A Comparative Analysis Of Natural Vs. Synthetic Polymer Scaffolds.
SEP 12, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer Scaffold Technology Evolution and Objectives
Polymer scaffolds have evolved significantly since their inception in the early 1980s, transforming from simple support structures to sophisticated bioactive platforms. Initially, scaffolds were primarily developed using synthetic polymers like polyglycolic acid (PGA) and polylactic acid (PLA) due to their predictable mechanical properties and ease of manufacturing. The 1990s marked a pivotal shift with the introduction of natural polymers such as collagen, chitosan, and alginate, which offered superior biocompatibility and cell recognition sites, albeit with less controllable degradation profiles.
The 2000s witnessed the emergence of hybrid scaffolds combining both natural and synthetic materials, attempting to harness the advantages of each while mitigating their respective limitations. This period also saw significant advancements in fabrication technologies, including electrospinning, 3D printing, and gas foaming, enabling precise control over scaffold architecture and porosity. These technological improvements facilitated better mimicry of the extracellular matrix (ECM) structure, crucial for effective cell attachment and proliferation.
Recent developments have focused on creating "smart" scaffolds with stimuli-responsive properties and controlled release mechanisms for growth factors and bioactive molecules. The integration of nanotechnology has further enhanced scaffold functionality, allowing for nanoscale surface modifications that can direct cell behavior and tissue formation with unprecedented precision. Additionally, advances in computational modeling have enabled the prediction of scaffold performance in various biological environments, streamlining the design process.
The primary objective of polymer scaffold technology is to create biocompatible, biodegradable structures that can effectively support cell growth and tissue regeneration while gradually degrading at a rate matching new tissue formation. Specific goals include optimizing mechanical properties to match those of native tissues, controlling degradation kinetics, enhancing bioactivity, and improving integration with host tissues. For natural versus synthetic polymer scaffolds, the aim is to determine which category—or what combination thereof—best serves specific tissue engineering applications.
Looking forward, the field is trending toward personalized scaffold designs tailored to individual patient needs, utilizing advanced manufacturing techniques and patient-specific data. There is also growing interest in developing scaffolds that can respond dynamically to the changing microenvironment during the healing process. The ultimate goal remains the creation of scaffolds that can seamlessly integrate with the body's natural healing processes, providing temporary support while facilitating the regeneration of fully functional tissues.
The 2000s witnessed the emergence of hybrid scaffolds combining both natural and synthetic materials, attempting to harness the advantages of each while mitigating their respective limitations. This period also saw significant advancements in fabrication technologies, including electrospinning, 3D printing, and gas foaming, enabling precise control over scaffold architecture and porosity. These technological improvements facilitated better mimicry of the extracellular matrix (ECM) structure, crucial for effective cell attachment and proliferation.
Recent developments have focused on creating "smart" scaffolds with stimuli-responsive properties and controlled release mechanisms for growth factors and bioactive molecules. The integration of nanotechnology has further enhanced scaffold functionality, allowing for nanoscale surface modifications that can direct cell behavior and tissue formation with unprecedented precision. Additionally, advances in computational modeling have enabled the prediction of scaffold performance in various biological environments, streamlining the design process.
The primary objective of polymer scaffold technology is to create biocompatible, biodegradable structures that can effectively support cell growth and tissue regeneration while gradually degrading at a rate matching new tissue formation. Specific goals include optimizing mechanical properties to match those of native tissues, controlling degradation kinetics, enhancing bioactivity, and improving integration with host tissues. For natural versus synthetic polymer scaffolds, the aim is to determine which category—or what combination thereof—best serves specific tissue engineering applications.
Looking forward, the field is trending toward personalized scaffold designs tailored to individual patient needs, utilizing advanced manufacturing techniques and patient-specific data. There is also growing interest in developing scaffolds that can respond dynamically to the changing microenvironment during the healing process. The ultimate goal remains the creation of scaffolds that can seamlessly integrate with the body's natural healing processes, providing temporary support while facilitating the regeneration of fully functional tissues.
