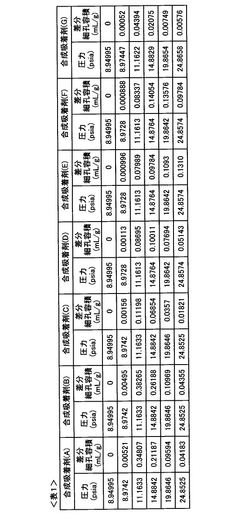
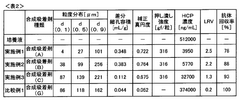
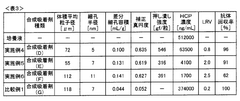
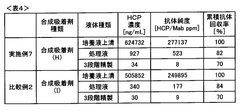
Adsorption Process Efficiency in Synthetic Biology Applications
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Adsorption Technology Evolution and Objectives
Adsorption technology has evolved significantly over the past century, transforming from simple physical separation processes to sophisticated molecular-level engineering applications. The journey began with basic activated carbon filtration systems in the early 20th century and has progressed to highly selective nanomaterial-based adsorbents capable of targeting specific biomolecules. This evolution has been particularly impactful in synthetic biology, where precise molecular interactions are essential for successful bioprocessing operations.
The integration of adsorption processes into synthetic biology applications represents a critical intersection of chemical engineering and biological sciences. Initially, adsorption in biological contexts focused primarily on downstream processing, particularly protein purification through chromatographic techniques. However, recent advances have expanded applications to include targeted delivery systems, biosensors, and biocatalyst immobilization platforms that enhance enzymatic stability and reusability.
Current technological objectives in this field center on addressing several key challenges. First, improving selectivity mechanisms to enable precise separation of biomolecules with similar physicochemical properties remains a priority. This is particularly relevant in synthetic biology where product purity directly impacts functionality and safety profiles. Second, enhancing adsorption capacity while maintaining selectivity presents an ongoing engineering challenge that requires innovative material design approaches.
Energy efficiency represents another critical objective, as traditional adsorption processes often demand significant energy inputs for regeneration cycles. Developing low-energy alternatives that maintain performance metrics would substantially improve the sustainability profile of synthetic biology manufacturing processes. Additionally, researchers are focusing on creating scalable adsorption technologies that maintain efficiency across production volumes, addressing a significant barrier to commercial implementation.
The convergence of computational modeling with experimental design has accelerated progress in this field. Machine learning algorithms now enable prediction of adsorption behaviors based on molecular characteristics, while molecular dynamics simulations provide insights into adsorbent-adsorbate interactions at the atomic level. These computational approaches are increasingly guiding rational design of novel adsorbent materials with tailored properties for specific synthetic biology applications.
Looking forward, the field aims to develop "programmable" adsorption systems that can dynamically adjust selectivity parameters in response to environmental cues or external stimuli. Such responsive systems would revolutionize bioprocessing by enabling adaptive separation processes that optimize for changing feedstock compositions or product specifications. This represents the frontier of adsorption technology in synthetic biology applications, where molecular engineering meets biological complexity.
The integration of adsorption processes into synthetic biology applications represents a critical intersection of chemical engineering and biological sciences. Initially, adsorption in biological contexts focused primarily on downstream processing, particularly protein purification through chromatographic techniques. However, recent advances have expanded applications to include targeted delivery systems, biosensors, and biocatalyst immobilization platforms that enhance enzymatic stability and reusability.
Current technological objectives in this field center on addressing several key challenges. First, improving selectivity mechanisms to enable precise separation of biomolecules with similar physicochemical properties remains a priority. This is particularly relevant in synthetic biology where product purity directly impacts functionality and safety profiles. Second, enhancing adsorption capacity while maintaining selectivity presents an ongoing engineering challenge that requires innovative material design approaches.
Energy efficiency represents another critical objective, as traditional adsorption processes often demand significant energy inputs for regeneration cycles. Developing low-energy alternatives that maintain performance metrics would substantially improve the sustainability profile of synthetic biology manufacturing processes. Additionally, researchers are focusing on creating scalable adsorption technologies that maintain efficiency across production volumes, addressing a significant barrier to commercial implementation.
The convergence of computational modeling with experimental design has accelerated progress in this field. Machine learning algorithms now enable prediction of adsorption behaviors based on molecular characteristics, while molecular dynamics simulations provide insights into adsorbent-adsorbate interactions at the atomic level. These computational approaches are increasingly guiding rational design of novel adsorbent materials with tailored properties for specific synthetic biology applications.
