sulphanilic acid is an aromatic amine sulfonic acid derived from aniline, widely used as an intermediate in dyes, pharmaceuticals, and sensing materials. Known for its zwitterionic structure and strong acid-base characteristics, this molecule exhibits excellent thermal stability, ionizability, and affinity for surface modification.
As industries strive for smarter diagnostics, greener catalysis, and sustainable material innovation, sulphanilic acid emerges as a multifunctional enabler. Its compatibility with hybrid systems, electrochemical sensors, and functional coatings makes it a candidate of growing interest across sectors.
This article delves into sulphanilic acid’s molecular properties, core industrial and academic application areas, performance advantages and drawbacks, emerging research trends, and strategic insights—all through PatSnap’s Eureka AI Agent.
Material Composition & Key Properties
- Molecular Formula: C6H7NO3S
- Structural Features: Contains an amino group (-NH2) and sulfonic acid group (-SO3H) on a benzene ring, forming a zwitterion in solid-state.
- Solubility: Soluble in water, low solubility in organic solvents
- Melting Point: ~288°C (decomposes)
- Key Properties:
- Strong acid-base interaction capability
- Zwitterionic nature for stable surface adhesion
- High thermal stability
- Reactive sites for diazotization and coupling reactions
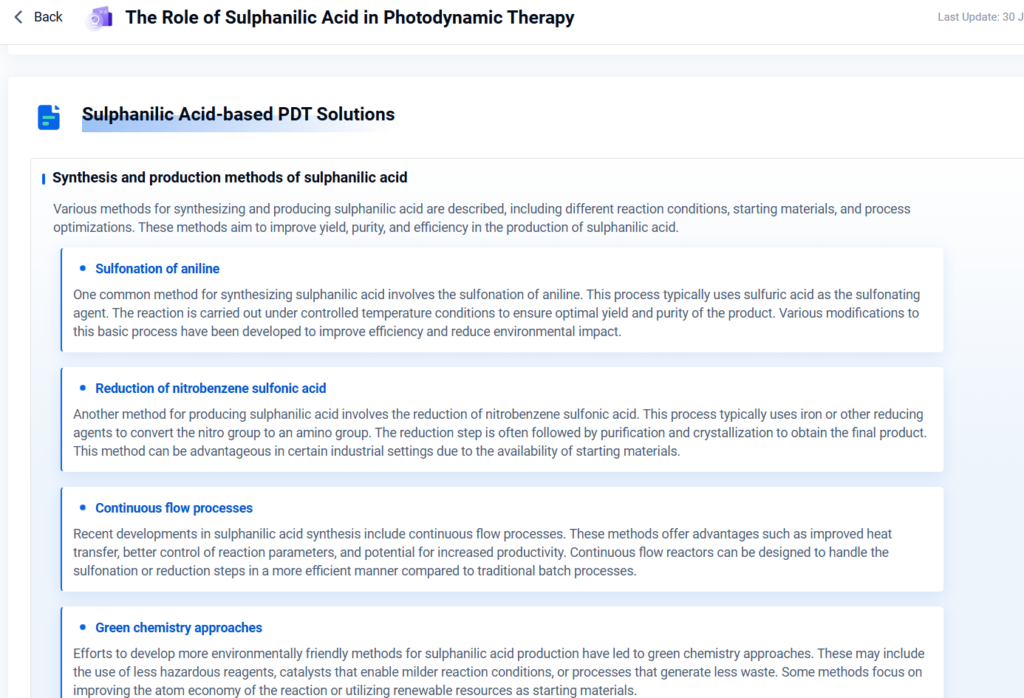
Synthesis Methods:
- Sulfonation of aniline with concentrated sulfuric acid
- Diazo coupling followed by purification or crystallization
Competitive Strengths:
- Excellent diazotization reactivity
- Biocompatible and low-toxicity precursor for biosensors
- Cost-effective and commercially available

Comparative Advantages & Limitations
Advantages:
- ✓ Versatile Functional Groups: Sulphanilic acid contains both amino and sulfonic acid groups, enabling diverse chemical reactions including azo coupling, coordination with metal ions, and polymer modifications.
- ✓ Excellent Water Solubility: Its high solubility facilitates aqueous-phase reactions, making it ideal for environmental and biomedical applications.
- ✓ Biocompatibility and Low Toxicity: Demonstrated safety in various biological assays supports its use in drug delivery systems and biosensors.
- ✓ Cost-Effective and Readily Available: As an industrially produced compound, it is affordable and accessible for large-scale applications.
- ✓ Strong Complexation Ability: It effectively binds heavy metals and acts as a corrosion inhibitor, useful in water treatment and metal protection.
- ✓ Photocatalytic and Electroluminescent Enhancement: Acts as a functional additive improving performance in light-emitting devices and catalytic processes.
