Carbolic Acid, also known as phenol, is an aromatic organic compound characterized by a hydroxyl group (-OH) attached to a benzene ring. Traditionally sourced from coal tar distillation, it is now widely produced via petrochemical processes. Renowned for its strong antiseptic and chemical reactivity, Carbolic Acid plays a critical role in modern industrial chemistry.
With industries continuously facing challenges such as antimicrobial resistance, environmental sustainability demands, and the need for advanced material functionalities, Carbolic Acid offers multifaceted solutions. Its unique chemical properties enable it to serve as a disinfectant, polymer additive, catalyst, and more. This article will delve into Carbolic Acid’s composition and properties, explore its broad application domains, discuss its advantages and limitations, and examine future research frontiers. We will also introduce how leveraging PatSnap’s Eureka AI Agent can accelerate innovation in this complex chemical landscape.
Material Composition & Key Properties
Carbolic Acid (C₆H₅OH) features a planar aromatic ring with an electronegative hydroxyl group, conferring both hydrophobic and polar characteristics. This duality underpins its reactivity and versatility. Its phenolic hydroxyl allows for hydrogen bonding, acidity, and radical scavenging, making it effective in antimicrobial and antioxidant applications.
Key physical and chemical properties include:
- Molecular Weight: 94.11 g/mol
- Melting Point: 40.5 °C
- Boiling Point: 181.7 °C
- Solubility: Moderately soluble in water; highly soluble in organic solvents
- Acidity: pKa ≈ 10, weakly acidic
- Reactivity: Undergoes electrophilic substitution, oxidation, and polymerization reactions
Carbolic Acid is synthesized industrially via the cumene process, yielding high purity phenol and acetone. Its structure enables functionalization, allowing derivatives tailored for specific applications such as disinfectants, resins, or pharmaceutical intermediates.
Compared with related aromatic compounds (e.g., cresols, resorcinol), Carbolic Acid provides a balanced combination of antimicrobial potency and chemical reactivity, making it indispensable across sectors.
Comparative Advantages & Limitations
Carbolic Acid (phenol) stands out as a highly versatile and widely used chemical across many industries. However, like any material, it exhibits both strengths and limitations. Understanding these factors is critical for optimizing its application and guiding future research.
Advantages ✓
- Broad-spectrum Antimicrobial Activity:
Carbolic Acid is a potent antimicrobial agent effective against a wide range of bacteria, fungi, and viruses. This makes it invaluable in disinfectants, antiseptics, and healthcare sanitation products. Its mechanism disrupts microbial cell membranes and denatures proteins, ensuring rapid biocidal action. - Versatile Chemical Reactivity:
The phenolic hydroxyl group imparts unique reactivity, allowing Carbolic Acid to participate in electrophilic substitution, polymerization, and oxidation reactions. This versatility enables its use as a precursor in synthesizing phenolic resins, antioxidants, and pharmaceutical intermediates. - Catalytic Efficiency in Industrial Processes:
Carbolic Acid acts as a catalyst or catalyst precursor in various chemical manufacturing pathways, enhancing reaction rates and selectivity. It supports green chemistry innovations by facilitating more efficient and sustainable synthetic routes. - Cost-effective Industrial Production:
The mature cumene process allows large-scale, cost-efficient production of Carbolic Acid with high purity. This affordability facilitates its widespread adoption across sectors. - Enhancement of Polymer and Material Properties:
When incorporated into polymer matrices, Carbolic Acid derivatives improve mechanical strength, thermal stability, and flame retardancy. This extends the lifespan and safety of products such as coatings, foams, and textiles. - Environmental Role in Biodegradation and Waste Treatment:
Carbolic Acid can enhance the biodegradability of certain polymers and organic wastes. Additionally, it participates in industrial wastewater treatments, breaking down hazardous compounds and reducing environmental impact.
