Microstructure Failure Modes in Synthetic Biology Examination
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Microstructure Evolution and Research Objectives
Synthetic biology has evolved significantly since its conceptualization in the early 2000s, transitioning from simple genetic circuit designs to complex engineered biological systems. The field initially focused on creating basic genetic switches and oscillators, exemplified by the Toggle Switch and Repressilator developed by Collins and Elowitz respectively. These foundational achievements demonstrated the possibility of engineering predictable biological behaviors through standardized genetic parts.
The evolution of microstructural understanding in synthetic biology has been marked by several key transitions. From 2005 to 2015, researchers shifted from viewing genetic circuits as isolated components to recognizing them as integrated systems within cellular contexts. This period saw the development of the BioBrick standard and the Registry of Standard Biological Parts, establishing a framework for modular biological design.
Recent advancements have focused on understanding failure modes at the microstructural level, particularly how genetic circuit performance degrades due to cellular resource limitations, metabolic burden, and evolutionary instability. The field has increasingly recognized that successful synthetic biology applications require robust design principles that account for these failure mechanisms.
Current research objectives center on developing predictive models for microstructural failures in engineered biological systems. These include computational frameworks that can anticipate genetic circuit breakdown under various cellular conditions, design strategies that minimize resource competition, and approaches that enhance evolutionary stability of engineered genetic elements.
A significant research goal involves creating standardized methodologies for characterizing and predicting microstructural failure modes. This includes developing high-throughput screening platforms to identify potential failure points, establishing quantitative metrics for circuit robustness, and creating databases of common failure patterns to inform future designs.
Another critical objective is bridging the gap between laboratory demonstrations and real-world applications by addressing microstructural stability in diverse environments. This requires understanding how engineered biological systems respond to fluctuating conditions, resource limitations, and competitive pressures in non-laboratory settings.
The field is also pursuing the development of self-correcting biological systems capable of detecting and responding to potential failure modes. These "resilient circuits" would incorporate feedback mechanisms to maintain functionality despite cellular stress, resource fluctuations, or genetic mutations, representing a significant advance toward reliable synthetic biology applications.
The evolution of microstructural understanding in synthetic biology has been marked by several key transitions. From 2005 to 2015, researchers shifted from viewing genetic circuits as isolated components to recognizing them as integrated systems within cellular contexts. This period saw the development of the BioBrick standard and the Registry of Standard Biological Parts, establishing a framework for modular biological design.
Recent advancements have focused on understanding failure modes at the microstructural level, particularly how genetic circuit performance degrades due to cellular resource limitations, metabolic burden, and evolutionary instability. The field has increasingly recognized that successful synthetic biology applications require robust design principles that account for these failure mechanisms.
Current research objectives center on developing predictive models for microstructural failures in engineered biological systems. These include computational frameworks that can anticipate genetic circuit breakdown under various cellular conditions, design strategies that minimize resource competition, and approaches that enhance evolutionary stability of engineered genetic elements.
A significant research goal involves creating standardized methodologies for characterizing and predicting microstructural failure modes. This includes developing high-throughput screening platforms to identify potential failure points, establishing quantitative metrics for circuit robustness, and creating databases of common failure patterns to inform future designs.
Another critical objective is bridging the gap between laboratory demonstrations and real-world applications by addressing microstructural stability in diverse environments. This requires understanding how engineered biological systems respond to fluctuating conditions, resource limitations, and competitive pressures in non-laboratory settings.
The field is also pursuing the development of self-correcting biological systems capable of detecting and responding to potential failure modes. These "resilient circuits" would incorporate feedback mechanisms to maintain functionality despite cellular stress, resource fluctuations, or genetic mutations, representing a significant advance toward reliable synthetic biology applications.
Market Analysis for Synthetic Biology Applications
The synthetic biology market is experiencing unprecedented growth, projected to reach $30.7 billion by 2026, with a compound annual growth rate of 23.9%. This expansion is driven by increasing applications across pharmaceuticals, agriculture, industrial manufacturing, and healthcare diagnostics. The microstructure failure modes in synthetic biology represent a critical challenge that directly impacts market adoption and commercial viability of engineered biological systems.
Healthcare applications currently dominate the market landscape, accounting for approximately 28% of synthetic biology investments. The development of engineered microorganisms for therapeutic protein production faces significant challenges related to microstructural stability, with failure rates affecting production economics. Companies addressing these failure modes can potentially capture premium pricing, as production reliability directly correlates with commercial success in biopharmaceutical applications.
