Analysis of Synthetic Biology in Context of Patent Regulations
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Research Objectives
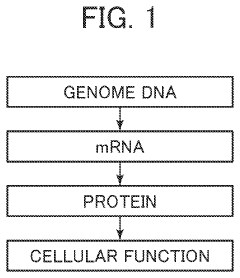
Synthetic biology has evolved significantly since its conceptual emergence in the 1970s, transitioning from theoretical frameworks to practical applications that merge biology with engineering principles. The field formally coalesced in the early 2000s when researchers began standardizing biological parts and developing modular approaches to genetic engineering. This evolution represents a paradigm shift from traditional genetic engineering to a more systematic, design-oriented discipline that treats genetic components as interchangeable parts within biological systems.
The technological progression of synthetic biology has been marked by several key milestones. The development of DNA synthesis and sequencing technologies dramatically reduced costs and increased throughput, enabling more complex genetic constructs. The establishment of the BioBrick standard in the early 2000s provided a framework for modular genetic assembly, while CRISPR-Cas9 gene editing technology revolutionized the precision with which genetic modifications could be implemented. More recently, advancements in computational tools and artificial intelligence have enhanced the predictive capabilities for designing biological systems.
Current research objectives in synthetic biology focus on addressing several critical challenges within the context of patent regulations. Researchers aim to develop standardized methods for characterizing biological parts that can be consistently reproduced across different laboratories, enhancing the reliability of patented technologies. There is also significant emphasis on creating robust regulatory frameworks that balance innovation protection through patents while preventing monopolistic control of fundamental biological processes.
Another key objective involves improving the predictability of engineered biological systems, as current limitations in modeling complex biological interactions create uncertainty in patent claims. Researchers are working to develop more sophisticated computational tools that can accurately predict the behavior of synthetic biological systems, thereby strengthening the specificity and enforceability of patents in this domain.
The field is also exploring ethical frameworks for responsible innovation, particularly regarding how patent regulations should address concerns about biosafety, biosecurity, and potential environmental impacts. This includes developing clear guidelines for what constitutes patentable subject matter in synthetic biology, especially for innovations that blur the line between naturally occurring and engineered biological systems.
Looking forward, synthetic biology research aims to establish international harmonization of patent regulations specific to biological technologies, addressing the current fragmentation in global approaches to intellectual property in this field. This would facilitate more effective technology transfer and commercialization of synthetic biology innovations across different jurisdictions.
The technological progression of synthetic biology has been marked by several key milestones. The development of DNA synthesis and sequencing technologies dramatically reduced costs and increased throughput, enabling more complex genetic constructs. The establishment of the BioBrick standard in the early 2000s provided a framework for modular genetic assembly, while CRISPR-Cas9 gene editing technology revolutionized the precision with which genetic modifications could be implemented. More recently, advancements in computational tools and artificial intelligence have enhanced the predictive capabilities for designing biological systems.
Current research objectives in synthetic biology focus on addressing several critical challenges within the context of patent regulations. Researchers aim to develop standardized methods for characterizing biological parts that can be consistently reproduced across different laboratories, enhancing the reliability of patented technologies. There is also significant emphasis on creating robust regulatory frameworks that balance innovation protection through patents while preventing monopolistic control of fundamental biological processes.
Another key objective involves improving the predictability of engineered biological systems, as current limitations in modeling complex biological interactions create uncertainty in patent claims. Researchers are working to develop more sophisticated computational tools that can accurately predict the behavior of synthetic biological systems, thereby strengthening the specificity and enforceability of patents in this domain.
The field is also exploring ethical frameworks for responsible innovation, particularly regarding how patent regulations should address concerns about biosafety, biosecurity, and potential environmental impacts. This includes developing clear guidelines for what constitutes patentable subject matter in synthetic biology, especially for innovations that blur the line between naturally occurring and engineered biological systems.
Looking forward, synthetic biology research aims to establish international harmonization of patent regulations specific to biological technologies, addressing the current fragmentation in global approaches to intellectual property in this field. This would facilitate more effective technology transfer and commercialization of synthetic biology innovations across different jurisdictions.
