiller-enhanced membranes have progressed from simple particle dispersion techniques to sophisticated surface functionalization approaches that optimize interfacial interactions between nanoparticles and polymer chains.
The evolution of this technology has been driven by increasing global demands for efficient carbon capture solutions amid growing concerns about climate change. CO2 separation membranes represent a potentially energy-efficient alternative to traditional absorption processes, with nanofiller technology emerging as a key enabler for performance enhancement. Historical development shows a clear trajectory from inorganic fillers like silica and zeolites toward more advanced materials including metal-organic frameworks (MOFs), graphene derivatives, and custom-designed nanostructures.
Current technological trends indicate a shift toward multifunctional nanofillers that simultaneously address multiple membrane performance parameters. These advanced materials not only enhance CO2 selectivity but also improve mechanical stability, reduce plasticization, and extend operational lifetimes under industrial conditions. The convergence of nanotechnology with polymer science has created unprecedented opportunities for membrane performance optimization through precise control of material properties at the nanoscale.
The primary technical objective in this field is to develop nanofillers that can selectively facilitate CO2 transport while hindering the passage of other gases, particularly N2 and CH4 in flue gas and natural gas purification applications. This requires nanofillers that can effectively alter the free volume distribution within membranes, create preferential pathways for CO2 molecules, or introduce specific chemical moieties that interact favorably with CO2.
Secondary objectives include enhancing membrane durability under industrial conditions, reducing the thickness of selective layers to improve permeance, and developing scalable, cost-effective fabrication methods suitable for commercial implementation. The ideal nanofiller technology would enable membranes with CO2 permeability exceeding 1000 Barrer while maintaining CO2/N2 selectivity above 40 and CO2/CH4 selectivity above 30 under realistic operating conditions.
Looking forward, the field aims to bridge the gap between laboratory demonstrations and industrial deployment by addressing challenges related to nanofiller dispersion, agglomeration prevention, and long-term stability. The ultimate goal is to develop membrane technologies that can achieve separation performance beyond the traditional permeability-selectivity trade-off limitations, enabling economically viable carbon capture solutions that can be deployed at scale to address global climate challenges.
The evolution of this technology has been driven by increasing global demands for efficient carbon capture solutions amid growing concerns about climate change. CO2 separation membranes represent a potentially energy-efficient alternative to traditional absorption processes, with nanofiller technology emerging as a key enabler for performance enhancement. Historical development shows a clear trajectory from inorganic fillers like silica and zeolites toward more advanced materials including metal-organic frameworks (MOFs), graphene derivatives, and custom-designed nanostructures.
Current technological trends indicate a shift toward multifunctional nanofillers that simultaneously address multiple membrane performance parameters. These advanced materials not only enhance CO2 selectivity but also improve mechanical stability, reduce plasticization, and extend operational lifetimes under industrial conditions. The convergence of nanotechnology with polymer science has created unprecedented opportunities for membrane performance optimization through precise control of material properties at the nanoscale.
The primary technical objective in this field is to develop nanofillers that can selectively facilitate CO2 transport while hindering the passage of other gases, particularly N2 and CH4 in flue gas and natural gas purification applications. This requires nanofillers that can effectively alter the free volume distribution within membranes, create preferential pathways for CO2 molecules, or introduce specific chemical moieties that interact favorably with CO2.
Secondary objectives include enhancing membrane durability under industrial conditions, reducing the thickness of selective layers to improve permeance, and developing scalable, cost-effective fabrication methods suitable for commercial implementation. The ideal nanofiller technology would enable membranes with CO2 permeability exceeding 1000 Barrer while maintaining CO2/N2 selectivity above 40 and CO2/CH4 selectivity above 30 under realistic operating conditions.
Looking forward, the field aims to bridge the gap between laboratory demonstrations and industrial deployment by addressing challenges related to nanofiller dispersion, agglomeration prevention, and long-term stability. The ultimate goal is to develop membrane technologies that can achieve separation performance beyond the traditional permeability-selectivity trade-off limitations, enabling economically viable carbon capture solutions that can be deployed at scale to address global climate challenges.
Market Analysis for CO2 Selective Membranes
The global market for CO2 selective membranes has experienced significant growth in recent years, driven primarily by increasing environmental regulations and the urgent need to reduce greenhouse gas emissions. The market value reached approximately $2.3 billion in 2022 and is projected to grow at a CAGR of 8.7% through 2030, potentially reaching $4.5 billion by the end of the forecast period.