Market Analysis for Biomedical Scaffold Applications
The global biomedical scaffold market has experienced significant growth in recent years, driven by increasing applications in tissue engineering, regenerative medicine, and drug delivery systems. As of 2023, the market is valued at approximately 1.5 billion USD, with projections indicating a compound annual growth rate (CAGR) of 8-10% over the next five years. This growth trajectory is primarily fueled by rising prevalence of chronic diseases, an aging global population, and advancements in biomaterial technologies.
Natural polymer scaffolds currently dominate the market share at roughly 60%, owing to their inherent biocompatibility, biodegradability, and similarity to native extracellular matrix components. Collagen-based scaffolds lead this segment, followed by hyaluronic acid, chitosan, and alginate derivatives. The natural polymer scaffold market is particularly strong in applications requiring minimal immune response and enhanced cell adhesion properties.
Synthetic polymer scaffolds, while holding a smaller market share of approximately 40%, are witnessing faster growth rates due to their customizable mechanical properties, batch-to-batch consistency, and extended shelf life. Poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyethylene glycol (PEG) based scaffolds are the primary contributors to this segment's expansion, especially in orthopedic and dental applications where mechanical strength is paramount.
Geographically, North America holds the largest market share (35-40%) due to substantial R&D investments, presence of major industry players, and favorable regulatory frameworks. Europe follows closely at 30%, with Asia-Pacific emerging as the fastest-growing region at a CAGR of 12-14%, driven by improving healthcare infrastructure, increasing medical tourism, and rising government initiatives in countries like China, Japan, and South Korea.
End-user segmentation reveals hospitals and surgical centers as the primary consumers (45%), followed by research institutions (30%) and pharmaceutical/biotechnology companies (25%). The orthopedic application segment currently leads market demand (35%), with cardiovascular (20%), dermatological (15%), and neurological applications (10%) following behind.
Key market restraints include high production costs, complex regulatory approval processes, and challenges in scaling up manufacturing while maintaining product consistency. Additionally, reimbursement limitations in certain regions pose significant barriers to market penetration, particularly for novel scaffold technologies with limited clinical validation.
Consumer trends indicate growing preference for personalized scaffold solutions that can be tailored to individual patient needs, creating opportunities for technologies that enable customization through 3D printing and other advanced manufacturing methods. Furthermore, increasing demand for dual-function scaffolds that combine structural support with drug delivery capabilities represents an emerging market segment with substantial growth potential.
Natural polymer scaffolds currently dominate the market share at roughly 60%, owing to their inherent biocompatibility, biodegradability, and similarity to native extracellular matrix components. Collagen-based scaffolds lead this segment, followed by hyaluronic acid, chitosan, and alginate derivatives. The natural polymer scaffold market is particularly strong in applications requiring minimal immune response and enhanced cell adhesion properties.
Synthetic polymer scaffolds, while holding a smaller market share of approximately 40%, are witnessing faster growth rates due to their customizable mechanical properties, batch-to-batch consistency, and extended shelf life. Poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and polyethylene glycol (PEG) based scaffolds are the primary contributors to this segment's expansion, especially in orthopedic and dental applications where mechanical strength is paramount.
Geographically, North America holds the largest market share (35-40%) due to substantial R&D investments, presence of major industry players, and favorable regulatory frameworks. Europe follows closely at 30%, with Asia-Pacific emerging as the fastest-growing region at a CAGR of 12-14%, driven by improving healthcare infrastructure, increasing medical tourism, and rising government initiatives in countries like China, Japan, and South Korea.
End-user segmentation reveals hospitals and surgical centers as the primary consumers (45%), followed by research institutions (30%) and pharmaceutical/biotechnology companies (25%). The orthopedic application segment currently leads market demand (35%), with cardiovascular (20%), dermatological (15%), and neurological applications (10%) following behind.
Key market restraints include high production costs, complex regulatory approval processes, and challenges in scaling up manufacturing while maintaining product consistency. Additionally, reimbursement limitations in certain regions pose significant barriers to market penetration, particularly for novel scaffold technologies with limited clinical validation.
Consumer trends indicate growing preference for personalized scaffold solutions that can be tailored to individual patient needs, creating opportunities for technologies that enable customization through 3D printing and other advanced manufacturing methods. Furthermore, increasing demand for dual-function scaffolds that combine structural support with drug delivery capabilities represents an emerging market segment with substantial growth potential.