Market Applications and Commercial Potential
The synthetic biology market is experiencing unprecedented growth, with projections indicating a global market value reaching $30.7 billion by 2026, growing at a CAGR of 23.9%. This rapid expansion is driven by diverse commercial applications across multiple sectors, creating significant economic opportunities while simultaneously raising complex patent regulation challenges.
Healthcare represents the largest commercial application sector, where synthetic biology enables the development of novel therapeutics, vaccines, and diagnostic tools. Companies like Moderna and BioNTech have demonstrated the transformative potential through mRNA technology, while engineered cell therapies for cancer treatment command premium pricing exceeding $400,000 per treatment. The biosimilars market alone is expected to reach $35.7 billion by 2025 as patent expirations for blockbuster biologics create new opportunities.
Agricultural applications constitute another substantial market segment, with engineered crops designed for enhanced yield, pest resistance, and climate adaptability. Companies like Bayer and Corteva are investing heavily in synthetic biology platforms to develop sustainable agricultural solutions. The bio-based materials sector is similarly expanding, with companies producing biodegradable plastics, synthetic textiles, and construction materials through engineered microorganisms.
Industrial biotechnology represents a rapidly growing commercial frontier, where engineered microorganisms produce chemicals, enzymes, and biofuels at industrial scale. This sector is projected to reach $262 billion by 2028, with particular growth in bio-based specialty chemicals that offer sustainable alternatives to petroleum-derived products.
The commercial landscape is characterized by both established multinational corporations and agile startups. Major pharmaceutical companies have invested over $5 billion in synthetic biology partnerships since 2018, while venture capital funding in the sector exceeded $8 billion in 2021 alone. This investment pattern reflects recognition of synthetic biology's disruptive potential across multiple industries.
Patent regulations significantly impact commercialization strategies, with companies adopting various approaches to intellectual property protection. Platform technologies often employ broad patent portfolios covering foundational methods, while application-specific innovations typically utilize more targeted protection strategies. The tension between open innovation and proprietary technology development creates complex dynamics in market development, particularly as standardized biological parts and processes become increasingly important for industry growth.
Healthcare represents the largest commercial application sector, where synthetic biology enables the development of novel therapeutics, vaccines, and diagnostic tools. Companies like Moderna and BioNTech have demonstrated the transformative potential through mRNA technology, while engineered cell therapies for cancer treatment command premium pricing exceeding $400,000 per treatment. The biosimilars market alone is expected to reach $35.7 billion by 2025 as patent expirations for blockbuster biologics create new opportunities.
Agricultural applications constitute another substantial market segment, with engineered crops designed for enhanced yield, pest resistance, and climate adaptability. Companies like Bayer and Corteva are investing heavily in synthetic biology platforms to develop sustainable agricultural solutions. The bio-based materials sector is similarly expanding, with companies producing biodegradable plastics, synthetic textiles, and construction materials through engineered microorganisms.
Industrial biotechnology represents a rapidly growing commercial frontier, where engineered microorganisms produce chemicals, enzymes, and biofuels at industrial scale. This sector is projected to reach $262 billion by 2028, with particular growth in bio-based specialty chemicals that offer sustainable alternatives to petroleum-derived products.
The commercial landscape is characterized by both established multinational corporations and agile startups. Major pharmaceutical companies have invested over $5 billion in synthetic biology partnerships since 2018, while venture capital funding in the sector exceeded $8 billion in 2021 alone. This investment pattern reflects recognition of synthetic biology's disruptive potential across multiple industries.
Patent regulations significantly impact commercialization strategies, with companies adopting various approaches to intellectual property protection. Platform technologies often employ broad patent portfolios covering foundational methods, while application-specific innovations typically utilize more targeted protection strategies. The tension between open innovation and proprietary technology development creates complex dynamics in market development, particularly as standardized biological parts and processes become increasingly important for industry growth.