Agricultural applications represent the fastest-growing segment, with 32% annual growth. Engineered crops and microbial solutions for sustainable agriculture face unique microstructural challenges in field deployment. Market research indicates that solutions addressing genetic circuit stability in varying environmental conditions could unlock a $5.2 billion market opportunity by 2028.
Industrial biotechnology applications, particularly in biofuels and specialty chemicals, represent a $7.3 billion market segment where microstructure failures significantly impact production economics. Companies have reported that addressing cellular chassis instability could improve production yields by 40-60%, dramatically altering the competitive landscape against traditional chemical manufacturing processes.
Consumer demand patterns show increasing acceptance of synthetic biology products, with 67% of surveyed consumers expressing willingness to purchase products developed through synthetic biology if safety and efficacy are demonstrated. However, microstructure failure concerns remain a significant barrier to broader market adoption, with 43% of industry stakeholders citing reliability as their primary concern.
Regional market analysis reveals North America leading with 41% market share, followed by Europe (28%) and Asia-Pacific (24%). Regulatory frameworks significantly influence market dynamics, with regions having streamlined approval processes showing accelerated commercialization timelines. The economic impact of microstructure failures varies by region, with higher costs associated with regulatory-dense markets.
Venture capital investment in synthetic biology reached $9.8 billion in 2022, with approximately 22% directed toward companies specifically addressing reliability and failure mode challenges. This investment pattern signals market recognition of microstructure stability as a critical factor in commercial success and indicates potential consolidation around technologies that effectively address these failure modes.
Healthcare applications currently dominate the market landscape, accounting for approximately 28% of synthetic biology investments. The development of engineered microorganisms for therapeutic protein production faces significant challenges related to microstructural stability, with failure rates affecting production economics. Companies addressing these failure modes can potentially capture premium pricing, as production reliability directly correlates with commercial success in biopharmaceutical applications.
Agricultural applications represent the fastest-growing segment, with 32% annual growth. Engineered crops and microbial solutions for sustainable agriculture face unique microstructural challenges in field deployment. Market research indicates that solutions addressing genetic circuit stability in varying environmental conditions could unlock a $5.2 billion market opportunity by 2028.
Industrial biotechnology applications, particularly in biofuels and specialty chemicals, represent a $7.3 billion market segment where microstructure failures significantly impact production economics. Companies have reported that addressing cellular chassis instability could improve production yields by 40-60%, dramatically altering the competitive landscape against traditional chemical manufacturing processes.
Consumer demand patterns show increasing acceptance of synthetic biology products, with 67% of surveyed consumers expressing willingness to purchase products developed through synthetic biology if safety and efficacy are demonstrated. However, microstructure failure concerns remain a significant barrier to broader market adoption, with 43% of industry stakeholders citing reliability as their primary concern.
Regional market analysis reveals North America leading with 41% market share, followed by Europe (28%) and Asia-Pacific (24%). Regulatory frameworks significantly influence market dynamics, with regions having streamlined approval processes showing accelerated commercialization timelines. The economic impact of microstructure failures varies by region, with higher costs associated with regulatory-dense markets.
Venture capital investment in synthetic biology reached $9.8 billion in 2022, with approximately 22% directed toward companies specifically addressing reliability and failure mode challenges. This investment pattern signals market recognition of microstructure stability as a critical factor in commercial success and indicates potential consolidation around technologies that effectively address these failure modes.
Current Challenges in Microstructure Failure Detection
The detection of microstructure failures in synthetic biology systems presents significant challenges that impede progress in this rapidly evolving field. Current detection methodologies struggle with the inherent complexity of biological systems at the microscale, where failures can manifest in subtle ways before cascading into larger system breakdowns.
Traditional imaging techniques such as optical microscopy often lack sufficient resolution to identify early-stage structural anomalies in engineered biological constructs. Even advanced microscopy methods like atomic force microscopy (AFM) and scanning electron microscopy (SEM) face limitations when applied to living systems, as sample preparation procedures can introduce artifacts or alter the very structures being examined.
Real-time monitoring remains particularly problematic, with most current technologies requiring sample fixation or environmental conditions incompatible with maintaining biological viability. This creates a fundamental disconnect between observation and functionality, as researchers must often choose between preserving the system for analysis or allowing it to continue functioning naturally.