Current Landscape and Challenges in Polymer Scaffold Development
The polymer scaffold landscape has evolved significantly over the past decade, with both natural and synthetic materials gaining prominence in tissue engineering and regenerative medicine applications. Currently, natural polymer scaffolds derived from sources such as collagen, gelatin, alginate, chitosan, and hyaluronic acid dominate certain biomedical applications due to their inherent biocompatibility and cell-recognition sites that promote cellular adhesion and proliferation.
Synthetic polymer scaffolds, including poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL), have simultaneously advanced through innovative manufacturing techniques and chemical modifications. These materials offer superior mechanical properties and more predictable degradation profiles compared to their natural counterparts.
Despite significant progress, several critical challenges persist in polymer scaffold development. Mechanical property optimization remains problematic, particularly for natural polymers which often exhibit insufficient strength for load-bearing applications. Conversely, synthetic polymers frequently lack the biological cues necessary for optimal cell attachment and tissue integration.
Degradation rate control presents another substantial hurdle. Natural polymers typically degrade rapidly and unpredictably in vivo, while synthetic polymers may produce acidic byproducts during degradation that can trigger inflammatory responses. Achieving synchronized degradation with tissue regeneration rates continues to challenge researchers across both material categories.
Scalability and manufacturing consistency represent significant obstacles, especially for natural polymers with batch-to-batch variability. While synthetic polymers offer better manufacturing reproducibility, complex scaffold architectures with biomimetic features remain difficult to produce at scale regardless of material type.
Immunogenicity concerns persist with certain natural polymers, particularly those derived from xenogeneic sources. Although synthetic polymers generally elicit minimal immune responses, their hydrophobic surfaces and lack of cell-recognition motifs can limit cellular infiltration and tissue integration.
The regulatory landscape adds another layer of complexity. Natural polymers often face stricter scrutiny due to potential pathogen transmission risks, while novel synthetic polymers require extensive safety validation before clinical translation. This regulatory burden significantly impacts commercialization timelines for both material types.
Recent developments have focused on hybrid approaches that combine natural and synthetic polymers to leverage the advantages of both while mitigating their respective limitations. These composite scaffolds represent a promising direction, though challenges in interface engineering and processing compatibility remain to be fully resolved.
Synthetic polymer scaffolds, including poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL), have simultaneously advanced through innovative manufacturing techniques and chemical modifications. These materials offer superior mechanical properties and more predictable degradation profiles compared to their natural counterparts.
Despite significant progress, several critical challenges persist in polymer scaffold development. Mechanical property optimization remains problematic, particularly for natural polymers which often exhibit insufficient strength for load-bearing applications. Conversely, synthetic polymers frequently lack the biological cues necessary for optimal cell attachment and tissue integration.
Degradation rate control presents another substantial hurdle. Natural polymers typically degrade rapidly and unpredictably in vivo, while synthetic polymers may produce acidic byproducts during degradation that can trigger inflammatory responses. Achieving synchronized degradation with tissue regeneration rates continues to challenge researchers across both material categories.
Scalability and manufacturing consistency represent significant obstacles, especially for natural polymers with batch-to-batch variability. While synthetic polymers offer better manufacturing reproducibility, complex scaffold architectures with biomimetic features remain difficult to produce at scale regardless of material type.
Immunogenicity concerns persist with certain natural polymers, particularly those derived from xenogeneic sources. Although synthetic polymers generally elicit minimal immune responses, their hydrophobic surfaces and lack of cell-recognition motifs can limit cellular infiltration and tissue integration.
The regulatory landscape adds another layer of complexity. Natural polymers often face stricter scrutiny due to potential pathogen transmission risks, while novel synthetic polymers require extensive safety validation before clinical translation. This regulatory burden significantly impacts commercialization timelines for both material types.
Recent developments have focused on hybrid approaches that combine natural and synthetic polymers to leverage the advantages of both while mitigating their respective limitations. These composite scaffolds represent a promising direction, though challenges in interface engineering and processing compatibility remain to be fully resolved.