Global Synthetic Biology Landscape and Barriers
Synthetic biology has emerged as a transformative field at the intersection of biology, engineering, and computer science. The global landscape of synthetic biology is characterized by rapid technological advancements, increasing investments, and expanding applications across various sectors. North America, particularly the United States, leads in synthetic biology research and commercialization, hosting approximately 40% of synthetic biology companies worldwide. The region benefits from robust academic-industry partnerships, substantial venture capital funding, and supportive regulatory frameworks.
Europe follows as the second-largest hub, with the United Kingdom, Germany, and Switzerland at the forefront. The European approach emphasizes precautionary principles in regulation while maintaining competitive research output. The Asia-Pacific region, led by China, Japan, and Singapore, is experiencing the fastest growth rate in synthetic biology development, with China particularly investing heavily in building infrastructure and talent pools to close the gap with Western counterparts.
Despite this global progress, significant barriers impede the full realization of synthetic biology's potential. Regulatory challenges constitute a primary obstacle, with inconsistent frameworks across jurisdictions creating uncertainty for companies operating internationally. Patent regulations present particular complexity, as different countries apply varying standards for patentability of biological inventions. The question of whether engineered biological systems constitute patentable subject matter remains contentious in many jurisdictions.
Technical barriers also persist, including limitations in DNA synthesis capabilities, standardization challenges, and difficulties in predicting biological system behaviors. The field struggles with reproducibility issues and scaling up laboratory successes to industrial applications. These technical constraints directly impact patentability, as inventions must demonstrate utility and enablement to qualify for patent protection.
Socioeconomic barriers further complicate the landscape. The high cost of research infrastructure creates entry barriers for developing nations and smaller entities. Additionally, public perception concerns regarding biosafety, biosecurity, and ethical implications influence regulatory decisions and market acceptance. The concentration of intellectual property in a few large corporations and academic institutions raises questions about equitable access to foundational technologies.
Geopolitical tensions add another layer of complexity, with increasing restrictions on international scientific collaboration and technology transfer. Countries increasingly view synthetic biology capabilities as strategic national assets, leading to protectionist policies that fragment the global innovation ecosystem. This fragmentation complicates international patent enforcement and technology licensing, creating additional hurdles for global commercialization of synthetic biology innovations.
Europe follows as the second-largest hub, with the United Kingdom, Germany, and Switzerland at the forefront. The European approach emphasizes precautionary principles in regulation while maintaining competitive research output. The Asia-Pacific region, led by China, Japan, and Singapore, is experiencing the fastest growth rate in synthetic biology development, with China particularly investing heavily in building infrastructure and talent pools to close the gap with Western counterparts.
Despite this global progress, significant barriers impede the full realization of synthetic biology's potential. Regulatory challenges constitute a primary obstacle, with inconsistent frameworks across jurisdictions creating uncertainty for companies operating internationally. Patent regulations present particular complexity, as different countries apply varying standards for patentability of biological inventions. The question of whether engineered biological systems constitute patentable subject matter remains contentious in many jurisdictions.
Technical barriers also persist, including limitations in DNA synthesis capabilities, standardization challenges, and difficulties in predicting biological system behaviors. The field struggles with reproducibility issues and scaling up laboratory successes to industrial applications. These technical constraints directly impact patentability, as inventions must demonstrate utility and enablement to qualify for patent protection.
Socioeconomic barriers further complicate the landscape. The high cost of research infrastructure creates entry barriers for developing nations and smaller entities. Additionally, public perception concerns regarding biosafety, biosecurity, and ethical implications influence regulatory decisions and market acceptance. The concentration of intellectual property in a few large corporations and academic institutions raises questions about equitable access to foundational technologies.
Geopolitical tensions add another layer of complexity, with increasing restrictions on international scientific collaboration and technology transfer. Countries increasingly view synthetic biology capabilities as strategic national assets, leading to protectionist policies that fragment the global innovation ecosystem. This fragmentation complicates international patent enforcement and technology licensing, creating additional hurdles for global commercialization of synthetic biology innovations.