- ✓ Chemical Stability and Thermal Resistance: Stable under a range of processing conditions, suitable for coatings, polymers, and composites.
Limitations:
- Limited Conductivity: By itself, sulphanilic acid has poor electrical conductivity, requiring combination with conductive materials for electronic applications.
- Susceptibility to Photodegradation: Under prolonged UV exposure, degradation can occur, potentially limiting outdoor or high-irradiation uses.
- Batch-to-Batch Variability: Industrial synthesis can result in purity variations, affecting reproducibility in sensitive applications.
- Moderate Thermal Stability Compared to Inorganic Analogues: While stable, it is less heat-resistant than some ceramic or inorganic materials used in harsh environments.
- Environmental Impact of Derivatives: Some sulphanilic acid-based azo dyes and derivatives pose ecological challenges due to persistence and toxicity.
- Complex Synthesis for Functionalized Derivatives: Tailoring sulphanilic acid for specific applications sometimes requires multi-step, costly synthesis routes.
- Limited Hydrophobicity: Its polar nature restricts use in hydrophobic polymer matrices unless chemically modified.
Comparative Analysis with Similar Materials:
| Feature | Sulphanilic Acid | Aniline Derivatives | Sulfamic Acid | 4-Aminobenzoic Acid |
|---|---|---|---|---|
| Functional Groups | Amino + Sulfonic Acid | Amino | Sulfonic Acid | Amino + Carboxylic Acid |
| Water Solubility | High | Moderate | Very High | Moderate |
| Biocompatibility | Good | Moderate | Good | Moderate |
| Stability (Thermal/UV) | Moderate | Moderate | High | Moderate |
| Complexation Ability | Strong (metal ions) | Moderate | Strong | Low |
| Cost and Availability | Affordable | Affordable | Affordable | Moderate |
Application Domains
⚡ Energy Storage & Conversion
In next‑generation energy systems, the demand for efficient, stable, and cost‑effective functional materials is growing—especially for thermoelectric, conductive, and catalyst‑based applications. Sulphanilic acid serves as an enabling molecule, allowing tuning of electronic properties, facilitating conductive polymer formation, and acting as a precursor in thermoelectric composites. Current research explores its incorporation into conductive inks, thermoelectric additives, and hybrid electrodes.
Research Frontlines:
Development of organic/inorganic composites, doped conductive networks, tailored energy harvesting coatings.
Related Reports:
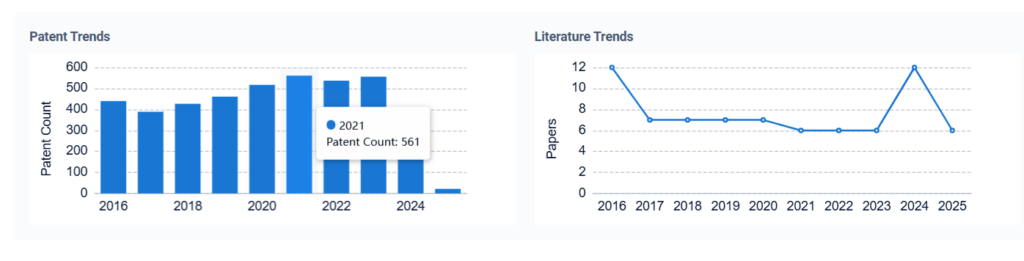
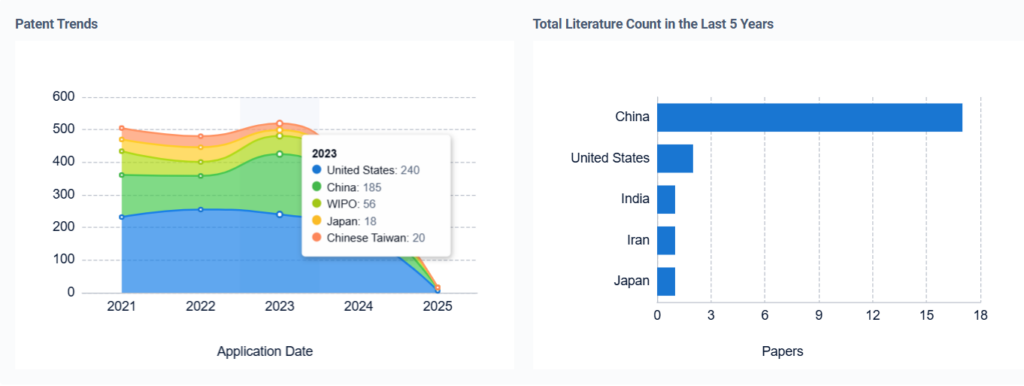
- How Sulphanilic Acid Enhances Electroluminescent Polymers
- Enhancing Thermoelectric Materials with Sulphanilic Acid-Based Compounds
- The Role of Sulphanilic Acid in Hydrogen Storage Materials
- Enhancing the Conductivity of Polymers with Sulphanilic Acid
- Study of Sulphanilic Acid-Modified Graphene in Energy Storage Applications
🧬 Biomedical Applications
In medical and life‑science domains, biocompatibility, sensing sensitivity, and antimicrobial functionality are essential. Sulphanilic acid and its derivatives function as fluorescent probes, enzymatic modulators, and smart hydrogel components with targeted drug‑release capabilities. Studies are exploring its roles in diagnostics, inflammation modulation, biosensing, and advanced hydrogel platforms.