Comparative Analysis of Natural and Synthetic Scaffold Solutions
01 Natural polymer scaffolds for tissue engineering
Natural polymers such as collagen, chitosan, and alginate are used to create biocompatible scaffolds for tissue engineering applications. These scaffolds provide a supportive environment for cell growth, proliferation, and differentiation. Natural polymers offer advantages including biodegradability, biocompatibility, and similarity to the extracellular matrix, making them ideal for tissue regeneration and wound healing applications.- Natural polymer scaffolds for tissue engineering: Natural polymers such as collagen, chitosan, and alginate are used to create biocompatible scaffolds for tissue engineering applications. These scaffolds provide a supportive environment for cell growth, proliferation, and differentiation. Natural polymers offer advantages including biodegradability, biocompatibility, and similarity to the extracellular matrix, making them ideal for tissue regeneration and wound healing applications.
- Synthetic polymer scaffolds with controlled properties: Synthetic polymers like polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL) are engineered to create scaffolds with precisely controlled physical and mechanical properties. These scaffolds can be designed with specific porosity, degradation rates, and mechanical strength to match the requirements of different tissue types. The ability to tailor these properties makes synthetic polymer scaffolds versatile for various biomedical applications.
- Hybrid and composite polymer scaffolds: Hybrid scaffolds combining natural and synthetic polymers leverage the advantages of both materials. These composite structures integrate the biocompatibility of natural polymers with the mechanical stability and customizable properties of synthetic polymers. By creating these hybrid systems, researchers can develop scaffolds with enhanced cell adhesion, controlled degradation rates, and improved mechanical properties for tissue engineering applications.
- 3D printing and fabrication techniques for polymer scaffolds: Advanced manufacturing techniques including 3D printing, electrospinning, and freeze-drying are used to fabricate polymer scaffolds with complex architectures. These techniques allow for precise control over scaffold geometry, porosity, and internal structure. The ability to create patient-specific scaffolds with biomimetic structures enhances cell infiltration, vascularization, and ultimately tissue regeneration outcomes.
- Functionalized polymer scaffolds with bioactive molecules: Polymer scaffolds can be functionalized with bioactive molecules such as growth factors, peptides, and drugs to enhance their biological performance. These bioactive components can be incorporated through various methods including surface modification, encapsulation, or chemical conjugation. Functionalized scaffolds provide instructive cues to cells, promoting specific cellular responses such as differentiation, migration, and extracellular matrix production for improved tissue regeneration.
02 Synthetic polymer scaffolds with controlled properties
Synthetic polymers like polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL) are engineered to create scaffolds with precisely controlled physical and mechanical properties. These scaffolds can be designed with specific porosity, degradation rates, and mechanical strength to match the requirements of different tissue types. The ability to tailor these properties makes synthetic polymer scaffolds versatile for various biomedical applications.Expand Specific Solutions03 Hybrid scaffolds combining natural and synthetic polymers
Hybrid scaffolds incorporate both natural and synthetic polymers to leverage the advantages of each material type. These composite scaffolds combine the biocompatibility and cell-recognition sites of natural polymers with the mechanical strength and customizable properties of synthetic polymers. This approach results in scaffolds with enhanced biological performance and structural integrity for tissue engineering applications.Expand Specific Solutions04 3D printing of polymer scaffolds
Advanced 3D printing technologies enable the fabrication of complex polymer scaffold architectures with precise control over internal structures. These techniques allow for patient-specific scaffold designs and the incorporation of multiple materials and bioactive components. 3D printed polymer scaffolds can mimic the hierarchical structure of natural tissues and include features like vascular networks to support cell viability in larger constructs.Expand Specific Solutions05 Functionalized polymer scaffolds with bioactive molecules
Polymer scaffolds can be functionalized with bioactive molecules such as growth factors, peptides, and drugs to enhance their biological performance. These bioactive components can be incorporated through various methods including surface modification, encapsulation, or chemical conjugation. Functionalized scaffolds provide biochemical cues that guide cell behavior, promote tissue formation, and can address specific therapeutic needs such as controlled drug release or antimicrobial properties.Expand Specific Solutions
Leading Organizations in Polymer Scaffold Research and Production
The polymer scaffold market is currently in a growth phase, characterized by increasing adoption in tissue engineering and regenerative medicine applications. The competitive landscape reveals a dynamic interplay between natural and synthetic polymer technologies, with market size projected to reach significant expansion due to rising demand in medical applications. Academic institutions like MIT, University of Washington, and Tianjin University are driving fundamental research, while companies including PolyNovo Biomaterials, Regentis Biomaterials, and Boston Scientific are commercializing advanced solutions. The technology maturity varies significantly between natural polymers (established but limited in customization) and synthetic polymers (rapidly evolving with enhanced mechanical properties and biocompatibility), creating opportunities for hybrid approaches that combine advantages of both material types.
