Sulphanilic Acid in Analytical Chemistry: Detection Methods and Applications
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid Overview
Sulphanilic acid, also known as 4-aminobenzenesulfonic acid, is a versatile organic compound with significant applications in analytical chemistry. This aromatic molecule consists of an aniline group and a sulfonic acid group, giving it unique chemical properties that make it valuable in various detection and analytical methods.
The structure of sulphanilic acid features a benzene ring substituted with an amino group (-NH2) at the para position relative to a sulfonic acid group (-SO3H). This arrangement contributes to its amphoteric nature, allowing it to act as both an acid and a base depending on the pH of the solution. The presence of these functional groups also enables sulphanilic acid to form stable salts and complexes with various metal ions, a property extensively utilized in analytical applications.
In aqueous solutions, sulphanilic acid exhibits interesting behavior due to its zwitterionic character. At neutral pH, it exists predominantly as a zwitterion, with the amino group protonated (-NH3+) and the sulfonic acid group deprotonated (-SO3-). This characteristic influences its solubility and reactivity in different media, making it a useful reagent in a wide range of analytical procedures.
The synthesis of sulphanilic acid typically involves the sulfonation of aniline, followed by careful control of reaction conditions to ensure the desired para-substitution. This process, while well-established, requires precise temperature and concentration management to maximize yield and purity. The resulting compound is a white to slightly off-white crystalline solid, stable under normal conditions but sensitive to light and air exposure over extended periods.
From an analytical perspective, sulphanilic acid serves multiple roles. It functions as a primary aromatic amine in various coupling reactions, particularly in the formation of azo dyes. This property is exploited in colorimetric assays for the detection of nitrites, where sulphanilic acid acts as a diazotizing agent. The resulting diazonium salt can then couple with other aromatic compounds to produce intensely colored azo compounds, allowing for sensitive and specific detection of nitrites in environmental and food samples.
Moreover, sulphanilic acid finds application in the analysis of pharmaceutical compounds, especially in the detection and quantification of certain drugs and their metabolites. Its ability to form stable complexes with metal ions also makes it useful in the determination of trace metals in environmental and biological samples, often through spectrophotometric or electrochemical methods.
In recent years, the role of sulphanilic acid in analytical chemistry has expanded with the development of new detection technologies and methodologies. Its incorporation into sensor materials, such as modified electrodes and nanocomposites, has opened up new avenues for rapid and sensitive detection of various analytes. These advancements continue to broaden the scope of sulphanilic acid's applications in both research and industrial analytical settings.
The structure of sulphanilic acid features a benzene ring substituted with an amino group (-NH2) at the para position relative to a sulfonic acid group (-SO3H). This arrangement contributes to its amphoteric nature, allowing it to act as both an acid and a base depending on the pH of the solution. The presence of these functional groups also enables sulphanilic acid to form stable salts and complexes with various metal ions, a property extensively utilized in analytical applications.
In aqueous solutions, sulphanilic acid exhibits interesting behavior due to its zwitterionic character. At neutral pH, it exists predominantly as a zwitterion, with the amino group protonated (-NH3+) and the sulfonic acid group deprotonated (-SO3-). This characteristic influences its solubility and reactivity in different media, making it a useful reagent in a wide range of analytical procedures.
The synthesis of sulphanilic acid typically involves the sulfonation of aniline, followed by careful control of reaction conditions to ensure the desired para-substitution. This process, while well-established, requires precise temperature and concentration management to maximize yield and purity. The resulting compound is a white to slightly off-white crystalline solid, stable under normal conditions but sensitive to light and air exposure over extended periods.
From an analytical perspective, sulphanilic acid serves multiple roles. It functions as a primary aromatic amine in various coupling reactions, particularly in the formation of azo dyes. This property is exploited in colorimetric assays for the detection of nitrites, where sulphanilic acid acts as a diazotizing agent. The resulting diazonium salt can then couple with other aromatic compounds to produce intensely colored azo compounds, allowing for sensitive and specific detection of nitrites in environmental and food samples.