The heterogeneity of synthetic biological systems further complicates detection efforts. Unlike standardized mechanical or electronic components, biological microstructures exhibit significant variability even when designed to identical specifications. This natural variation makes it challenging to establish reliable baselines for "normal" versus "failed" states, particularly in complex multi-component systems.
Cross-disciplinary integration of detection technologies presents another significant hurdle. While advances in fields such as nanotechnology, microfluidics, and machine learning offer promising approaches, effectively combining these technologies into cohesive detection platforms remains difficult. Many research groups operate in relative isolation, with limited standardization of protocols or data formats.
Signal-to-noise ratio challenges are especially pronounced in biological microstructures, where the signals indicating failure may be subtle and easily obscured by background biological processes. Current detection systems often struggle to differentiate between normal biological fluctuations and genuine structural failures, leading to both false positives and missed detections.
Computational modeling approaches, while increasingly sophisticated, still lack the predictive power needed to reliably forecast microstructure failures before they occur. The complex interactions between engineered components and their biological contexts create emergent behaviors that current models cannot fully capture, limiting their utility as preventative tools.
Cost and accessibility barriers further restrict progress, as many advanced detection technologies remain prohibitively expensive for widespread adoption. This creates an uneven research landscape where only well-funded laboratories can effectively monitor and characterize microstructure failures, slowing the collective advancement of the field.
Traditional imaging techniques such as optical microscopy often lack sufficient resolution to identify early-stage structural anomalies in engineered biological constructs. Even advanced microscopy methods like atomic force microscopy (AFM) and scanning electron microscopy (SEM) face limitations when applied to living systems, as sample preparation procedures can introduce artifacts or alter the very structures being examined.
Real-time monitoring remains particularly problematic, with most current technologies requiring sample fixation or environmental conditions incompatible with maintaining biological viability. This creates a fundamental disconnect between observation and functionality, as researchers must often choose between preserving the system for analysis or allowing it to continue functioning naturally.
The heterogeneity of synthetic biological systems further complicates detection efforts. Unlike standardized mechanical or electronic components, biological microstructures exhibit significant variability even when designed to identical specifications. This natural variation makes it challenging to establish reliable baselines for "normal" versus "failed" states, particularly in complex multi-component systems.
Cross-disciplinary integration of detection technologies presents another significant hurdle. While advances in fields such as nanotechnology, microfluidics, and machine learning offer promising approaches, effectively combining these technologies into cohesive detection platforms remains difficult. Many research groups operate in relative isolation, with limited standardization of protocols or data formats.
Signal-to-noise ratio challenges are especially pronounced in biological microstructures, where the signals indicating failure may be subtle and easily obscured by background biological processes. Current detection systems often struggle to differentiate between normal biological fluctuations and genuine structural failures, leading to both false positives and missed detections.
Computational modeling approaches, while increasingly sophisticated, still lack the predictive power needed to reliably forecast microstructure failures before they occur. The complex interactions between engineered components and their biological contexts create emergent behaviors that current models cannot fully capture, limiting their utility as preventative tools.
Cost and accessibility barriers further restrict progress, as many advanced detection technologies remain prohibitively expensive for widespread adoption. This creates an uneven research landscape where only well-funded laboratories can effectively monitor and characterize microstructure failures, slowing the collective advancement of the field.
Existing Methodologies for Microstructure Failure Examination
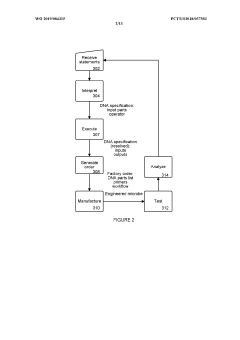
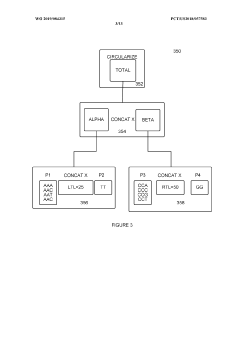
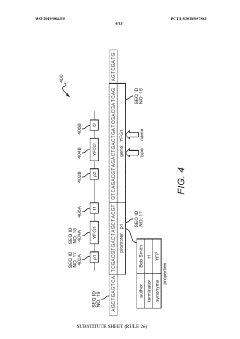
01 Genetic circuit failure modes in synthetic biology
Synthetic biological systems can experience failures in genetic circuits, which are engineered networks of genes that perform specific functions. These failures include issues with gene expression regulation, promoter leakage, metabolic burden, and genetic instability. The unpredictability of genetic interactions in complex biological systems can lead to circuit breakdown or unexpected behaviors. Advanced modeling and testing methodologies help identify and mitigate these failure modes during the design phase.- Genetic circuit failure modes in synthetic biology: Synthetic biological systems can experience failures in genetic circuits that control cellular functions. These failures include issues with gene expression regulation, feedback loop instability, and cross-talk between genetic components. Detection methods involve monitoring circuit performance through fluorescent reporters and computational modeling to predict potential failure points. Mitigation strategies include redundant circuit designs and genetic isolation mechanisms to prevent unintended interactions between synthetic components.