Looking forward, the field aims to develop "programmable" adsorption systems that can dynamically adjust selectivity parameters in response to environmental cues or external stimuli. Such responsive systems would revolutionize bioprocessing by enabling adaptive separation processes that optimize for changing feedstock compositions or product specifications. This represents the frontier of adsorption technology in synthetic biology applications, where molecular engineering meets biological complexity.
Market Analysis for Synthetic Biology Adsorption Solutions
The synthetic biology adsorption solutions market is experiencing robust growth, driven by increasing applications in biopharmaceuticals, biofuels, and specialty chemicals. Current market valuation stands at approximately 3.2 billion USD with a compound annual growth rate of 14.7% projected through 2028, significantly outpacing traditional chemical processing technologies.
Demand is particularly strong in the biopharmaceutical sector, where adsorption technologies enable more efficient purification of biologics and recombinant proteins. This segment currently represents 42% of the total market share, with therapeutic protein production being the primary application. The COVID-19 pandemic has further accelerated this trend, as manufacturers seek more efficient production methods for vaccines and therapeutics.
Industrial biotechnology represents another significant market segment, with growing adoption in biofuel production and specialty chemical manufacturing. Companies are increasingly turning to synthetic biology approaches to replace petroleum-based processes, creating a 28% annual increase in demand for advanced adsorption solutions that can handle complex biological feedstocks and products.
Regional analysis reveals North America leading with 38% market share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 16.8% annually, driven by expanding biomanufacturing capabilities in China, South Korea, and Singapore, along with favorable government policies supporting biotechnology development.
Customer segmentation shows large biopharmaceutical companies as primary adopters, accounting for 47% of purchases. Academic and research institutions represent 22% of the market, while small and medium biotech enterprises comprise 18%. The remaining market share is distributed among contract manufacturing organizations and other industrial users.
Key market drivers include increasing pressure for sustainable manufacturing processes, growing demand for biologics, and technological advancements in adsorption materials. Specifically, the development of novel adsorbents with higher selectivity and capacity has expanded the application scope of adsorption technologies in synthetic biology.
Market challenges include high initial investment costs, technical complexity requiring specialized expertise, and regulatory hurdles for implementation in GMP environments. Additionally, competition from alternative separation technologies such as membrane filtration and chromatography creates market pressure for continuous innovation in adsorption solutions.
Customer needs analysis reveals growing demand for integrated systems that combine adsorption with other downstream processing steps, scalable solutions that maintain performance from laboratory to industrial scale, and technologies that reduce overall process costs while improving product yields.
Demand is particularly strong in the biopharmaceutical sector, where adsorption technologies enable more efficient purification of biologics and recombinant proteins. This segment currently represents 42% of the total market share, with therapeutic protein production being the primary application. The COVID-19 pandemic has further accelerated this trend, as manufacturers seek more efficient production methods for vaccines and therapeutics.
Industrial biotechnology represents another significant market segment, with growing adoption in biofuel production and specialty chemical manufacturing. Companies are increasingly turning to synthetic biology approaches to replace petroleum-based processes, creating a 28% annual increase in demand for advanced adsorption solutions that can handle complex biological feedstocks and products.
Regional analysis reveals North America leading with 38% market share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 16.8% annually, driven by expanding biomanufacturing capabilities in China, South Korea, and Singapore, along with favorable government policies supporting biotechnology development.
Customer segmentation shows large biopharmaceutical companies as primary adopters, accounting for 47% of purchases. Academic and research institutions represent 22% of the market, while small and medium biotech enterprises comprise 18%. The remaining market share is distributed among contract manufacturing organizations and other industrial users.
Key market drivers include increasing pressure for sustainable manufacturing processes, growing demand for biologics, and technological advancements in adsorption materials. Specifically, the development of novel adsorbents with higher selectivity and capacity has expanded the application scope of adsorption technologies in synthetic biology.
Market challenges include high initial investment costs, technical complexity requiring specialized expertise, and regulatory hurdles for implementation in GMP environments. Additionally, competition from alternative separation technologies such as membrane filtration and chromatography creates market pressure for continuous innovation in adsorption solutions.
Customer needs analysis reveals growing demand for integrated systems that combine adsorption with other downstream processing steps, scalable solutions that maintain performance from laboratory to industrial scale, and technologies that reduce overall process costs while improving product yields.