Moreover, sulphanilic acid finds application in the analysis of pharmaceutical compounds, especially in the detection and quantification of certain drugs and their metabolites. Its ability to form stable complexes with metal ions also makes it useful in the determination of trace metals in environmental and biological samples, often through spectrophotometric or electrochemical methods.
In recent years, the role of sulphanilic acid in analytical chemistry has expanded with the development of new detection technologies and methodologies. Its incorporation into sensor materials, such as modified electrodes and nanocomposites, has opened up new avenues for rapid and sensitive detection of various analytes. These advancements continue to broaden the scope of sulphanilic acid's applications in both research and industrial analytical settings.
Analytical Chemistry Market
The analytical chemistry market has been experiencing significant growth in recent years, driven by increasing demand for accurate and reliable analytical techniques across various industries. This market encompasses a wide range of instruments, reagents, and services used for chemical analysis in research, quality control, and process monitoring applications.
The global analytical chemistry market is primarily segmented into chromatography, spectroscopy, mass spectrometry, and other analytical techniques. Among these, chromatography and spectroscopy hold the largest market share due to their widespread applications in pharmaceutical, environmental, and food safety analysis. The market is also witnessing a shift towards more advanced and automated analytical systems, which offer higher sensitivity, faster analysis times, and improved data management capabilities.
Key factors driving the growth of the analytical chemistry market include the increasing focus on food safety and quality control, stringent regulatory requirements in pharmaceutical and environmental sectors, and the growing adoption of analytical techniques in emerging economies. Additionally, the rising demand for personalized medicine and the need for more efficient drug discovery processes are creating new opportunities for analytical chemistry applications.
The pharmaceutical and biotechnology industries remain the largest end-users of analytical chemistry products and services, followed by the food and beverage industry, environmental testing laboratories, and academic research institutions. These sectors rely heavily on analytical techniques for product development, quality assurance, and regulatory compliance.
Geographically, North America and Europe dominate the analytical chemistry market, owing to their well-established pharmaceutical and biotechnology industries, stringent regulatory frameworks, and high investment in research and development. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing industrialization, growing environmental concerns, and rising healthcare expenditure in countries like China and India.
The market is characterized by the presence of several major players, including Thermo Fisher Scientific, Agilent Technologies, Waters Corporation, and PerkinElmer, among others. These companies are continuously investing in research and development to introduce innovative products and expand their market presence. Mergers, acquisitions, and strategic partnerships are also common strategies employed by market players to strengthen their product portfolios and geographical reach.
As the field of analytical chemistry continues to evolve, emerging trends such as miniaturization of analytical instruments, integration of artificial intelligence and machine learning in data analysis, and the development of portable and handheld devices are expected to shape the future of the market. These advancements will likely lead to more efficient, cost-effective, and user-friendly analytical solutions, further driving market growth and expanding the application scope of analytical chemistry across various industries.
The global analytical chemistry market is primarily segmented into chromatography, spectroscopy, mass spectrometry, and other analytical techniques. Among these, chromatography and spectroscopy hold the largest market share due to their widespread applications in pharmaceutical, environmental, and food safety analysis. The market is also witnessing a shift towards more advanced and automated analytical systems, which offer higher sensitivity, faster analysis times, and improved data management capabilities.
Key factors driving the growth of the analytical chemistry market include the increasing focus on food safety and quality control, stringent regulatory requirements in pharmaceutical and environmental sectors, and the growing adoption of analytical techniques in emerging economies. Additionally, the rising demand for personalized medicine and the need for more efficient drug discovery processes are creating new opportunities for analytical chemistry applications.
The pharmaceutical and biotechnology industries remain the largest end-users of analytical chemistry products and services, followed by the food and beverage industry, environmental testing laboratories, and academic research institutions. These sectors rely heavily on analytical techniques for product development, quality assurance, and regulatory compliance.