Limitations
- Toxicity and Safety Concerns:
Despite its benefits, Carbolic Acid is corrosive and toxic at elevated concentrations. Prolonged exposure poses health risks including skin burns, respiratory irritation, and systemic toxicity. Strict handling protocols and exposure limits are required in industrial and laboratory settings. - Environmental Persistence and Ecotoxicity:
Carbolic Acid and some of its derivatives degrade slowly in natural environments, potentially leading to accumulation and adverse ecological effects. This limits its use in open systems without adequate treatment or containment. - Limited Water Solubility:
Its moderate solubility in water can restrict certain aqueous applications or necessitate formulation adjustments (e.g., emulsifiers or solvents) to improve dispersion and efficacy. - Batch Variability in Derivatives:
Production inconsistencies in substituted Carbolic Acid derivatives can affect product performance, especially in pharmaceuticals or specialized polymers requiring precise chemical profiles. - Corrosive Nature Affecting Equipment:
Carbolic Acid’s corrosiveness can degrade metal processing equipment, increasing maintenance costs and limiting certain manufacturing configurations. - Competitive Alternatives in Specific Applications:
Emerging antimicrobial agents, biodegradable additives, and green catalysts sometimes outperform Carbolic Acid in select niches due to better safety profiles or enhanced sustainability.
Comparative Analysis with Related Chemicals
| Property/Aspect | Carbolic Acid (Phenol) | Cresols (Methylphenols) | Resorcinol (1,3-Dihydroxybenzene) | Bisphenol A |
|---|---|---|---|---|
| Antimicrobial Effectiveness | High | Moderate | Low | Negligible |
| Chemical Reactivity | High (versatile) | Moderate | High (multiple hydroxyl groups) | High (for polymerization) |
| Toxicity | Moderate to High | Similar to phenol | Moderate | Lower but controversial |
| Environmental Persistence | Moderate | Moderate | Moderate | High |
| Industrial Cost Efficiency | High | Moderate | Moderate | Variable |
| Polymer Property Enhancement | Effective flame retardancy | Moderate | High (crosslinking agent) | High (polycarbonate precursor) |
Carbolic Acid balances antimicrobial potency, chemical versatility, and cost-effectiveness better than many related compounds, though it faces limitations in toxicity and environmental persistence that require ongoing management and innovation.
Application Domains
Disinfection & Antimicrobial Applications
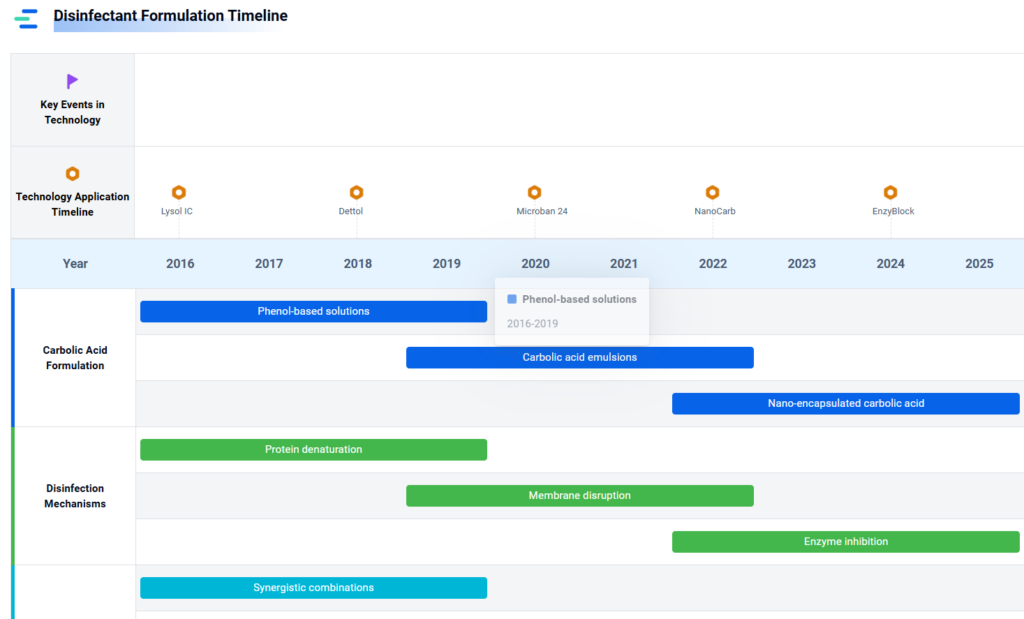
Industrial and healthcare sectors require potent disinfectants and antiseptics to combat resistant pathogens and ensure safety. Carbolic Acid, known for its strong antibacterial properties, acts as a key active ingredient in disinfectant formulations and antiseptic products. Current research focuses on optimizing Carbolic Acid derivatives for enhanced efficacy, developing novel antiseptics for healthcare settings, and comparative analyses of antibacterial performance.