PolyNovo Biomaterials Pty Ltd.
Technical Solution: PolyNovo专注于开发基于NovoSorb技术平台的合成聚合物支架。其核心技术是一种生物可降解的聚氨酯聚合物,可形成多孔结构支架,促进组织再生。该公司的NovoSorb BTM (Biodegradable Temporizing Matrix)产品是一种双层合成聚合物支架,上层为密封膜防止细菌侵入和水分流失,下层为生物可降解多孔基质,允许细胞迁移和血管生成。与天然聚合物支架相比,NovoSorb技术提供了可预测的降解速率和机械性能,同时减少了免疫原性问题。临床研究表明,这种合成支架在严重烧伤和复杂伤口治疗中表现出色,支持细胞迁移和新血管形成,最终被自体组织替代。
优势:合成聚合物支架具有可控的降解速率、一致的机械性能和低免疫原性;生产过程可扩展性强,批次间差异小;可根据特定应用需求调整物理和化学性质。劣势:缺乏天然聚合物固有的生物活性信号;可能需要额外的生物活性分子修饰以增强细胞粘附和功能;某些合成聚合物降解产物可能引起局部炎症反应。
Regentis Biomaterials Ltd.
Technical Solution: Regentis Biomaterials开发了GelrinC技术,这是一种结合天然与合成聚合物优势的混合支架系统。该技术基于聚乙二醇二丙烯酸酯(PEG-DA)与变性纤维蛋白原的结合,通过光交联形成半合成水凝胶支架。这种独特的混合方法利用了天然纤维蛋白原的生物相容性和细胞粘附特性,同时结合了合成PEG的可调控性和机械稳定性。GelrinC支架最初以液态形式注入软骨缺损部位,然后通过紫外线照射原位固化,形成与周围组织完美贴合的三维支架。随着时间推移,支架逐渐降解,被新生软骨组织替代。临床研究显示,这种混合支架在软骨修复中表现优异,能促进软骨细胞分化和细胞外基质产生,为关节软骨损伤提供有效治疗方案。
优势:结合了天然聚合物的生物活性和合成聚合物的机械稳定性;可注射性使其能够填充不规则形状的缺损;原位固化技术减少了手术创伤。劣势:光交联过程可能对某些生物活性分子造成损害;长期稳定性和机械性能可能不如纯合成支架;生产和质量控制复杂度高于单一成分支架。
Key Patents and Innovations in Polymer Scaffold Technology
Electrospun blends of natural and synthetic polymer fibers as tissue engineering scaffolds
PatentInactiveUS8048446B2
Innovation
- Electrospun biocompatible biomatrices composed of a blend of synthetic polymers like PLGA and natural proteins such as gelatin and elastin, which do not require crosslinking, creating a fibrous matrix that supports cell penetration and proliferation without disintegration in aqueous environments.
Mechanically competent natural polymer based porous grafts for bone repair and regeneration
PatentInactiveUS20100249931A1
Innovation
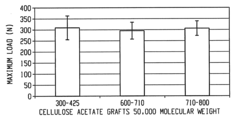
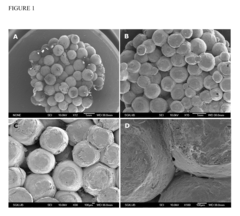
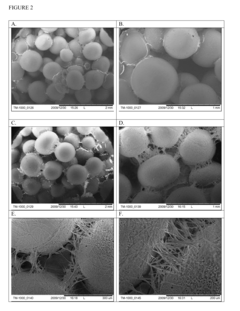
- Development of bone replacement scaffolds fabricated from naturally derived polysaccharide microspheres, such as ethyl cellulose and cellulose acetate, using a solvent/non-solvent sintering method, which provides enhanced mechanical properties and biocompatibility, and functionalization with collagen nanofibers for improved cell attachment and tissue regeneration.