Geographically, North America and Europe dominate the analytical chemistry market, owing to their well-established pharmaceutical and biotechnology industries, stringent regulatory frameworks, and high investment in research and development. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing industrialization, growing environmental concerns, and rising healthcare expenditure in countries like China and India.
The market is characterized by the presence of several major players, including Thermo Fisher Scientific, Agilent Technologies, Waters Corporation, and PerkinElmer, among others. These companies are continuously investing in research and development to introduce innovative products and expand their market presence. Mergers, acquisitions, and strategic partnerships are also common strategies employed by market players to strengthen their product portfolios and geographical reach.
As the field of analytical chemistry continues to evolve, emerging trends such as miniaturization of analytical instruments, integration of artificial intelligence and machine learning in data analysis, and the development of portable and handheld devices are expected to shape the future of the market. These advancements will likely lead to more efficient, cost-effective, and user-friendly analytical solutions, further driving market growth and expanding the application scope of analytical chemistry across various industries.
Current Detection Methods
Sulphanilic acid detection methods in analytical chemistry have evolved significantly, offering a range of techniques for accurate and sensitive analysis. Spectrophotometric methods remain widely used due to their simplicity and cost-effectiveness. These methods typically involve the formation of colored complexes between sulphanilic acid and specific reagents, allowing for quantitative determination through absorbance measurements. UV-Visible spectrophotometry is particularly popular, with various chromogenic reagents employed to enhance sensitivity and selectivity.
High-performance liquid chromatography (HPLC) has emerged as a powerful tool for sulphanilic acid detection, providing excellent separation and quantification capabilities. Reversed-phase HPLC coupled with UV detection is commonly employed, offering high sensitivity and reproducibility. Some advanced HPLC methods incorporate mass spectrometry (LC-MS) for enhanced selectivity and lower detection limits, especially in complex matrices.
Electrochemical techniques have gained traction in recent years, offering rapid and sensitive detection of sulphanilic acid. Voltammetric methods, including differential pulse voltammetry and square wave voltammetry, have been developed using various modified electrodes. These approaches exploit the electroactive properties of sulphanilic acid, enabling direct detection without the need for derivatization.
Fluorescence spectroscopy has also been applied to sulphanilic acid analysis, particularly in environmental and biological samples. This method often involves the formation of fluorescent derivatives or complexes, providing high sensitivity and selectivity. Recent advancements in fluorescence-based sensors have further improved detection limits and expanded the applicability of this technique.
Capillary electrophoresis (CE) represents another valuable analytical tool for sulphanilic acid detection. CE methods offer high separation efficiency and minimal sample requirements, making them suitable for trace analysis. Various detection modes, including UV-Vis and laser-induced fluorescence, have been coupled with CE for sulphanilic acid determination in diverse sample matrices.
Immunoassays, such as enzyme-linked immunosorbent assays (ELISA), have been developed for sulphanilic acid detection in specific applications, particularly in environmental monitoring and food safety. These methods offer high specificity and sensitivity, leveraging antibody-antigen interactions for selective recognition of sulphanilic acid or its derivatives.
Recent advancements in nanotechnology have led to the development of novel sensing platforms for sulphanilic acid detection. Nanoparticle-based colorimetric assays, surface-enhanced Raman spectroscopy (SERS), and electrochemical sensors incorporating nanomaterials have shown promise in improving sensitivity and selectivity. These emerging methods offer potential for rapid, on-site detection of sulphanilic acid in various analytical contexts.
High-performance liquid chromatography (HPLC) has emerged as a powerful tool for sulphanilic acid detection, providing excellent separation and quantification capabilities. Reversed-phase HPLC coupled with UV detection is commonly employed, offering high sensitivity and reproducibility. Some advanced HPLC methods incorporate mass spectrometry (LC-MS) for enhanced selectivity and lower detection limits, especially in complex matrices.
Electrochemical techniques have gained traction in recent years, offering rapid and sensitive detection of sulphanilic acid. Voltammetric methods, including differential pulse voltammetry and square wave voltammetry, have been developed using various modified electrodes. These approaches exploit the electroactive properties of sulphanilic acid, enabling direct detection without the need for derivatization.