Current Adsorption Techniques and Bottlenecks
Adsorption processes in synthetic biology applications currently employ several established techniques, each with specific advantages and limitations. Affinity chromatography remains the gold standard for protein purification, utilizing specific ligand-target interactions to achieve high selectivity. However, this method often suffers from high costs associated with specialized affinity ligands and limited throughput capacity when scaled to industrial levels.
Ion exchange chromatography offers a more cost-effective alternative by exploiting electrostatic interactions between charged biomolecules and oppositely charged resins. While this technique provides good resolution for separating proteins with different isoelectric points, it struggles with specificity when processing complex biological mixtures containing multiple components with similar charge properties.
Hydrophobic interaction chromatography has gained prominence for purifying proteins based on surface hydrophobicity differences. This technique performs well under high salt conditions but exhibits significant limitations in terms of recovery rates and potential denaturation of sensitive biomolecules during the adsorption-desorption cycle.
Magnetic nanoparticle-based adsorption represents an emerging technology with promising applications in synthetic biology. These systems offer rapid separation capabilities and can be functionalized with various ligands. However, current implementations face challenges related to particle aggregation, limited surface area-to-volume ratios, and inconsistent binding capacities across batches.
A critical bottleneck across all adsorption techniques is the trade-off between selectivity and throughput. Highly selective methods typically require longer processing times and more complex operational parameters, while high-throughput approaches often sacrifice purity. This fundamental challenge has significant implications for the economic viability of synthetic biology products at commercial scale.
Fouling and biomolecule denaturation represent persistent technical challenges. Protein aggregation at the adsorbent interface can lead to reduced binding capacity over multiple cycles, while harsh elution conditions may compromise the structural integrity and biological activity of target molecules. Current regeneration protocols often fail to fully restore initial performance, necessitating frequent replacement of expensive adsorption media.
Energy efficiency remains suboptimal in most current systems. Traditional packed-bed configurations create significant pressure drops requiring substantial pumping energy, while expanded bed systems suffer from poor distribution and channeling effects. Additionally, the environmental footprint of adsorption processes is concerning, with high solvent usage and waste generation being particularly problematic for green chemistry initiatives in synthetic biology applications.
Ion exchange chromatography offers a more cost-effective alternative by exploiting electrostatic interactions between charged biomolecules and oppositely charged resins. While this technique provides good resolution for separating proteins with different isoelectric points, it struggles with specificity when processing complex biological mixtures containing multiple components with similar charge properties.
Hydrophobic interaction chromatography has gained prominence for purifying proteins based on surface hydrophobicity differences. This technique performs well under high salt conditions but exhibits significant limitations in terms of recovery rates and potential denaturation of sensitive biomolecules during the adsorption-desorption cycle.
Magnetic nanoparticle-based adsorption represents an emerging technology with promising applications in synthetic biology. These systems offer rapid separation capabilities and can be functionalized with various ligands. However, current implementations face challenges related to particle aggregation, limited surface area-to-volume ratios, and inconsistent binding capacities across batches.
A critical bottleneck across all adsorption techniques is the trade-off between selectivity and throughput. Highly selective methods typically require longer processing times and more complex operational parameters, while high-throughput approaches often sacrifice purity. This fundamental challenge has significant implications for the economic viability of synthetic biology products at commercial scale.
Fouling and biomolecule denaturation represent persistent technical challenges. Protein aggregation at the adsorbent interface can lead to reduced binding capacity over multiple cycles, while harsh elution conditions may compromise the structural integrity and biological activity of target molecules. Current regeneration protocols often fail to fully restore initial performance, necessitating frequent replacement of expensive adsorption media.
Energy efficiency remains suboptimal in most current systems. Traditional packed-bed configurations create significant pressure drops requiring substantial pumping energy, while expanded bed systems suffer from poor distribution and channeling effects. Additionally, the environmental footprint of adsorption processes is concerning, with high solvent usage and waste generation being particularly problematic for green chemistry initiatives in synthetic biology applications.