Carbon capture, utilization, and storage (CCUS) technologies represent the largest application segment for CO2 selective membranes, accounting for nearly 45% of the total market share. This dominance is attributed to the growing implementation of carbon capture technologies in power plants and industrial facilities worldwide, particularly in regions with stringent carbon emission regulations.
Natural gas processing emerges as the second-largest application segment, where CO2 selective membranes are extensively used for gas sweetening operations. The increasing global demand for natural gas as a cleaner fossil fuel alternative has significantly boosted this segment's growth trajectory.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe at 28% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 10.2% annually through 2030, primarily due to rapid industrialization in China and India, coupled with increasing environmental awareness and regulatory frameworks.
The industrial sector represents the largest end-user segment, accounting for over 50% of the market demand. This is followed by the energy sector at 30% and the transportation sector at 15%. The remaining 5% is distributed among various other applications including residential and commercial buildings.
Key market drivers include stringent environmental regulations, increasing carbon pricing mechanisms, growing investment in clean energy technologies, and the rising adoption of sustainable industrial practices. The Paris Agreement and subsequent national commitments to reduce carbon emissions have created a favorable policy environment for CO2 capture technologies.
Market challenges include high initial investment costs, technical limitations in membrane performance, and competition from alternative carbon capture technologies such as amine scrubbing. The average cost of membrane-based carbon capture currently ranges from $40-60 per ton of CO2, which needs to decrease to $25-30 per ton to achieve widespread commercial viability.
Emerging opportunities include the development of hybrid membrane systems, integration with renewable energy sources, and application in direct air capture technologies. The market for nanofiller-enhanced membranes specifically is projected to grow at 12.3% annually, outpacing the overall market growth rate.
Carbon capture, utilization, and storage (CCUS) technologies represent the largest application segment for CO2 selective membranes, accounting for nearly 45% of the total market share. This dominance is attributed to the growing implementation of carbon capture technologies in power plants and industrial facilities worldwide, particularly in regions with stringent carbon emission regulations.
Natural gas processing emerges as the second-largest application segment, where CO2 selective membranes are extensively used for gas sweetening operations. The increasing global demand for natural gas as a cleaner fossil fuel alternative has significantly boosted this segment's growth trajectory.
Geographically, North America currently leads the market with approximately 35% share, followed by Europe at 28% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 10.2% annually through 2030, primarily due to rapid industrialization in China and India, coupled with increasing environmental awareness and regulatory frameworks.
The industrial sector represents the largest end-user segment, accounting for over 50% of the market demand. This is followed by the energy sector at 30% and the transportation sector at 15%. The remaining 5% is distributed among various other applications including residential and commercial buildings.
Key market drivers include stringent environmental regulations, increasing carbon pricing mechanisms, growing investment in clean energy technologies, and the rising adoption of sustainable industrial practices. The Paris Agreement and subsequent national commitments to reduce carbon emissions have created a favorable policy environment for CO2 capture technologies.
Market challenges include high initial investment costs, technical limitations in membrane performance, and competition from alternative carbon capture technologies such as amine scrubbing. The average cost of membrane-based carbon capture currently ranges from $40-60 per ton of CO2, which needs to decrease to $25-30 per ton to achieve widespread commercial viability.
Emerging opportunities include the development of hybrid membrane systems, integration with renewable energy sources, and application in direct air capture technologies. The market for nanofiller-enhanced membranes specifically is projected to grow at 12.3% annually, outpacing the overall market growth rate.
Current Challenges in Membrane Selectivity Enhancement
Despite significant advancements in membrane technology for CO2 separation, several critical challenges persist in enhancing membrane selectivity. The fundamental trade-off between permeability and selectivity, known as the Robeson upper bound, continues to limit membrane performance. As membranes become more selective for CO2, they typically experience reduced permeability, hampering overall separation efficiency and industrial applicability.
Material stability presents another significant challenge, particularly in industrial settings where membranes must withstand harsh operating conditions. Many promising membrane materials exhibit performance degradation when exposed to high pressures, elevated temperatures, or contaminants commonly found in flue gas streams such as SOx, NOx, and water vapor. This degradation often manifests as plasticization, where CO2 sorption causes polymer chain mobility to increase, resulting in diminished selectivity over time.
Scalability remains problematic for many nanofiller-enhanced membranes. Laboratory-scale successes frequently fail to translate to industrial-scale manufacturing due to challenges in achieving uniform nanofiller dispersion across large membrane areas. Agglomeration of nanoparticles during scale-up often creates defects and non-selective voids that compromise the membrane's separation performance.