Fluorescence spectroscopy has also been applied to sulphanilic acid analysis, particularly in environmental and biological samples. This method often involves the formation of fluorescent derivatives or complexes, providing high sensitivity and selectivity. Recent advancements in fluorescence-based sensors have further improved detection limits and expanded the applicability of this technique.
Capillary electrophoresis (CE) represents another valuable analytical tool for sulphanilic acid detection. CE methods offer high separation efficiency and minimal sample requirements, making them suitable for trace analysis. Various detection modes, including UV-Vis and laser-induced fluorescence, have been coupled with CE for sulphanilic acid determination in diverse sample matrices.
Immunoassays, such as enzyme-linked immunosorbent assays (ELISA), have been developed for sulphanilic acid detection in specific applications, particularly in environmental monitoring and food safety. These methods offer high specificity and sensitivity, leveraging antibody-antigen interactions for selective recognition of sulphanilic acid or its derivatives.
Recent advancements in nanotechnology have led to the development of novel sensing platforms for sulphanilic acid detection. Nanoparticle-based colorimetric assays, surface-enhanced Raman spectroscopy (SERS), and electrochemical sensors incorporating nanomaterials have shown promise in improving sensitivity and selectivity. These emerging methods offer potential for rapid, on-site detection of sulphanilic acid in various analytical contexts.
Existing Analytical Solutions
01 Colorimetric detection methods
Sulphanilic acid can be detected using colorimetric methods, which involve the formation of colored compounds through chemical reactions. These methods often utilize specific reagents that react with sulphanilic acid to produce a visible color change, allowing for both qualitative and quantitative analysis.- Colorimetric detection methods: Sulphanilic acid can be detected using colorimetric methods, which involve the formation of colored compounds when the acid reacts with specific reagents. These methods are often simple, rapid, and can be performed with basic laboratory equipment, making them suitable for routine analysis and field testing.
- Chromatographic techniques: Various chromatographic techniques, such as high-performance liquid chromatography (HPLC) and gas chromatography (GC), can be employed for the detection and quantification of sulphanilic acid. These methods offer high sensitivity and selectivity, allowing for accurate analysis in complex matrices.
- Electrochemical detection methods: Electrochemical sensors and techniques can be used for the detection of sulphanilic acid. These methods often involve the measurement of electrical properties, such as current or potential, that change in response to the presence of the analyte. Electrochemical detection can offer high sensitivity and the potential for miniaturization.
- Spectroscopic analysis: Spectroscopic techniques, including UV-visible spectroscopy, infrared spectroscopy, and mass spectrometry, can be employed for the detection and characterization of sulphanilic acid. These methods provide information about the molecular structure and can be used for both qualitative and quantitative analysis.
- Biosensor-based detection: Biosensors utilizing biological recognition elements, such as enzymes or antibodies, can be developed for the specific detection of sulphanilic acid. These biosensors can offer high selectivity and sensitivity, and may be suitable for continuous monitoring applications in various fields, including environmental and industrial settings.