Research Frontlines:
Fluorometric assays, enzymatic activity modulation, hydrogel engineering for drug delivery.
Related Reports:
- Biological Evaluation of Anti-inflammatory Properties of Sulphanilic Acid Derivatives
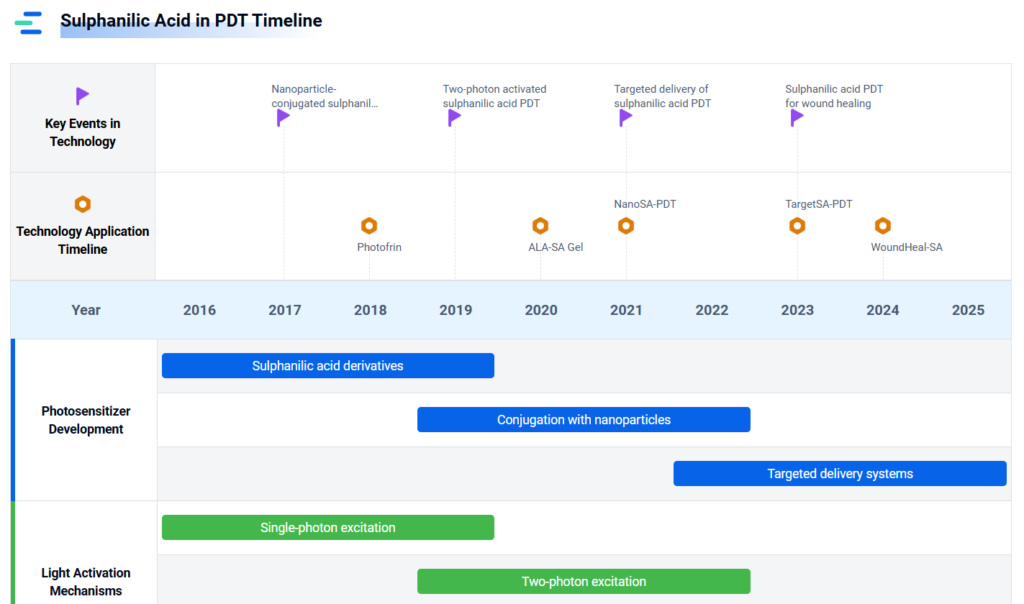
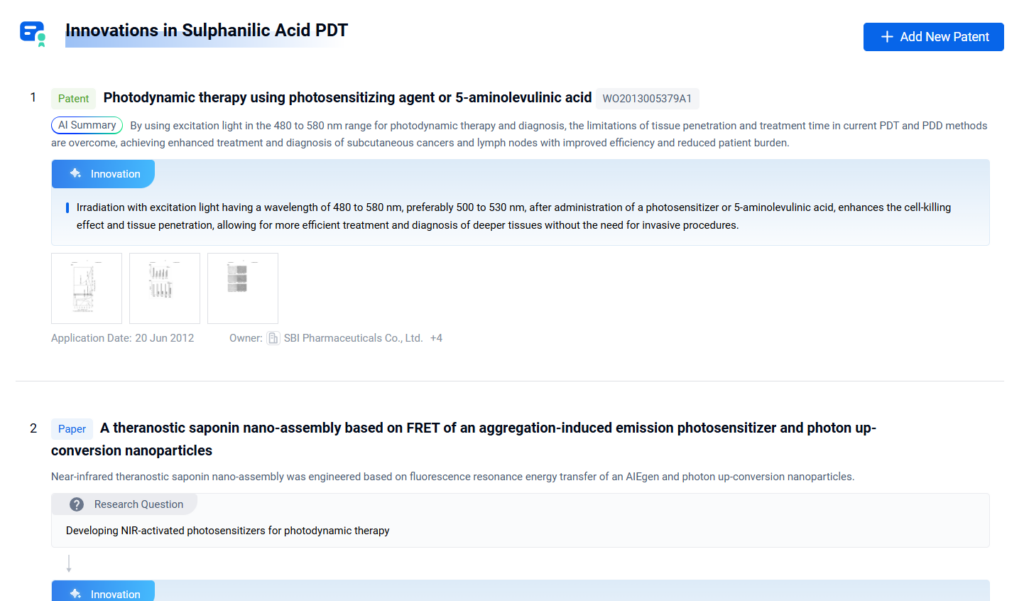
- The Role of Sulphanilic Acid in Photodynamic Therapy
- Engineering Hydrogels with Sulphanilic Acid for Biomedical Use
🧪 Advanced Electronics & Sensors
Semiconductors and sensor technologies require functional monomers with tunable optoelectronic properties. Sulphanilic acid is employed in the design of gas sensors, light‑emitting diodes (LED), and surface coatings for high‑selectivity detection. Reports investigate its polymer synthesis routes, LED applications, semiconductor stabilization, and integration into photonic and signal devices.