Interface engineering between nanofillers and polymer matrices represents another significant hurdle. Poor compatibility often leads to the formation of non-selective voids at the nanofiller-polymer interface, creating pathways for undesired gases and reducing overall selectivity. While surface modification techniques exist, developing universally effective and economically viable modification methods remains challenging.
Long-term performance stability constitutes a critical concern for commercial viability. Many nanofiller-enhanced membranes show promising initial performance but experience significant selectivity decline over operational lifetimes. This deterioration often stems from nanofiller leaching, polymer aging, or structural changes under continuous operation conditions.
Cost-effectiveness presents perhaps the most significant barrier to widespread adoption. Many high-performance nanofillers involve complex synthesis procedures and expensive precursors, making them economically prohibitive for large-scale applications. The additional processing steps required for proper nanofiller incorporation further increase manufacturing costs, challenging the economic viability of these advanced membranes in competitive markets.
Addressing these interconnected challenges requires multidisciplinary approaches combining materials science, chemical engineering, and process optimization to develop next-generation membranes that can overcome the current limitations in CO2 selectivity enhancement.
Material stability presents another significant challenge, particularly in industrial settings where membranes must withstand harsh operating conditions. Many promising membrane materials exhibit performance degradation when exposed to high pressures, elevated temperatures, or contaminants commonly found in flue gas streams such as SOx, NOx, and water vapor. This degradation often manifests as plasticization, where CO2 sorption causes polymer chain mobility to increase, resulting in diminished selectivity over time.
Scalability remains problematic for many nanofiller-enhanced membranes. Laboratory-scale successes frequently fail to translate to industrial-scale manufacturing due to challenges in achieving uniform nanofiller dispersion across large membrane areas. Agglomeration of nanoparticles during scale-up often creates defects and non-selective voids that compromise the membrane's separation performance.
Interface engineering between nanofillers and polymer matrices represents another significant hurdle. Poor compatibility often leads to the formation of non-selective voids at the nanofiller-polymer interface, creating pathways for undesired gases and reducing overall selectivity. While surface modification techniques exist, developing universally effective and economically viable modification methods remains challenging.
Long-term performance stability constitutes a critical concern for commercial viability. Many nanofiller-enhanced membranes show promising initial performance but experience significant selectivity decline over operational lifetimes. This deterioration often stems from nanofiller leaching, polymer aging, or structural changes under continuous operation conditions.
Cost-effectiveness presents perhaps the most significant barrier to widespread adoption. Many high-performance nanofillers involve complex synthesis procedures and expensive precursors, making them economically prohibitive for large-scale applications. The additional processing steps required for proper nanofiller incorporation further increase manufacturing costs, challenging the economic viability of these advanced membranes in competitive markets.
Addressing these interconnected challenges requires multidisciplinary approaches combining materials science, chemical engineering, and process optimization to develop next-generation membranes that can overcome the current limitations in CO2 selectivity enhancement.
Current Nanofiller Solutions for CO2 Separation
01 Nanofiller types for enhanced membrane selectivity
Various types of nanofillers can be incorporated into membranes to enhance their selectivity properties. These include metal-organic frameworks (MOFs), zeolites, carbon nanotubes, and metal oxide nanoparticles. The specific nanofiller type can be selected based on the target separation application, with each offering unique molecular sieving capabilities and surface interactions that improve the membrane's ability to selectively transport certain molecules while rejecting others.- Metal-organic framework (MOF) nanofillers for enhanced selectivity: Metal-organic frameworks (MOFs) can be incorporated as nanofillers in membrane materials to significantly enhance gas and liquid separation selectivity. These crystalline porous materials provide uniform pore size distribution and high surface area, allowing for molecular sieving effects. When integrated into polymer matrices, MOF nanofillers create preferential pathways for specific molecules while blocking others, thereby improving the membrane's overall selectivity for applications in gas separation, water purification, and chemical processing.
- Carbon-based nanofillers for selective membrane performance: Carbon-based nanofillers such as graphene, carbon nanotubes, and carbon quantum dots can be incorporated into membrane structures to enhance selectivity. These materials provide unique transport channels at the nanoscale that facilitate selective permeation of certain molecules while rejecting others. The two-dimensional structure of graphene derivatives creates nanochannels that allow for precise molecular sieving, while carbon nanotubes offer frictionless transport pathways with diameter-dependent selectivity, making them effective for applications in water desalination, gas separation, and pharmaceutical purification.