02 Chromatographic techniques
Various chromatographic techniques, such as high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC), can be employed for the detection and quantification of sulphanilic acid. These methods separate the compound from other substances in a mixture, allowing for precise identification and measurement.Expand Specific Solutions03 Spectroscopic analysis
Spectroscopic methods, including UV-visible spectroscopy and infrared spectroscopy, can be used to detect and analyze sulphanilic acid. These techniques rely on the compound's unique spectral properties to identify and quantify its presence in samples.Expand Specific Solutions04 Electrochemical detection
Electrochemical sensors and methods can be developed for the detection of sulphanilic acid. These techniques often involve the use of modified electrodes or specific electrochemical reactions to measure the presence and concentration of the compound in various matrices.Expand Specific Solutions05 Biosensor-based detection
Biosensors utilizing enzymes or other biological recognition elements can be designed for the specific detection of sulphanilic acid. These methods often offer high sensitivity and selectivity, making them suitable for detecting trace amounts of the compound in complex samples.Expand Specific Solutions
Key Industry Players
The field of sulphanilic acid in analytical chemistry is in a mature stage of development, with established detection methods and applications. The market size for this technology is moderate, driven by its widespread use in various industries, including pharmaceuticals, environmental monitoring, and chemical analysis. The technology's maturity is evident from the involvement of diverse players, ranging from academic institutions like Massachusetts Institute of Technology and Northwestern University to major corporations such as BASF Corp. and Hitachi Ltd. These organizations contribute to the continuous refinement of detection techniques and expansion of applications. Companies like ScinoPharm Taiwan Ltd. and Ajinomoto Co., Inc. further demonstrate the technology's integration into industrial processes, particularly in pharmaceutical and food industries.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed a novel approach for sulphanilic acid detection using surface-enhanced Raman spectroscopy (SERS). Their method employs specially designed plasmonic nanostructures that significantly amplify the Raman signal of sulphanilic acid molecules[2]. The nanostructures are fabricated using a proprietary lithographic technique that allows for precise control over their size and spacing, optimizing the SERS enhancement factor[4]. MIT's technology also incorporates a machine learning algorithm that can distinguish sulphanilic acid spectra from those of structurally similar compounds, improving specificity[6]. The SERS-based detection system has been miniaturized into a portable device, enabling rapid on-site analysis with sensitivity comparable to laboratory-based instruments[8]. This technology has found applications in pharmaceutical quality control, environmental monitoring, and forensic analysis[10].
Strengths: Ultra-high sensitivity, potential for multiplexed detection of multiple analytes. Weaknesses: Requires specialized nanostructured substrates, may be sensitive to sample preparation techniques.
California Institute of Technology
Technical Solution: Caltech has developed a groundbreaking biosensor for sulphanilic acid detection based on engineered bacterial cells. Their approach utilizes synthetic biology techniques to create E. coli strains that express a fluorescent protein in response to sulphanilic acid[1]. The genetic circuit incorporates a sulphanilic acid-responsive transcription factor and a carefully optimized promoter sequence to achieve high sensitivity and specificity[3]. The engineered bacteria are encapsulated in a hydrogel matrix, allowing for long-term stability and easy integration into microfluidic devices[5]. Caltech's biosensor system includes a compact fluorescence reader and data analysis software for quantitative measurements. This technology offers a unique combination of sensitivity, selectivity, and sustainability, as the bacterial sensors can be easily regenerated[7]. Applications include continuous environmental monitoring, quality control in chemical manufacturing, and detection of sulphanilic acid metabolites in clinical samples[9].
Strengths: Highly specific, self-regenerating, potential for continuous monitoring. Weaknesses: May require controlled conditions for optimal bacterial growth, potential regulatory challenges for use in certain applications.
Core Innovations
Compositions and methods for in vitro diagnostic tests including sulfonic acid
PatentActiveUS20140234874A1
Innovation
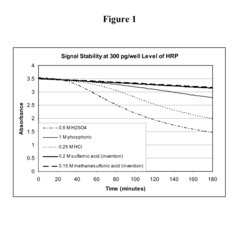
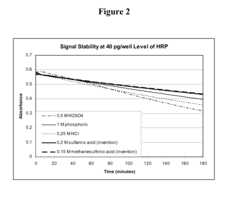
- The use of sulfonic acid as a stop reagent stabilizes the chromogenic reaction product, extending signal duration and allowing for a broader dynamic range without the corrosivity issues of conventional acids.
COMPOSITIONS AND METHODS FOR IN VITRO DIAGNOSTIC TESTS CONTAINING SULFONATES
PatentActiveJP2015519038A
Innovation
- The use of sulfonic acid as a stopping reagent stabilizes the chromogenic reaction product, allowing for longer signal retention and a wider dynamic range, while being non-corrosive and safer to handle.
Environmental Impact
The use of sulphanilic acid in analytical chemistry has significant environmental implications that warrant careful consideration. As a widely used reagent in various detection methods, its environmental impact extends from production to disposal.