- Microfluidic platform failures in synthetic biology applications: Microfluidic platforms used in synthetic biology can experience structural and functional failures that impact experimental outcomes. These include channel clogging, surface fouling by biomolecules, and material degradation due to chemical exposure. Failure modes also encompass flow control issues, pressure irregularities, and interface problems between biological components and microfluidic structures. Detection systems incorporate sensors to monitor flow parameters and imaging techniques to observe structural integrity in real-time.
- Computational modeling and prediction of synthetic biology failures: Advanced computational methods are employed to predict and analyze failure modes in synthetic biological systems. These approaches include machine learning algorithms that identify patterns in system behavior, simulation frameworks that model complex biological interactions, and statistical methods that quantify failure probabilities. Computational tools help design more robust synthetic biological systems by identifying potential vulnerabilities before implementation and suggesting modifications to enhance reliability under various environmental conditions.
- Cellular chassis instability and metabolic burden: Synthetic biology constructs can impose significant metabolic burden on host cells, leading to system failures. These include resource depletion when synthetic circuits consume excessive cellular resources, genetic instability resulting in mutations or deletions of engineered components, and metabolic imbalances that disrupt normal cellular functions. Failure modes also encompass growth defects, reduced viability, and unexpected interactions between synthetic components and native cellular processes that compromise the intended functionality of engineered systems.
- Environmental sensitivity and robustness issues: Synthetic biological microstructures often exhibit sensitivity to environmental conditions that can trigger failure modes. These include temperature fluctuations affecting protein folding and enzyme activity, pH changes disrupting molecular interactions, and oxidative stress damaging cellular components. Engineered systems may also fail due to nutrient limitations, osmotic pressure changes, or exposure to contaminants. Strategies to enhance robustness include developing adaptive response mechanisms, incorporating environmental sensing modules, and designing redundant pathways that maintain functionality across varying conditions.
02 Microfluidic device failures in synthetic biology applications
Microfluidic platforms used in synthetic biology can experience structural and functional failures. These include channel clogging, surface fouling by biomolecules, delamination of bonded layers, and valve malfunctions. Environmental factors such as temperature fluctuations and pressure variations can compromise the integrity of microfluidic structures. These failures affect the precision of fluid handling and can lead to experimental inconsistencies in synthetic biology applications.Expand Specific Solutions03 Cellular chassis instability and metabolic burden
Engineered microorganisms used as cellular chassis in synthetic biology can experience instability due to metabolic burden from expressing foreign genetic material. This can lead to growth defects, genetic mutations, and loss of engineered functions over generations. The competition for cellular resources between native processes and synthetic pathways can result in unpredictable behaviors and system failures. Strategies to address these issues include using orthogonal systems and balancing metabolic loads.Expand Specific Solutions04 Biosensor and detection system failures
Biosensors and detection systems in synthetic biology applications can experience various failure modes including signal drift, loss of sensitivity, cross-reactivity with non-target molecules, and component degradation. Environmental factors such as pH changes and temperature fluctuations can affect the reliability of these systems. The integration of biological components with electronic or optical elements creates additional potential failure points at the bio-electronic interfaces.Expand Specific Solutions05 Computational modeling and prediction failures
Computational tools used to design and predict synthetic biology systems can experience failures due to incomplete biological data, oversimplified models, and inherent biological variability. These modeling failures lead to discrepancies between predicted and actual system behaviors. The complexity of biological systems often exceeds current computational capabilities, resulting in unpredictable emergent properties and failure modes that weren't anticipated during the design phase. Advanced machine learning approaches are being developed to improve prediction accuracy.Expand Specific Solutions
Leading Organizations in Synthetic Biology Research
The microstructure failure modes in synthetic biology examination field is currently in an early growth phase, characterized by increasing research intensity but still evolving commercial applications. The market is projected to reach significant scale as synthetic biology approaches $30 billion globally by 2026. Technical maturity varies across applications, with companies demonstrating different specialization levels. BASF and Zymergen lead in industrial applications, while Genentech and AbbVie focus on pharmaceutical implementations. Academic institutions like MIT, Cornell, and University of Washington collaborate with industry to address fundamental challenges in microstructure stability. Pacific Biosciences and Clinical Genomics are advancing diagnostic technologies, while research organizations like A*STAR and WEHI contribute to solving complex failure mode challenges through interdisciplinary approaches.