Related topics:
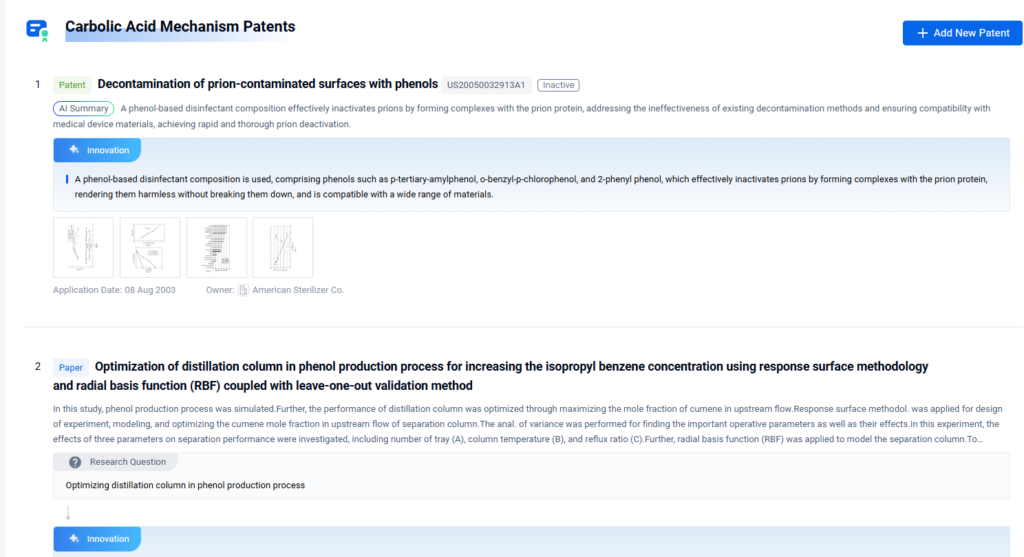
- Mechanisms of Carbolic Acid in Industrial Disinfectant Formulations
- Comparative Analysis of Carbolic Acid Derivatives in Antibacterial Efficacy
- Carbolic Acid-Based Innovations in Antiviral Surface Coatings
Polymer & Material Enhancements
Materials science demands additives and catalysts that improve durability, flame retardancy, and functional properties of polymers. Carbolic Acid contributes as a catalyst or additive in polymer synthesis, cross-linking, and enhancing epoxy resin and foam materials. Research advances include developing biodegradable plastics, flame retardant composites, and resilient flooring, alongside sustainable polymer blending and coating innovations.
Related topics:
- Carbolic Acid in Phenolic Compound Synthesis Pathway Optimization
- The Role of Carbolic Acid in Enhancing Epoxy Resin Properties
- Developing Biodegradable Carbolic Acid-Based Plastics
Environmental & Waste Treatment
Sustainability and eco-friendly chemical practices drive the need for materials aiding pollutant degradation and waste processing. Carbolic Acid is studied for its influence on biodegradation rates, environmental degradation byproducts, and its integration in wastewater and industrial waste treatments. Nanotechnology approaches to stabilize Carbolic Acid in aqueous solutions further improve its application scope.
Related topics:
- Environmental Impact of Carbolic Acid Degradation Byproducts
- How Carbolic Acid Affects Biodegradation Rates in Wastewater Treatment
- Enhancing Carbolic Acid Stability in Aqueous Solutions through Nanotechnology
- Developing Sustainable Water Treatment Solutions with Carbolic Acid
Industrial Catalysis & Chemical Synthesis
Carbolic Acid functions as an important catalyst and intermediate in organic synthesis and industrial chemical processes. Its role in phenolic compound production, esterification, heterogenous catalysis, and pharmaceutical synthesis is widely investigated. Studies emphasize optimization of catalytic pathways, reaction efficiencies, and green chemistry applications to enhance industrial sustainability.