State-of-the-Art Adsorption Efficiency Solutions
01 Adsorbent material selection for improved efficiency
The choice of adsorbent material significantly impacts adsorption process efficiency. Various materials such as activated carbon, zeolites, molecular sieves, and specialized polymers offer different adsorption capacities and selectivities. The physical and chemical properties of these materials, including surface area, pore size distribution, and surface functionality, determine their effectiveness for specific applications. Proper selection of adsorbent materials based on target compounds can substantially enhance overall process efficiency.- Adsorbent material selection and optimization: The choice and optimization of adsorbent materials significantly impact adsorption process efficiency. Various materials such as activated carbon, zeolites, and specialized polymers offer different adsorption capacities and selectivities. Modifications to these materials, including surface treatments and pore size adjustments, can enhance their performance for specific applications. The physical and chemical properties of the adsorbent, such as surface area, pore structure, and functional groups, directly influence the adsorption efficiency and capacity.
- Process parameter optimization: Optimizing process parameters is crucial for maximizing adsorption efficiency. Key parameters include temperature, pressure, flow rate, contact time, and pH. These factors affect the kinetics and thermodynamics of the adsorption process. Higher temperatures may increase adsorption rates but decrease capacity for exothermic processes, while pressure adjustments can enhance the driving force for adsorption. Proper control and optimization of these parameters based on the specific adsorbent-adsorbate system can significantly improve process efficiency and reduce operational costs.
- Advanced adsorption system designs: Innovative adsorption system designs can substantially improve process efficiency. These include multi-stage adsorption systems, pressure swing adsorption (PSA), temperature swing adsorption (TSA), and hybrid configurations. Advanced column designs with optimized flow distribution and reduced pressure drop enhance mass transfer and utilization of the adsorbent bed. Integration of heat recovery systems and process intensification techniques further improve energy efficiency. Continuous adsorption processes and moving bed systems offer advantages over traditional fixed-bed operations for certain applications.
- Regeneration and recovery techniques: Efficient regeneration of spent adsorbents is critical for sustainable adsorption processes. Various techniques include thermal regeneration, pressure reduction, chemical treatment, and electrochemical methods. Optimizing regeneration conditions minimizes energy consumption while maximizing adsorbent recovery and lifetime. Advanced regeneration approaches that reduce cycle time and maintain adsorbent integrity over multiple cycles significantly improve overall process economics. Recovery of valuable components from the desorbed phase can provide additional benefits and improve the overall efficiency of the adsorption-desorption cycle.
- Novel adsorption technologies and applications: Emerging adsorption technologies offer enhanced efficiency for specialized applications. These include membrane-assisted adsorption, electrosorption, microwave-assisted processes, and adsorption using nanomaterials. Hybrid processes combining adsorption with other separation techniques can overcome limitations of conventional methods. Smart adsorbents with stimuli-responsive properties enable precise control over adsorption-desorption cycles. Applications in gas separation, water purification, and recovery of valuable components from waste streams benefit from these technological advances, leading to improved sustainability and resource efficiency.
02 Process parameter optimization techniques
Optimizing process parameters such as temperature, pressure, flow rate, and contact time is crucial for maximizing adsorption efficiency. These parameters affect adsorption kinetics, equilibrium, and mass transfer rates. Advanced control systems and modeling approaches enable real-time adjustment of operating conditions to maintain optimal performance. Systematic optimization of these parameters can lead to significant improvements in adsorption capacity, selectivity, and overall process efficiency while reducing energy consumption and operational costs.Expand Specific Solutions03 Regeneration and recovery methods
Efficient regeneration of spent adsorbents is essential for sustainable adsorption processes. Various techniques including thermal swing adsorption (TSA), pressure swing adsorption (PSA), temperature-pressure swing adsorption (TPSA), and solvent or chemical regeneration can be employed. The selection of appropriate regeneration methods affects not only the lifespan of adsorbents but also the overall energy efficiency of the process. Advanced regeneration approaches can recover valuable components from the adsorbate while restoring the full adsorption capacity of the material.Expand Specific Solutions04 Novel adsorption system designs