Current Patent Strategies and Protection Methods
01 Genetic engineering and DNA manipulation techniques
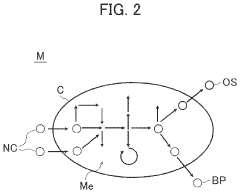
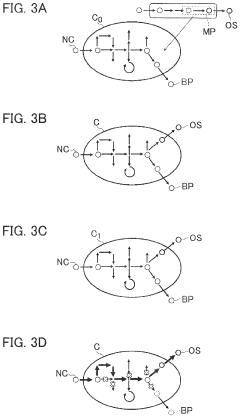
Synthetic biology employs various genetic engineering techniques to manipulate DNA sequences for creating novel biological functions. These techniques include gene editing, DNA synthesis, and assembly methods that allow researchers to design and construct artificial genetic circuits. The engineered biological systems can perform specific functions not found in nature, enabling applications in medicine, agriculture, and industrial biotechnology.- Genetic engineering and DNA manipulation techniques: Synthetic biology employs various genetic engineering techniques to manipulate DNA sequences for creating novel biological functions. These techniques include gene editing, DNA synthesis, and assembly methods that allow researchers to design and construct artificial genetic circuits. The engineered genetic systems can be used to program cells with new capabilities, enabling applications in medicine, agriculture, and industrial biotechnology.
- Biosensors and detection systems: Synthetic biology enables the development of advanced biosensors that can detect specific molecules, environmental conditions, or biological threats. These engineered biological systems translate the presence of target analytes into measurable signals, providing sensitive and specific detection methods. Applications include medical diagnostics, environmental monitoring, and security screening for pathogens or toxins.
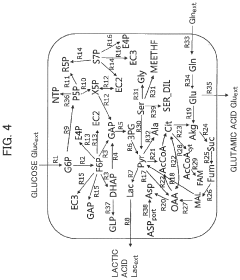
- Metabolic engineering for bioproduction: Synthetic biology approaches are used to redesign metabolic pathways in microorganisms to produce valuable compounds such as pharmaceuticals, biofuels, and specialty chemicals. By introducing new enzymatic reactions or optimizing existing pathways, researchers can transform simple feedstocks into complex molecules. These engineered biological factories offer sustainable alternatives to traditional chemical synthesis methods.
- Computational tools and artificial intelligence in synthetic biology: Advanced computational tools and artificial intelligence are increasingly integrated into synthetic biology workflows to design genetic circuits, predict protein functions, and optimize biological systems. These digital technologies enable more efficient design-build-test cycles and help researchers navigate the complex design space of biological systems. Machine learning algorithms can identify patterns in biological data and suggest novel designs with desired properties.
- Cell-free synthetic biology systems: Cell-free synthetic biology involves the use of cellular machinery outside the context of living cells to perform biological reactions. These systems provide a simplified and controlled environment for studying biological processes, prototyping genetic circuits, and producing proteins. By eliminating cell walls and other cellular components, researchers can directly access and manipulate biochemical reactions, enabling rapid testing and optimization of synthetic biological designs.
02 Biosensors and detection systems
Synthetic biology enables the development of advanced biosensors that can detect specific molecules, environmental conditions, or biological threats. These engineered biological systems translate the presence of target analytes into measurable signals, providing sensitive and specific detection capabilities. Applications include medical diagnostics, environmental monitoring, and biosecurity, where rapid and accurate detection is crucial.Expand Specific Solutions03 Computational tools and artificial intelligence in synthetic biology
The integration of computational tools and artificial intelligence with synthetic biology has accelerated the design and optimization of biological systems. These technologies enable the prediction of genetic circuit behavior, protein structure, and metabolic pathway performance. Machine learning algorithms analyze large biological datasets to identify patterns and optimize synthetic biological designs, reducing development time and improving success rates.Expand Specific Solutions04 Metabolic engineering and bioproduction systems
Synthetic biology approaches are used to engineer metabolic pathways in microorganisms for the production of valuable compounds. By redesigning existing pathways or introducing new ones, researchers can create cellular factories that efficiently convert feedstocks into pharmaceuticals, biofuels, chemicals, and materials. These engineered production systems offer sustainable alternatives to traditional chemical synthesis methods.Expand Specific Solutions05 Synthetic biology platforms and standardized biological parts
The development of standardized biological parts and assembly platforms has enabled more efficient and reproducible synthetic biology applications. These platforms include collections of well-characterized genetic components that can be combined in modular ways to create complex biological systems. Standardization facilitates collaboration, accelerates development cycles, and improves the reliability of engineered biological systems across various applications.Expand Specific Solutions
Industry Leaders and Competitive Dynamics
Synthetic biology is currently in a growth phase, with the market expected to reach significant expansion in the coming years. The competitive landscape features a diverse mix of academic institutions (California Institute of Technology, MIT, Columbia University), pharmaceutical giants (Eli Lilly, Pfizer, GlaxoSmithKline), and specialized biotech companies (Twist Bioscience, Viridos, Genentech). Patent regulations remain a critical challenge as the field matures, with major players adopting different strategies: established pharmaceutical companies acquiring IP portfolios, while research institutions focus on fundamental innovations. The technology is approaching commercial viability in several applications, though regulatory frameworks are still evolving to address the unique challenges of engineered biological systems.