Related topics:
- Carbolic Acid in Phenolic Compound Synthesis Pathway Optimization
- Carbolic Acid Interaction with Common Industrial Catalysts
- Carbolic Acid in Organic Synthesis: Efficiency and Limitations
- Carbolic Acid Derivatives in Pharmaceutical Synthesis: A Green Approach
- Carbolic Acid Catalyzed Reactions in Organic Electronics Fabrication
- Advanced Carbolic Acid Applications in Smart Glass Technologies
Coatings, Surface Treatments & Textiles
Surface protection and functional coatings are critical in multiple industries including automotive, construction, and textiles. Carbolic Acid enhances adhesion, flame retardancy, antimicrobial properties, and durability in coatings and textile treatments. Research highlights include development of low-VOC and eco-friendly coatings, textile fire retardants, and surface gloss improvements.
Related topics:
Here are the topics embedded with links according to the rule:
- Carbolic Acid Impact on Maritime Biofouling Prevention Coatings
- The Role of Carbolic Acid in Textile Industry Antimicrobial Treatments
- Innovative Uses of Carbolic Acid in Surface Coating Technologies
- Carbolic Acid Contributions to Non-VOC Paint Innovations
- Carbolic Acid Utilization to Enhance Surface Gloss in Automotive Paints
Energy & Advanced Technologies
Emerging energy technologies and high-performance materials benefit from Carbolic Acid’s catalytic and additive roles. Its applications span solar panel manufacturing, fuel additives for combustion efficiency, lithium-ion battery electrode stability, and oil recovery enhancement. Cutting-edge studies explore Carbolic Acid’s integration in renewable energy systems and electrochemical sensor technologies.
Related topics:
- How Carbolic Acid Enhances Fuel Efficiency in Combustion Engines
- How Carbolic Acid Affects Lithium-Ion Battery Electrode Stability
- The Function of Carbolic Acid in Electrochemical Sensor Technologies
- Carbolic Acid Applications in Developing Energy-Saving Coatings
Emerging Biomedical Frontiers with Carbolic Acid
Carbolic acid—once revolutionary in antiseptic surgery—is now finding new life in advanced biomedical materials and pharmaceutical formulations. But what about its role in:
Phenol-based carriers for targeted cancer drug delivery?
Smart wound dressings with sustained antimicrobial activity?
Biocompatible polymers for implantable devices?
Antiviral surface coatings in high-contact healthcare settings?
And beyond?
These aren’t just speculative uses—they’re shaping the next wave of biomedicine.
Curious how carbolic acid is being re-engineered for precision therapeutics, infection control, and regenerative systems?
👉 Explore how carbolic acid is paving the way for next-gen therapeutics, biomedical coatings, and advanced healthcare materials through PatSnap Eureka AI Agent.
Future Outlook & Research Frontiers
Looking ahead, Carbolic Acid’s role is set to expand in multiple high-potential areas:
- Sustainable Chemistry: Green synthesis routes and biodegradable derivatives will reduce environmental footprints.
- Biomedical Innovations: Targeted drug delivery and tissue engineering scaffolds will leverage functionalized Carbolic Acid polymers.
- Smart Materials: Integration in self-healing polymers and smart coatings to improve durability and responsiveness.
- Energy Technologies: Enhancements in battery electrodes and solar panel materials to increase efficiency.
- Advanced Catalysis: AI-guided catalyst design and reaction pathway optimization for higher yields and lower waste.

Industrial challenges remain, particularly in toxicity mitigation and scalable green manufacturing. However, advances in nanotechnology, AI-assisted material design, and bio-based derivatives offer promising solutions.
Conclusion & Key Takeaways
Carbolic Acid is a foundational chemical with multifaceted applications spanning disinfection, materials science, environmental management, energy, agriculture, and biomedical fields. Its versatile chemical properties enable innovation in both traditional and emerging technologies. Given ongoing global demands for sustainable and effective materials, now is a critical time for researchers and industry leaders to deepen engagement with Carbolic Acid technologies.
Whether you are developing next-generation polymers, eco-friendly coatings, or advanced biomedical devices, Carbolic Acid offers a scalable, adaptable foundation to accelerate breakthroughs.
Navigating the complexity of Carbolic Acid innovation requires powerful tools. PatSnap Eureka AI Agent empowers R&D teams to explore patent landscapes, identify emerging trends, and benchmark competitive technologies — all in one intuitive platform. Discover untapped white spaces, optimize research direction, and drive faster, smarter innovation with data-driven insights.
👉 Request a demo today to unlock the full potential of Carbolic Acid and revolutionize your R&D.