- Inorganic nanoparticle fillers for selective membrane separation: Inorganic nanoparticles such as silica, titanium dioxide, and zeolites can be incorporated into membrane matrices to enhance selectivity through multiple mechanisms. These nanofillers create tortuous paths for molecules to travel through the membrane, allowing smaller molecules to pass while blocking larger ones. Additionally, the surface chemistry of these nanoparticles can be modified to introduce specific interactions with target molecules, further enhancing separation selectivity. The rigid structure of inorganic nanofillers also helps maintain membrane stability under harsh operating conditions.
- Polymer-nanofiller interface engineering for selectivity control: The interface between polymer matrices and nanofillers plays a crucial role in determining membrane selectivity. By engineering this interface through surface modifications, compatibilizers, or chemical bonding, non-selective voids can be eliminated while creating selective transport channels. Techniques such as grafting functional groups onto nanofillers, using coupling agents, or incorporating reactive nanoparticles can optimize the polymer-nanofiller interaction, leading to enhanced selectivity for specific molecules or ions while maintaining desirable permeation rates.
- Stimuli-responsive nanofillers for dynamic selectivity control: Stimuli-responsive nanofillers can be incorporated into membranes to achieve dynamic control over selectivity in response to external triggers such as pH, temperature, light, or electrical signals. These smart nanofillers undergo reversible changes in their properties, altering pore sizes or surface chemistry of the membrane on demand. This allows for switchable selectivity where the membrane can be tuned to selectively permeate different molecules under different conditions, enabling advanced separation processes with adaptable performance for complex separation challenges.
02 Nanofiller surface modification techniques
Surface modification of nanofillers is crucial for improving their compatibility with polymer matrices and enhancing membrane selectivity. Techniques include functionalization with organic groups, silane coupling agents, or polymer grafting to create specific interactions with target molecules. These modifications can prevent nanofiller agglomeration, improve dispersion, and create selective transport pathways through the membrane, resulting in enhanced separation performance for specific applications.Expand Specific Solutions03 Nanofiller concentration and distribution effects
The concentration and distribution of nanofillers within membrane matrices significantly impact selectivity performance. Optimal loading levels must be determined to balance increased selectivity with other membrane properties. Too low concentrations may not provide sufficient enhancement, while excessive loading can lead to agglomeration and defect formation. Uniform distribution of nanofillers creates consistent selective pathways throughout the membrane, while controlled gradient distributions can create directional transport properties.Expand Specific Solutions04 Mixed-matrix membrane architectures
Mixed-matrix membranes (MMMs) incorporate nanofillers within polymer matrices to create hybrid structures with enhanced selectivity. These architectures combine the processability of polymers with the selective transport properties of nanofillers. Various design approaches include layered structures, core-shell configurations, and interpenetrating networks. The interface between nanofillers and polymer matrix is critical for preventing non-selective voids while maintaining desired molecular transport pathways.Expand Specific Solutions05 Application-specific nanofiller selection for gas and liquid separations
Selecting appropriate nanofillers based on the specific separation application is essential for optimizing membrane selectivity. For gas separations, nanofillers with precise pore sizes matching molecular dimensions of target gases are preferred. In liquid separations, nanofillers with specific surface chemistries that enhance interactions with target molecules are utilized. The selection process considers factors such as molecular size differences, chemical affinities, and operating conditions to achieve maximum separation efficiency.Expand Specific Solutions
Key Nanofiller Mechanisms for Enhanced CO2 Selectivity
Carbon capture material containing ultrathin MOF nanosheets as well as preparation and application of carbon capture material
PatentPendingCN117225208A
Innovation
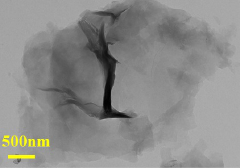
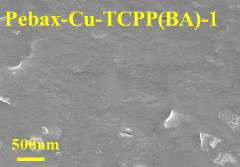
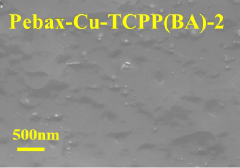
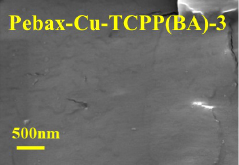
- The ultra-thin two-dimensional MOF material Cu-TCPP (BA) is used as a filler, combined with the polyether polyimide block Pebax to form a carbon capture material, which is connected through the paddle wheel-like structure and weak interaction of Cu-TCPP (BA) Construct hydrophobic nanochannels, build hydrophilic and hydrophobic transmission paths, and improve CO2 penetration and selectivity.