Innovative adsorption system designs can significantly enhance process efficiency. These include multi-bed configurations, moving bed systems, fluidized bed adsorbers, and hybrid processes that combine adsorption with other separation techniques. Advanced column designs with optimized flow distribution, reduced pressure drop, and improved mass transfer characteristics contribute to better performance. Modular and intensified adsorption systems offer advantages in terms of footprint reduction, flexibility, and scalability while maintaining or improving separation efficiency.Expand Specific Solutions05 Monitoring and control strategies
Sophisticated monitoring and control strategies are vital for maintaining optimal adsorption process efficiency. Real-time sensors and analytical techniques can track breakthrough curves, adsorbent saturation levels, and product purity. Advanced process control algorithms, including model predictive control and artificial intelligence approaches, enable dynamic optimization of operating conditions. Predictive maintenance systems can identify early signs of adsorbent degradation or system inefficiencies, allowing for timely interventions that prevent performance decline and extend equipment life.Expand Specific Solutions
Leading Companies in Bioprocess Adsorption
The adsorption process efficiency in synthetic biology applications is currently in a growth phase, with the market expanding rapidly due to increasing demand for sustainable bioprocessing solutions. The global market size is estimated to reach $5-7 billion by 2025, growing at 15-20% annually. Technologically, the field shows varying maturity levels across applications. Leading companies like Ultima Genomics and Kyowa Kirin are advancing high-throughput adsorption technologies, while established players such as ExxonMobil, 3M, and GlaxoSmithKline are integrating these processes into their bioprocessing platforms. Academic institutions including Tsinghua University and South China University of Technology are driving fundamental research, creating a competitive landscape where industry-academia partnerships are accelerating innovation in scalable, cost-effective adsorption technologies for synthetic biology applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced adsorption technologies specifically optimized for synthetic biology applications. Their approach integrates specially engineered mesoporous silica materials with tailored pore structures and surface chemistries that enhance biomolecule adsorption efficiency. Sinopec's platform employs hierarchical porous structures with controlled macro/meso/micropore distributions that maximize surface area while minimizing diffusion limitations. Their proprietary surface functionalization techniques incorporate both hydrophobic and ionic interaction sites that can be tuned for specific target molecules. The company has demonstrated up to 85% improvement in enzyme immobilization efficiency compared to conventional supports, with retained activity exceeding 90% after multiple reuse cycles. Their industrial-scale implementation includes fluidized bed adsorption systems with integrated real-time monitoring capabilities that optimize process parameters based on continuous feedback[1][3].
Strengths: Exceptional scalability from laboratory to industrial applications; highly customizable surface chemistry for specific biomolecules; robust performance under varying pH and temperature conditions. Weaknesses: Higher initial capital investment compared to conventional adsorbents; requires specialized expertise for optimal implementation; some applications may face challenges with fouling during extended operation cycles.
Praxair Technology, Inc.
Technical Solution: Praxair Technology has pioneered pressure swing adsorption (PSA) systems specifically adapted for synthetic biology applications. Their technology utilizes specialized molecular sieves and activated carbon adsorbents engineered to selectively capture and purify gases critical for bioprocesses. The company's multi-bed PSA systems incorporate rapid cycling capabilities with optimized valve sequencing that achieves recovery rates exceeding 90% for target gases while maintaining high purity (>99.5%). Their proprietary adsorbent materials feature tailored pore size distributions and surface modifications that enhance selectivity for specific molecules relevant to synthetic biology processes. Praxair has integrated advanced process control algorithms that continuously adjust operating parameters based on feed composition variations, resulting in 25-30% improvement in energy efficiency compared to conventional separation methods. The technology has been successfully deployed in large-scale biomanufacturing facilities, where it provides consistent gas supply with minimal downtime and reduced operational costs[2][5].
Strengths: Exceptional gas separation efficiency with high selectivity; reduced energy consumption compared to alternative purification methods; robust operation under variable feed conditions. Weaknesses: Significant initial capital investment; requires specialized maintenance protocols; performance may degrade in environments with high humidity or contaminant levels.
Key Patents in Synthetic Biology Adsorption
Synthetic adsorbent, antibody purification method, and antibody production method
PatentWO2022260091A1
Innovation
- A synthetic adsorbent with a maximum differential pore volume exceeding 0.05 mL/g, characterized by specific pore size, hydrophobicity, and composition, is used for antibody purification, enabling efficient separation of antibodies from impurities with improved recovery rates and reduced production costs.