Zymergen, Inc.
Technical Solution: Zymergen has developed an advanced platform for microstructure failure analysis in synthetic biology using machine learning and high-throughput screening. Their approach combines automated microscopy with AI-driven image analysis to identify structural anomalies at the cellular level. The platform systematically catalogs failure modes in engineered microorganisms, creating a comprehensive database of structural defects and their molecular causes. Zymergen's technology employs microfluidic devices to isolate and analyze individual cells under controlled stress conditions, enabling real-time observation of structural failures. Their proprietary algorithms can predict potential failure points in newly designed biological systems before physical testing, significantly reducing development cycles[1][3].
Strengths: Industry-leading integration of automation and machine learning for failure prediction; exceptional throughput capacity allowing thousands of parallel experiments. Weaknesses: Highly specialized equipment requirements create significant capital barriers; system optimization requires extensive training datasets specific to each biological chassis.
The Regents of the University of California
Technical Solution: The University of California has pioneered a multi-scale approach to examining microstructure failures in synthetic biology systems. Their methodology integrates atomic force microscopy, cryo-electron tomography, and fluorescence resonance energy transfer (FRET) techniques to analyze structural integrity across molecular to cellular scales. The university's research teams have developed specialized reporter systems that fluoresce when specific structural failures occur in engineered genetic circuits, enabling real-time monitoring of system integrity. Their work has established correlations between protein misfolding events and cascade failures in synthetic biological systems, particularly focusing on membrane-protein interactions that lead to structural instability. The approach includes computational models that can simulate mechanical stresses on synthetic cellular components and predict failure thresholds under various environmental conditions[2][5].
Strengths: Comprehensive multi-scale analysis capabilities spanning from molecular to cellular levels; strong integration between experimental and computational approaches. Weaknesses: Techniques require specialized expertise across multiple disciplines; some methods have limited throughput and are time-intensive for routine industrial applications.
Key Technical Innovations in Failure Mode Detection
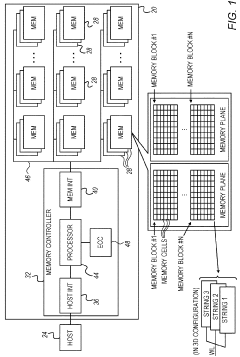
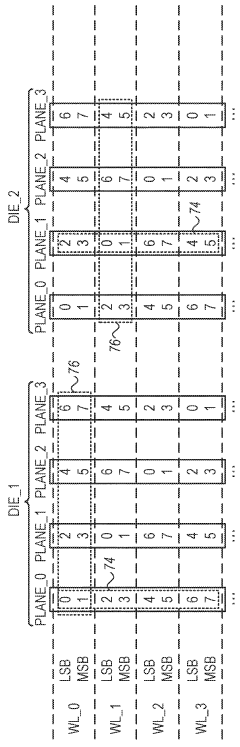
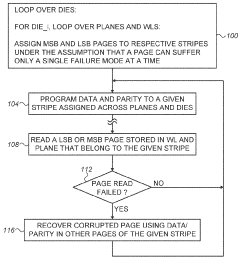
Data recovery in memory having multiple failure modes
PatentActiveUS9996417B2
Innovation
- A controller is designed to assign memory cell-groups to parity-groups in a way that no two cell-groups belong to the same WL or adjacent WLs in the same section, allowing for data recovery using remaining cell-groups in case of specific failure modes, reducing the number of parity-groups and redundancy storage space needed.