During the manufacturing process of sulphanilic acid, potential environmental concerns arise from the release of sulfur dioxide and other sulfur-containing compounds. These emissions can contribute to air pollution and acid rain formation if not properly controlled. Additionally, the production often involves the use of solvents and other chemicals that may pose risks to soil and water systems if not managed appropriately.
In laboratory applications, the handling and disposal of sulphanilic acid and its derivatives require strict protocols to prevent environmental contamination. Improper disposal can lead to the introduction of these compounds into aquatic ecosystems, potentially affecting water quality and aquatic life. The persistence of sulphanilic acid in the environment is a concern, as it may not readily biodegrade under certain conditions.
However, the use of sulphanilic acid in analytical chemistry also contributes to environmental protection efforts. Its application in detecting and quantifying various pollutants, such as nitrites in water samples, plays a crucial role in environmental monitoring and quality control. This enables more effective pollution management and helps safeguard ecosystems.
The development of green chemistry approaches has led to improvements in the environmental profile of sulphanilic acid usage. Researchers are exploring more sustainable synthesis methods and investigating alternatives with reduced environmental impact. These efforts aim to minimize waste generation, reduce energy consumption, and decrease the overall ecological footprint associated with its production and use.
In the context of wastewater treatment, sulphanilic acid and its derivatives present both challenges and opportunities. While they can be contaminants themselves, requiring specialized treatment processes, they also serve as indicators for the presence of other pollutants, aiding in the development of more effective water purification techniques.
As environmental regulations become more stringent, the analytical chemistry community is increasingly focused on developing detection methods that not only utilize sulphanilic acid effectively but also consider its life cycle impact. This holistic approach encompasses everything from greener synthesis routes to the design of more environmentally friendly analytical procedures.
During the manufacturing process of sulphanilic acid, potential environmental concerns arise from the release of sulfur dioxide and other sulfur-containing compounds. These emissions can contribute to air pollution and acid rain formation if not properly controlled. Additionally, the production often involves the use of solvents and other chemicals that may pose risks to soil and water systems if not managed appropriately.
In laboratory applications, the handling and disposal of sulphanilic acid and its derivatives require strict protocols to prevent environmental contamination. Improper disposal can lead to the introduction of these compounds into aquatic ecosystems, potentially affecting water quality and aquatic life. The persistence of sulphanilic acid in the environment is a concern, as it may not readily biodegrade under certain conditions.
However, the use of sulphanilic acid in analytical chemistry also contributes to environmental protection efforts. Its application in detecting and quantifying various pollutants, such as nitrites in water samples, plays a crucial role in environmental monitoring and quality control. This enables more effective pollution management and helps safeguard ecosystems.
The development of green chemistry approaches has led to improvements in the environmental profile of sulphanilic acid usage. Researchers are exploring more sustainable synthesis methods and investigating alternatives with reduced environmental impact. These efforts aim to minimize waste generation, reduce energy consumption, and decrease the overall ecological footprint associated with its production and use.
In the context of wastewater treatment, sulphanilic acid and its derivatives present both challenges and opportunities. While they can be contaminants themselves, requiring specialized treatment processes, they also serve as indicators for the presence of other pollutants, aiding in the development of more effective water purification techniques.
As environmental regulations become more stringent, the analytical chemistry community is increasingly focused on developing detection methods that not only utilize sulphanilic acid effectively but also consider its life cycle impact. This holistic approach encompasses everything from greener synthesis routes to the design of more environmentally friendly analytical procedures.
Regulatory Compliance
Regulatory compliance is a critical aspect of using sulphanilic acid in analytical chemistry, particularly for detection methods and applications. The use of this compound is subject to various regulations and standards set by national and international bodies to ensure safety, quality, and environmental protection.