Regulatory Framework for Biomaterial Approval and Commercialization
The regulatory landscape for biomaterial scaffolds represents a complex and evolving framework that significantly impacts the development and commercialization of both natural and synthetic polymer scaffolds. In the United States, the FDA regulates these materials primarily through the Center for Devices and Radiological Health (CDRH) or the Center for Biologics Evaluation and Research (CBER), depending on their intended use and mechanism of action. Natural polymer scaffolds derived from biological sources often face additional scrutiny regarding source material consistency, potential immunogenicity, and pathogen transmission risks.
The approval pathway typically involves classification into Class I, II, or III medical devices, with most scaffolds falling into Class II or III, requiring either 510(k) clearance or Premarket Approval (PMA). Synthetic polymer scaffolds may benefit from more straightforward regulatory processes due to their controlled manufacturing processes and consistent composition, potentially reducing the burden of safety testing compared to natural alternatives.
In the European Union, the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR) govern biomaterial approval, with particular emphasis on clinical evidence requirements and post-market surveillance. The classification system differs from the US approach, categorizing devices into Classes I, IIa, IIb, and III based on risk profiles. Notably, the EU regulatory framework has recently strengthened requirements for clinical evidence and traceability, affecting both natural and synthetic scaffold developers.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to standardize requirements across major markets, though significant regional variations persist. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation for innovative medical technologies, potentially accelerating approval for novel scaffold technologies.
Quality management systems compliance, particularly ISO 13485, remains fundamental across all jurisdictions. For natural polymer scaffolds, additional standards related to animal-derived materials (ISO 22442) apply, creating an extra layer of compliance requirements not applicable to fully synthetic alternatives.
Reimbursement considerations also significantly impact commercialization strategies. While regulatory approval enables market access, securing favorable reimbursement decisions from public and private payers determines commercial viability. Evidence requirements for reimbursement often exceed regulatory standards, necessitating robust clinical and economic data demonstrating value over existing treatments.
Recent regulatory trends indicate movement toward more adaptive frameworks that accommodate rapidly evolving biomaterial technologies while maintaining safety standards. Risk-based approaches and real-world evidence utilization are becoming increasingly important in regulatory decision-making, potentially benefiting innovative scaffold technologies with strong safety profiles regardless of their natural or synthetic origin.
The approval pathway typically involves classification into Class I, II, or III medical devices, with most scaffolds falling into Class II or III, requiring either 510(k) clearance or Premarket Approval (PMA). Synthetic polymer scaffolds may benefit from more straightforward regulatory processes due to their controlled manufacturing processes and consistent composition, potentially reducing the burden of safety testing compared to natural alternatives.
In the European Union, the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR) govern biomaterial approval, with particular emphasis on clinical evidence requirements and post-market surveillance. The classification system differs from the US approach, categorizing devices into Classes I, IIa, IIb, and III based on risk profiles. Notably, the EU regulatory framework has recently strengthened requirements for clinical evidence and traceability, affecting both natural and synthetic scaffold developers.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) aim to standardize requirements across major markets, though significant regional variations persist. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation for innovative medical technologies, potentially accelerating approval for novel scaffold technologies.
Quality management systems compliance, particularly ISO 13485, remains fundamental across all jurisdictions. For natural polymer scaffolds, additional standards related to animal-derived materials (ISO 22442) apply, creating an extra layer of compliance requirements not applicable to fully synthetic alternatives.
Reimbursement considerations also significantly impact commercialization strategies. While regulatory approval enables market access, securing favorable reimbursement decisions from public and private payers determines commercial viability. Evidence requirements for reimbursement often exceed regulatory standards, necessitating robust clinical and economic data demonstrating value over existing treatments.
Recent regulatory trends indicate movement toward more adaptive frameworks that accommodate rapidly evolving biomaterial technologies while maintaining safety standards. Risk-based approaches and real-world evidence utilization are becoming increasingly important in regulatory decision-making, potentially benefiting innovative scaffold technologies with strong safety profiles regardless of their natural or synthetic origin.