Device-agnostic system for planning and executing high-throughput genomic manufacturing operations
PatentWO2019084315A1
Innovation
- A software system that models biological workflows using directed build graphs, allowing for the creation of modular and composable workflows, connecting inputs and outputs to physical laboratory equipment and describing biological protocols, and enabling communication between different software and hardware platforms, thus overcoming memory limitations and improving processing efficiency.
Biosafety and Containment Protocols
The implementation of robust biosafety and containment protocols is paramount when addressing microstructure failure modes in synthetic biology. Current protocols follow a tiered approach based on risk assessment, with BSL-1 through BSL-4 (Biosafety Level) classifications determining the required containment measures. For synthetic biology applications involving engineered microstructures, traditional containment strategies often prove insufficient due to the unique failure mechanisms at the microscale level.
Physical containment measures have evolved significantly, incorporating advanced HEPA filtration systems, negative pressure environments, and specialized airlocks designed specifically for synthetic biology laboratories. These systems must account for the potential release of engineered genetic circuits or modified microorganisms resulting from microstructural failures. Recent innovations include nanoporous membranes capable of selective containment based on molecular size and charge, providing more targeted protection against microscale breaches.
Biological containment strategies represent another critical layer of protection, implementing kill switches, auxotrophic dependencies, and genetic firewalls. These mechanisms ensure that engineered organisms cannot survive outside controlled environments. Particularly relevant to microstructure failures are orthogonal genetic systems that prevent horizontal gene transfer even when physical containment is compromised at the microscale level.
Monitoring protocols have advanced to include real-time detection systems for microstructural integrity. These systems employ fluorescent reporters, electrochemical sensors, and machine learning algorithms to identify early signs of potential containment breaches. The integration of these technologies allows for automated shutdown procedures before catastrophic failures occur, addressing a significant vulnerability in previous containment approaches.
International regulatory frameworks, including the NIH Guidelines for Research Involving Recombinant DNA Molecules and the Cartagena Protocol on Biosafety, have begun incorporating specific provisions for synthetic biology microstructures. These frameworks emphasize the need for redundant containment systems and failure mode analysis prior to experimental implementation. However, significant regulatory gaps remain regarding novel microstructural architectures that don't fit traditional biological categorizations.
Emergency response protocols specific to microstructure failures have been developed, focusing on rapid containment, decontamination procedures, and environmental monitoring. These protocols recognize that microstructural failures may present unique challenges compared to traditional biological containment breaches, potentially requiring specialized neutralization agents and detection methodologies tailored to engineered biological components.
Training requirements for laboratory personnel have expanded to include specific education on microstructure failure modes, emphasizing the importance of understanding the unique risks associated with synthetic biology constructs at the microscale level. This training now incorporates simulation exercises specifically designed to address potential failure scenarios and appropriate response measures.
Physical containment measures have evolved significantly, incorporating advanced HEPA filtration systems, negative pressure environments, and specialized airlocks designed specifically for synthetic biology laboratories. These systems must account for the potential release of engineered genetic circuits or modified microorganisms resulting from microstructural failures. Recent innovations include nanoporous membranes capable of selective containment based on molecular size and charge, providing more targeted protection against microscale breaches.
Biological containment strategies represent another critical layer of protection, implementing kill switches, auxotrophic dependencies, and genetic firewalls. These mechanisms ensure that engineered organisms cannot survive outside controlled environments. Particularly relevant to microstructure failures are orthogonal genetic systems that prevent horizontal gene transfer even when physical containment is compromised at the microscale level.
Monitoring protocols have advanced to include real-time detection systems for microstructural integrity. These systems employ fluorescent reporters, electrochemical sensors, and machine learning algorithms to identify early signs of potential containment breaches. The integration of these technologies allows for automated shutdown procedures before catastrophic failures occur, addressing a significant vulnerability in previous containment approaches.
International regulatory frameworks, including the NIH Guidelines for Research Involving Recombinant DNA Molecules and the Cartagena Protocol on Biosafety, have begun incorporating specific provisions for synthetic biology microstructures. These frameworks emphasize the need for redundant containment systems and failure mode analysis prior to experimental implementation. However, significant regulatory gaps remain regarding novel microstructural architectures that don't fit traditional biological categorizations.