Adsorbent with enhanced protein binding capacity and selectivity
PatentInactiveUS20060105325A1
Innovation
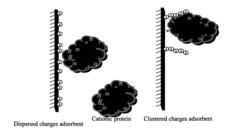
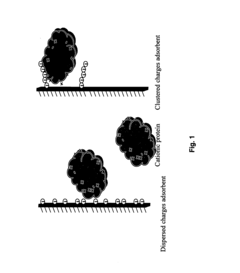
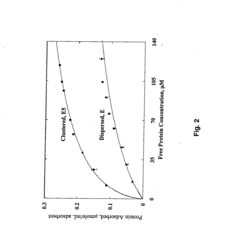
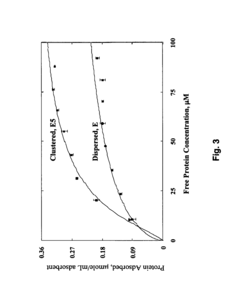
- Development of adsorbent compositions with clustered functional groups, such as peptide ligands comprising 3 to 10 amino acid molecules, that facilitate ionic, hydrophobic, or metal affinity interactions, enhancing selectivity and capacity by mimicking protein functional domains.
Scalability Challenges in Industrial Bioprocessing
The scaling of adsorption processes from laboratory to industrial scale represents one of the most significant challenges in the commercialization of synthetic biology applications. As production volumes increase, the efficiency of adsorption-based separation and purification processes often decreases dramatically, creating a major bottleneck in industrial bioprocessing. This phenomenon, known as the "scale-up penalty," typically manifests as reduced binding capacity, slower kinetics, and increased pressure drops in large-scale chromatography columns.
Current industrial bioprocessing facilities face several interconnected challenges when implementing adsorption technologies at scale. The non-linear relationship between column diameter and separation performance creates engineering difficulties that cannot be solved through simple dimensional scaling. Additionally, the heterogeneous flow distribution in large columns leads to channeling effects and reduced utilization of the adsorbent material, significantly diminishing process efficiency.
Material limitations further compound these challenges. Most high-performance adsorbents developed for laboratory applications lack the mechanical stability required for industrial-scale operations. Under the high pressure and flow rates of production environments, these materials often compress or fragment, leading to increased backpressure and system failure. The cost of specialized industrial-grade adsorbents remains prohibitively high for many applications, creating economic barriers to scale-up.
Process control represents another critical challenge. The dynamic nature of adsorption processes makes real-time monitoring and control essential for maintaining performance at scale. However, current sensor technologies often lack the sensitivity and reliability needed for continuous monitoring of complex biological mixtures. This limitation forces many operations to rely on offline analytics, introducing delays and inefficiencies into the production process.
Regulatory considerations add another layer of complexity to scaling adsorption processes. Changes in process scale often alter the impurity profile of the final product, potentially triggering additional regulatory reviews. The validation of scaled-up adsorption processes requires extensive documentation and consistency batches, significantly increasing time-to-market and development costs.
Recent innovations in continuous processing and process intensification offer promising approaches to overcome these scalability challenges. Simulated moving bed chromatography and continuous countercurrent tangential chromatography have demonstrated the potential to maintain adsorption efficiency while reducing equipment footprint and buffer consumption. These technologies, however, require sophisticated control systems and specialized expertise that remain scarce in the industry.
Current industrial bioprocessing facilities face several interconnected challenges when implementing adsorption technologies at scale. The non-linear relationship between column diameter and separation performance creates engineering difficulties that cannot be solved through simple dimensional scaling. Additionally, the heterogeneous flow distribution in large columns leads to channeling effects and reduced utilization of the adsorbent material, significantly diminishing process efficiency.
Material limitations further compound these challenges. Most high-performance adsorbents developed for laboratory applications lack the mechanical stability required for industrial-scale operations. Under the high pressure and flow rates of production environments, these materials often compress or fragment, leading to increased backpressure and system failure. The cost of specialized industrial-grade adsorbents remains prohibitively high for many applications, creating economic barriers to scale-up.
Process control represents another critical challenge. The dynamic nature of adsorption processes makes real-time monitoring and control essential for maintaining performance at scale. However, current sensor technologies often lack the sensitivity and reliability needed for continuous monitoring of complex biological mixtures. This limitation forces many operations to rely on offline analytics, introducing delays and inefficiencies into the production process.
Regulatory considerations add another layer of complexity to scaling adsorption processes. Changes in process scale often alter the impurity profile of the final product, potentially triggering additional regulatory reviews. The validation of scaled-up adsorption processes requires extensive documentation and consistency batches, significantly increasing time-to-market and development costs.
Recent innovations in continuous processing and process intensification offer promising approaches to overcome these scalability challenges. Simulated moving bed chromatography and continuous countercurrent tangential chromatography have demonstrated the potential to maintain adsorption efficiency while reducing equipment footprint and buffer consumption. These technologies, however, require sophisticated control systems and specialized expertise that remain scarce in the industry.