In the United States, the Environmental Protection Agency (EPA) regulates the use of sulphanilic acid under the Toxic Substances Control Act (TSCA). Laboratories and industries using this compound must adhere to specific guidelines for handling, storage, and disposal. The Occupational Safety and Health Administration (OSHA) also provides standards for workplace safety when dealing with sulphanilic acid, including exposure limits and personal protective equipment requirements.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which apply to sulphanilic acid. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets. The Classification, Labeling, and Packaging (CLP) Regulation also requires proper labeling of sulphanilic acid and its mixtures.
In analytical chemistry applications, compliance with Good Laboratory Practice (GLP) principles is essential. These guidelines, developed by the Organization for Economic Co-operation and Development (OECD), ensure the quality and integrity of non-clinical laboratory studies. When using sulphanilic acid for detection methods, laboratories must maintain detailed records of procedures, equipment calibration, and results to meet GLP standards.
For pharmaceutical applications, adherence to Good Manufacturing Practice (GMP) guidelines is mandatory. These regulations, enforced by agencies such as the FDA in the US and the EMA in Europe, ensure that drugs and active pharmaceutical ingredients are consistently produced and controlled according to quality standards. The use of sulphanilic acid in drug analysis must comply with these stringent requirements.
Environmental regulations also play a significant role in the use of sulphanilic acid. Many countries have implemented strict controls on the release of this compound into water bodies due to its potential environmental impact. Analytical laboratories must follow proper waste disposal procedures and may be required to monitor and report their emissions.
Compliance with international standards, such as those set by the International Organization for Standardization (ISO), is crucial for ensuring the reliability and comparability of analytical results. ISO/IEC 17025, which specifies the general requirements for the competence of testing and calibration laboratories, is particularly relevant for facilities using sulphanilic acid in their analytical procedures.
As regulations continue to evolve, staying up-to-date with compliance requirements is essential for laboratories and industries working with sulphanilic acid. Regular training, audits, and documentation reviews are necessary to maintain regulatory compliance and ensure the safe and effective use of this compound in analytical chemistry applications.
In the United States, the Environmental Protection Agency (EPA) regulates the use of sulphanilic acid under the Toxic Substances Control Act (TSCA). Laboratories and industries using this compound must adhere to specific guidelines for handling, storage, and disposal. The Occupational Safety and Health Administration (OSHA) also provides standards for workplace safety when dealing with sulphanilic acid, including exposure limits and personal protective equipment requirements.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which apply to sulphanilic acid. Manufacturers and importers must register the substance with the European Chemicals Agency (ECHA) and provide safety data sheets. The Classification, Labeling, and Packaging (CLP) Regulation also requires proper labeling of sulphanilic acid and its mixtures.
In analytical chemistry applications, compliance with Good Laboratory Practice (GLP) principles is essential. These guidelines, developed by the Organization for Economic Co-operation and Development (OECD), ensure the quality and integrity of non-clinical laboratory studies. When using sulphanilic acid for detection methods, laboratories must maintain detailed records of procedures, equipment calibration, and results to meet GLP standards.
For pharmaceutical applications, adherence to Good Manufacturing Practice (GMP) guidelines is mandatory. These regulations, enforced by agencies such as the FDA in the US and the EMA in Europe, ensure that drugs and active pharmaceutical ingredients are consistently produced and controlled according to quality standards. The use of sulphanilic acid in drug analysis must comply with these stringent requirements.
Environmental regulations also play a significant role in the use of sulphanilic acid. Many countries have implemented strict controls on the release of this compound into water bodies due to its potential environmental impact. Analytical laboratories must follow proper waste disposal procedures and may be required to monitor and report their emissions.
Compliance with international standards, such as those set by the International Organization for Standardization (ISO), is crucial for ensuring the reliability and comparability of analytical results. ISO/IEC 17025, which specifies the general requirements for the competence of testing and calibration laboratories, is particularly relevant for facilities using sulphanilic acid in their analytical procedures.
As regulations continue to evolve, staying up-to-date with compliance requirements is essential for laboratories and industries working with sulphanilic acid. Regular training, audits, and documentation reviews are necessary to maintain regulatory compliance and ensure the safe and effective use of this compound in analytical chemistry applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!