Gas sensor nanocomposite membranes
PatentInactiveAU2022241614A1
Innovation
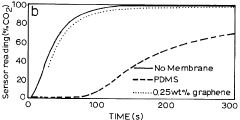
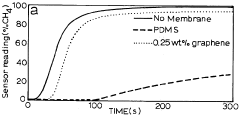
- Development of nano-composite membranes incorporating film-forming polymers like polydimethylsiloxane and polyacetylene with embedded silver nanoparticles that catalytically interact with gases, forming nano-voids for selective permeability and antimicrobial properties to enhance sensor performance.
Environmental Impact and Sustainability Considerations
The integration of nanofillers in CO2 selective membranes presents significant environmental implications that must be carefully evaluated. These advanced materials offer promising solutions for carbon capture and separation technologies, potentially reducing greenhouse gas emissions on a global scale. By enhancing membrane selectivity for CO2, nanofiller-enhanced membranes can contribute to more efficient carbon capture from industrial emissions, power plants, and other major CO2 sources, thereby mitigating climate change impacts.
However, the environmental footprint of nanofiller production requires thorough assessment. Manufacturing processes for nanoparticles often involve energy-intensive methods and potentially hazardous chemicals. The life cycle analysis of nanofiller-enhanced membranes must account for energy consumption, resource utilization, and emissions associated with nanomaterial synthesis, membrane fabrication, operation, and end-of-life disposal.
Sustainability considerations extend to the longevity and durability of these enhanced membranes. Nanofillers that improve membrane resistance to plasticization, fouling, and degradation can significantly extend operational lifetimes, reducing replacement frequency and associated material consumption. This aspect represents a critical sustainability advantage that may offset initial production impacts.
The potential release of nanomaterials into the environment during membrane production, use, or disposal raises additional concerns. Certain nanoparticles may exhibit toxicity to aquatic organisms or persist in ecosystems. Developing encapsulation strategies or selecting biodegradable nanofillers could mitigate these risks while maintaining performance benefits.
Water usage in membrane manufacturing and operation represents another environmental consideration. Nanofiller-enhanced membranes that operate efficiently under lower pressure differentials could reduce energy requirements for gas separation processes, translating to lower water consumption for cooling systems in industrial applications.
From a circular economy perspective, research into recyclable or recoverable nanofillers deserves priority. Designing membranes with end-of-life recovery options for valuable nanomaterials could improve the overall sustainability profile while potentially reducing production costs through material reclamation.
Regulatory frameworks worldwide are increasingly addressing nanomaterial safety and environmental impacts. Membrane technology developers must navigate these evolving regulations while demonstrating environmental benefits through comprehensive impact assessments. Standardized testing protocols for evaluating nanofiller leaching, degradation products, and ecological effects would strengthen sustainability claims and facilitate regulatory approval.
However, the environmental footprint of nanofiller production requires thorough assessment. Manufacturing processes for nanoparticles often involve energy-intensive methods and potentially hazardous chemicals. The life cycle analysis of nanofiller-enhanced membranes must account for energy consumption, resource utilization, and emissions associated with nanomaterial synthesis, membrane fabrication, operation, and end-of-life disposal.
Sustainability considerations extend to the longevity and durability of these enhanced membranes. Nanofillers that improve membrane resistance to plasticization, fouling, and degradation can significantly extend operational lifetimes, reducing replacement frequency and associated material consumption. This aspect represents a critical sustainability advantage that may offset initial production impacts.
The potential release of nanomaterials into the environment during membrane production, use, or disposal raises additional concerns. Certain nanoparticles may exhibit toxicity to aquatic organisms or persist in ecosystems. Developing encapsulation strategies or selecting biodegradable nanofillers could mitigate these risks while maintaining performance benefits.
Water usage in membrane manufacturing and operation represents another environmental consideration. Nanofiller-enhanced membranes that operate efficiently under lower pressure differentials could reduce energy requirements for gas separation processes, translating to lower water consumption for cooling systems in industrial applications.
From a circular economy perspective, research into recyclable or recoverable nanofillers deserves priority. Designing membranes with end-of-life recovery options for valuable nanomaterials could improve the overall sustainability profile while potentially reducing production costs through material reclamation.