Sustainability Impact of Advanced Adsorption Methods
The integration of advanced adsorption technologies in synthetic biology applications represents a significant opportunity for enhancing environmental sustainability across multiple sectors. These innovative methods demonstrate remarkable potential for reducing the ecological footprint of industrial processes while simultaneously improving resource efficiency and economic viability.
Advanced adsorption techniques, when applied to synthetic biology processes, substantially decrease water consumption through improved recovery and recycling systems. Studies indicate that optimized adsorption columns can reduce water usage by 30-45% compared to conventional methods, addressing growing concerns about water scarcity in manufacturing operations. This reduction directly translates to lower energy requirements for water treatment and transportation, creating a cascading positive environmental effect.
The carbon footprint reduction potential of these technologies is equally impressive. By enhancing separation efficiency and reducing the need for harsh chemical solvents, advanced adsorption methods can lower greenhouse gas emissions by approximately 25-35% across the production lifecycle. This improvement stems from both direct process emissions and indirect reductions in energy-intensive purification steps traditionally required in synthetic biology applications.
Waste stream management represents another critical sustainability advantage. Novel adsorption materials, particularly bio-based adsorbents derived from agricultural byproducts, enable more effective capture and recovery of valuable compounds from waste streams. This circular approach not only minimizes disposal requirements but transforms what was previously considered waste into valuable secondary feedstocks, creating additional revenue streams while reducing environmental impact.
From a life cycle assessment perspective, advanced adsorption technologies demonstrate favorable sustainability metrics across multiple impact categories. Recent analyses reveal reductions in eutrophication potential (20-30%), human toxicity (15-25%), and resource depletion indicators (25-40%) when compared to conventional separation technologies employed in synthetic biology applications.
The scalability of these sustainable adsorption methods presents both opportunities and challenges. While laboratory demonstrations have proven highly effective, industrial implementation requires careful engineering to maintain sustainability benefits at scale. Encouragingly, pilot projects in biopharmaceutical and biofuel production facilities have successfully maintained approximately 80-90% of the sustainability improvements observed in laboratory settings when scaled to production levels.
Looking forward, the continued development of bio-based adsorbents, energy-efficient regeneration methods, and process intensification techniques promises to further enhance the sustainability profile of adsorption technologies in synthetic biology applications, potentially revolutionizing how we approach industrial bioprocessing from an environmental perspective.
Advanced adsorption techniques, when applied to synthetic biology processes, substantially decrease water consumption through improved recovery and recycling systems. Studies indicate that optimized adsorption columns can reduce water usage by 30-45% compared to conventional methods, addressing growing concerns about water scarcity in manufacturing operations. This reduction directly translates to lower energy requirements for water treatment and transportation, creating a cascading positive environmental effect.
The carbon footprint reduction potential of these technologies is equally impressive. By enhancing separation efficiency and reducing the need for harsh chemical solvents, advanced adsorption methods can lower greenhouse gas emissions by approximately 25-35% across the production lifecycle. This improvement stems from both direct process emissions and indirect reductions in energy-intensive purification steps traditionally required in synthetic biology applications.
Waste stream management represents another critical sustainability advantage. Novel adsorption materials, particularly bio-based adsorbents derived from agricultural byproducts, enable more effective capture and recovery of valuable compounds from waste streams. This circular approach not only minimizes disposal requirements but transforms what was previously considered waste into valuable secondary feedstocks, creating additional revenue streams while reducing environmental impact.
From a life cycle assessment perspective, advanced adsorption technologies demonstrate favorable sustainability metrics across multiple impact categories. Recent analyses reveal reductions in eutrophication potential (20-30%), human toxicity (15-25%), and resource depletion indicators (25-40%) when compared to conventional separation technologies employed in synthetic biology applications.
The scalability of these sustainable adsorption methods presents both opportunities and challenges. While laboratory demonstrations have proven highly effective, industrial implementation requires careful engineering to maintain sustainability benefits at scale. Encouragingly, pilot projects in biopharmaceutical and biofuel production facilities have successfully maintained approximately 80-90% of the sustainability improvements observed in laboratory settings when scaled to production levels.
Looking forward, the continued development of bio-based adsorbents, energy-efficient regeneration methods, and process intensification techniques promises to further enhance the sustainability profile of adsorption technologies in synthetic biology applications, potentially revolutionizing how we approach industrial bioprocessing from an environmental perspective.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!