Sustainability and Environmental Impact of Scaffold Materials
The sustainability profile of scaffold materials represents a critical consideration in biomedical engineering applications, with significant differences observed between natural and synthetic polymer options. Natural polymers such as collagen, chitosan, and alginate generally demonstrate superior environmental credentials due to their biodegradability and renewable sourcing. These materials typically decompose through natural biological processes without generating toxic byproducts, significantly reducing their environmental footprint compared to many synthetic alternatives.
Synthetic polymers, while offering advantages in terms of customization and mechanical properties, often present greater sustainability challenges. Many conventional synthetic scaffolds derive from petroleum-based resources, contributing to carbon emissions and resource depletion. Their production frequently involves energy-intensive processes and potentially hazardous chemicals, raising concerns about manufacturing sustainability. Additionally, non-biodegradable synthetic polymers may persist in the environment for decades or centuries after disposal.
Recent advancements have focused on developing more sustainable synthetic options, including biodegradable synthetic polymers like polylactic acid (PLA) and polyglycolic acid (PGA). These materials offer improved end-of-life characteristics while maintaining the performance advantages of synthetic materials. However, life cycle assessments indicate they still generally carry higher environmental burdens than their natural counterparts.
Manufacturing processes represent another significant sustainability differentiator. Natural polymer extraction typically requires fewer chemical inputs and less energy than synthetic polymer production, though scale and standardization remain challenging. Conversely, synthetic polymer manufacturing benefits from established industrial processes but often generates more waste and emissions. Recent green chemistry initiatives have made progress in reducing solvent use and developing more environmentally benign synthesis routes for both material categories.
End-of-life considerations strongly favor natural polymers, which can be metabolized by biological systems or composted under appropriate conditions. Synthetic non-biodegradable scaffolds may require specialized disposal procedures, potentially contributing to medical waste challenges. Biodegradable synthetics occupy a middle ground, offering controlled degradation but sometimes requiring specific conditions to fully break down.
Regulatory frameworks increasingly incorporate sustainability metrics into material approval processes, creating market incentives for greener scaffold technologies. This trend has accelerated research into hybrid materials that combine the performance advantages of synthetics with the environmental benefits of natural polymers. Such innovations may represent the most promising path forward, potentially delivering scaffolds that minimize environmental impact without compromising therapeutic efficacy.
Synthetic polymers, while offering advantages in terms of customization and mechanical properties, often present greater sustainability challenges. Many conventional synthetic scaffolds derive from petroleum-based resources, contributing to carbon emissions and resource depletion. Their production frequently involves energy-intensive processes and potentially hazardous chemicals, raising concerns about manufacturing sustainability. Additionally, non-biodegradable synthetic polymers may persist in the environment for decades or centuries after disposal.
Recent advancements have focused on developing more sustainable synthetic options, including biodegradable synthetic polymers like polylactic acid (PLA) and polyglycolic acid (PGA). These materials offer improved end-of-life characteristics while maintaining the performance advantages of synthetic materials. However, life cycle assessments indicate they still generally carry higher environmental burdens than their natural counterparts.
Manufacturing processes represent another significant sustainability differentiator. Natural polymer extraction typically requires fewer chemical inputs and less energy than synthetic polymer production, though scale and standardization remain challenging. Conversely, synthetic polymer manufacturing benefits from established industrial processes but often generates more waste and emissions. Recent green chemistry initiatives have made progress in reducing solvent use and developing more environmentally benign synthesis routes for both material categories.
End-of-life considerations strongly favor natural polymers, which can be metabolized by biological systems or composted under appropriate conditions. Synthetic non-biodegradable scaffolds may require specialized disposal procedures, potentially contributing to medical waste challenges. Biodegradable synthetics occupy a middle ground, offering controlled degradation but sometimes requiring specific conditions to fully break down.
Regulatory frameworks increasingly incorporate sustainability metrics into material approval processes, creating market incentives for greener scaffold technologies. This trend has accelerated research into hybrid materials that combine the performance advantages of synthetics with the environmental benefits of natural polymers. Such innovations may represent the most promising path forward, potentially delivering scaffolds that minimize environmental impact without compromising therapeutic efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!