Regulatory frameworks worldwide are increasingly addressing nanomaterial safety and environmental impacts. Membrane technology developers must navigate these evolving regulations while demonstrating environmental benefits through comprehensive impact assessments. Standardized testing protocols for evaluating nanofiller leaching, degradation products, and ecological effects would strengthen sustainability claims and facilitate regulatory approval.
Scalability and Industrial Implementation Challenges
The transition from laboratory-scale membrane development to industrial-scale production represents a significant challenge in the commercialization of nanofiller-enhanced membranes for CO2 separation. Current laboratory synthesis methods typically produce small membrane samples (10-100 cm²), whereas industrial applications require membrane areas of hundreds or thousands of square meters. This scaling disparity necessitates the development of continuous manufacturing processes that can maintain consistent nanofiller dispersion and membrane quality.
Uniform dispersion of nanofillers presents a major hurdle in large-scale production. As batch sizes increase, achieving homogeneous distribution becomes exponentially more difficult, leading to potential agglomeration and reduced performance. Advanced mixing technologies and dispersion techniques must be optimized specifically for different nanofiller types, considering their unique surface chemistries and interaction behaviors with polymer matrices.
Manufacturing consistency also poses significant challenges. Minor variations in production parameters can lead to substantial differences in membrane performance. Establishing robust quality control protocols and in-line monitoring systems is essential to ensure batch-to-batch consistency. Parameters such as nanofiller loading, dispersion quality, membrane thickness, and defect density must be continuously monitored throughout the production process.
Cost considerations further complicate industrial implementation. Many high-performance nanofillers (graphene derivatives, metal-organic frameworks, etc.) remain prohibitively expensive for large-scale applications. Economic viability requires either cost reduction of these materials through improved synthesis methods or the development of equally effective alternatives using more abundant and affordable materials.
Environmental and safety concerns associated with nanomaterial handling at industrial scales present additional challenges. Potential risks include airborne nanoparticle release during manufacturing and the environmental impact of nanomaterial waste. Implementing appropriate containment systems, worker protection protocols, and waste management strategies is crucial for responsible industrial deployment.
Regulatory frameworks for nanomaterial-enhanced products remain underdeveloped in many regions, creating uncertainty for industrial implementation. Companies must navigate evolving regulations while demonstrating both the safety and efficacy of their membrane technologies. This regulatory landscape varies significantly across different markets, complicating global commercialization efforts.
Integration with existing industrial infrastructure represents another implementation barrier. New membrane technologies must be compatible with current gas separation systems or offer sufficient performance improvements to justify infrastructure modifications. This requires careful engineering of membrane modules and consideration of operational parameters such as pressure requirements, fouling resistance, and long-term stability under industrial conditions.
Uniform dispersion of nanofillers presents a major hurdle in large-scale production. As batch sizes increase, achieving homogeneous distribution becomes exponentially more difficult, leading to potential agglomeration and reduced performance. Advanced mixing technologies and dispersion techniques must be optimized specifically for different nanofiller types, considering their unique surface chemistries and interaction behaviors with polymer matrices.
Manufacturing consistency also poses significant challenges. Minor variations in production parameters can lead to substantial differences in membrane performance. Establishing robust quality control protocols and in-line monitoring systems is essential to ensure batch-to-batch consistency. Parameters such as nanofiller loading, dispersion quality, membrane thickness, and defect density must be continuously monitored throughout the production process.
Cost considerations further complicate industrial implementation. Many high-performance nanofillers (graphene derivatives, metal-organic frameworks, etc.) remain prohibitively expensive for large-scale applications. Economic viability requires either cost reduction of these materials through improved synthesis methods or the development of equally effective alternatives using more abundant and affordable materials.
Environmental and safety concerns associated with nanomaterial handling at industrial scales present additional challenges. Potential risks include airborne nanoparticle release during manufacturing and the environmental impact of nanomaterial waste. Implementing appropriate containment systems, worker protection protocols, and waste management strategies is crucial for responsible industrial deployment.
Regulatory frameworks for nanomaterial-enhanced products remain underdeveloped in many regions, creating uncertainty for industrial implementation. Companies must navigate evolving regulations while demonstrating both the safety and efficacy of their membrane technologies. This regulatory landscape varies significantly across different markets, complicating global commercialization efforts.
Integration with existing industrial infrastructure represents another implementation barrier. New membrane technologies must be compatible with current gas separation systems or offer sufficient performance improvements to justify infrastructure modifications. This requires careful engineering of membrane modules and consideration of operational parameters such as pressure requirements, fouling resistance, and long-term stability under industrial conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!