Research Frontlines:
Custom sensor materials, functional polymers for LEDs and gas detection, and conductive coatings.
Related Reports:
- Sulphanilic Acid in Light Emitting Diodes: Advances and Perspectives
- Application of Sulphanilic Acid in Air Quality Monitoring Devices
- How Sulphanilic Acid Enhances the Properties of Carbon-Based Electrodes
- Engineering Conductive Inks Using Sulphanilic Acid
- Developing Electrochromic Devices with Sulphanilic Acid
♻️ Environmental & Water Treatment
Real‑world water and wastewater remediation face industrial challenges: removing nitrates/nitrites, degrading pollutants, and inhibiting microbial growth. Sulphanilic acid‑based materials offer potential as reactive probes, pollutant absorbers, and catalysts in advanced oxidation processes. Research focuses on its role in nitrite removal, pollutant photocatalysis, soil microbial modulation, and environmental sensor integration.
Research Frontlines:
Combined pollutant removal and real‑time detection, functional coatings for remediation, and microbial impact studies.
Related Reports:
- Analysis of Sulphanilic Acid Interactions with Heavy Metal Ions in Aqueous Solutions
- Application of Sulphanilic Acid in Water Treatment: Nitrite Removal Mechanisms
- Photocatalytic Degradation of Pollutants Using Sulphanilic Acid-Derived Catalysts
- How Sulphanilic Acid Modulates Biofilm Formation in Bacteria
🧫 Materials Science & Additives
Polymeric systems, coatings, and structural composites benefit from additives that reinforce strength, stability, and functional performance. Sulphanilic acid is used to create flame-retardant formulations, enhance mechanical rigidity, control crystal growth, and improve polymer degradation profiles. Cutting-edge studies explore its use in MOFs, supramolecular assemblies, corrosion inhibition, and fire-retardant coatings.
Research Frontlines:
Smart coatings, additive manufacturing of high‑performance materials, and sustainable polymer functionalization.
Related Reports:
- Role of Sulphanilic Acid in Polymeric Material Strengthening
- The Role of Sulphanilic Acid in Developing Fire Retardant Materials
- Characterization of Supramolecular Assemblies of Sulphanilic Acid
- Potential of Sulphanilic Acid as a Corrosion Inhibitor in Metal Industries
🧪 Biomedical Sensors & Diagnostics (optional cross‑over domain filler)
Sensor miniaturization and biosignal clarity rely on stable fluorophores and specific binding molecules. Sulphanilic acid enhances signal detection, allows sensitive aptamer or ligand interactions, and stabilizes protein binding. Applications include glucose detection, protein assays, and enzymatic signal amplification in diagnostic platforms.
Research Frontlines:
Sensor coatings, biolabeling, and signal amplification platforms.
🔍 Want to track innovation in biomedical sensors using sulphanilic acid?
👉 Use PatSnap Eureka AI Agent to explore patented diagnostics, real-time academic research, and emerging biotech applications.
Uncover competitive benchmarks, functional material applications, and cross-industry collaboration patterns — all in one place.


Future Outlook & Research Frontiers
Sulphanilic acid is expected to expand its utility in hybrid nanomaterials, eco-safe dyes, and intelligent biomedical systems. Key frontiers include:
- Neural biointerfaces with self-healing hydrogels
- AI-guided co-polymer design incorporating sulphanilic units
- Solvent-free synthesis pathways for greener production
- Surface-modified electrochemical sensors
- Antimicrobial smart textiles

As research pushes toward multifunctional and adaptive materials, sulphanilic acid will remain a key building block for scalable and sustainable innovation.
Conclusion & Key Takeaways
Sulphanilic acid is more than a dye precursor—it’s a bridge between molecular functionality and real-world applications. From biosensing to catalysis and organic electronics, its adaptability offers broad industrial relevance. Now is the time to explore its full innovation potential across research and product pipelines.
Sulphanilic acid’s cross-disciplinary relevance makes tracking its evolution critical. PatSnap Eureka AI Agent empowers your R&D and IP strategy by:
- Mapping global patent and research frontiers
- Benchmarking leading players and institutions
- Surfacing white space and collaboration opportunities
👉 Unlock the future of functional molecules