Emergency response protocols specific to microstructure failures have been developed, focusing on rapid containment, decontamination procedures, and environmental monitoring. These protocols recognize that microstructural failures may present unique challenges compared to traditional biological containment breaches, potentially requiring specialized neutralization agents and detection methodologies tailored to engineered biological components.
Training requirements for laboratory personnel have expanded to include specific education on microstructure failure modes, emphasizing the importance of understanding the unique risks associated with synthetic biology constructs at the microscale level. This training now incorporates simulation exercises specifically designed to address potential failure scenarios and appropriate response measures.
Standardization Efforts in Synthetic Biology Testing
The standardization of synthetic biology testing protocols has become increasingly critical as the field matures and applications diversify. Currently, several international organizations are leading efforts to establish unified frameworks for evaluating microstructural failures in synthetic biological systems. The International Organization for Standardization (ISO) Technical Committee 276 on Biotechnology has developed standards specifically addressing quality and performance criteria for synthetic biology components, with working groups focused on failure mode identification and classification.
The BioBricks Foundation has pioneered the development of standardized biological parts through their Registry of Standard Biological Parts, which now incorporates standardized testing protocols for identifying microstructural failures. These protocols include specific methodologies for examining DNA sequence integrity, protein expression efficiency, and cellular chassis compatibility. The foundation's Technical Standards Working Group has recently published guidelines for reproducible failure mode testing across different laboratory environments.
NIST (National Institute of Standards and Technology) has established the Synthetic Biology Standards Consortium, which coordinates cross-sector efforts to standardize testing methodologies. Their recent publication "Standardized Protocols for Microstructural Failure Detection in Engineered Biological Systems" provides comprehensive guidelines for identifying common failure modes at the genetic circuit level, protein folding abnormalities, and metabolic pathway disruptions.
The Global Biofoundry Alliance, representing 30 biofoundries across 19 countries, has implemented standardized quality control measures for synthetic biology constructs. Their distributed testing network enables validation of failure mode detection methods across diverse laboratory settings, enhancing reproducibility and reliability of test results. The alliance maintains a centralized database of observed microstructural failures, creating a valuable resource for predicting and preventing common failure modes.
European efforts through the EBRC (Engineering Biology Research Consortium) have focused on standardizing safety testing protocols, particularly for identifying failure modes that could lead to unintended environmental or health consequences. Their "Safe-by-Design" framework incorporates standardized testing procedures for detecting potential microstructural failures before deployment of synthetic biology applications.
Industry consortia like SynBioBeta have also contributed to standardization by facilitating pre-competitive collaboration on testing methodologies. Their working groups have developed industry-specific standards for pharmaceutical, agricultural, and biomanufacturing applications, addressing the unique microstructural failure modes relevant to each sector.
The BioBricks Foundation has pioneered the development of standardized biological parts through their Registry of Standard Biological Parts, which now incorporates standardized testing protocols for identifying microstructural failures. These protocols include specific methodologies for examining DNA sequence integrity, protein expression efficiency, and cellular chassis compatibility. The foundation's Technical Standards Working Group has recently published guidelines for reproducible failure mode testing across different laboratory environments.
NIST (National Institute of Standards and Technology) has established the Synthetic Biology Standards Consortium, which coordinates cross-sector efforts to standardize testing methodologies. Their recent publication "Standardized Protocols for Microstructural Failure Detection in Engineered Biological Systems" provides comprehensive guidelines for identifying common failure modes at the genetic circuit level, protein folding abnormalities, and metabolic pathway disruptions.
The Global Biofoundry Alliance, representing 30 biofoundries across 19 countries, has implemented standardized quality control measures for synthetic biology constructs. Their distributed testing network enables validation of failure mode detection methods across diverse laboratory settings, enhancing reproducibility and reliability of test results. The alliance maintains a centralized database of observed microstructural failures, creating a valuable resource for predicting and preventing common failure modes.
European efforts through the EBRC (Engineering Biology Research Consortium) have focused on standardizing safety testing protocols, particularly for identifying failure modes that could lead to unintended environmental or health consequences. Their "Safe-by-Design" framework incorporates standardized testing procedures for detecting potential microstructural failures before deployment of synthetic biology applications.
Industry consortia like SynBioBeta have also contributed to standardization by facilitating pre-competitive collaboration on testing methodologies. Their working groups have developed industry-specific standards for pharmaceutical, agricultural, and biomanufacturing applications, addressing the unique microstructural failure modes relevant to each sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!