The Regents of the University of California
Technical Solution: UC system has implemented a multi-layered approach to synthetic biology patent protection focusing on both foundational technologies and application-specific innovations. Their strategy includes securing broad patents on core CRISPR-Cas9 gene editing technology[1], developing novel biosafety containment mechanisms for engineered organisms[2], and creating standardized regulatory elements for precise genetic control[3]. UC's patent portfolio encompasses both tools and applications, with particular strength in agricultural biotechnology and biopharmaceuticals. Their approach includes strategic international filing to secure protection in key markets while maintaining research exemptions for academic use. UC has pioneered novel licensing models that balance commercial development with public access, including humanitarian use provisions for applications in developing countries and tiered royalty structures based on market applications.
Strengths: Extensive patent portfolio covering fundamental synthetic biology tools; sophisticated technology transfer infrastructure; strong position in international patent disputes. Weaknesses: Complex institutional structure can slow decision-making; ongoing litigation with other institutions over overlapping claims; challenges in balancing public mission with commercial interests.
Twist Bioscience Corp.
Technical Solution: Twist Bioscience has developed a proprietary silicon-based DNA synthesis platform that revolutionizes synthetic biology patent strategies through unprecedented scale and precision. Their technology enables the synthesis of DNA sequences up to 1.8 million base pairs with error rates below 1:1000[1], facilitating rapid prototyping of synthetic genes and genomes. Twist's patent strategy centers on process innovations in DNA synthesis rather than end-product applications, creating a technology platform that serves multiple markets while maintaining strong IP protection. Their approach includes securing patents on silicon-chip based synthesis methods, enzymatic error correction techniques, and high-throughput quality control systems[2]. Twist has strategically positioned itself as an enabling technology provider, developing licensing models that allow customers to access their synthesis platform while maintaining rights to downstream applications, effectively navigating the complex patent landscape by focusing on tools rather than end-products.
Strengths: Dominant position in DNA synthesis technology; scalable platform applicable across multiple industries; business model that minimizes direct patent conflicts with customers. Weaknesses: Vulnerability to alternative synthesis technologies; dependence on customers' success in navigating application patents; challenges in protecting process innovations globally.
Key Intellectual Property Frameworks Analysis
Designing synthetic biological circuits using optimality and nonequilibrium thermodynamics
PatentInactiveUS20120117518A1
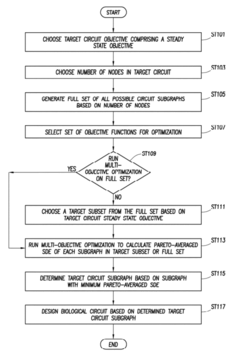
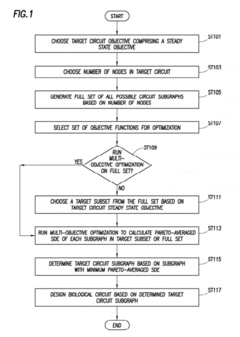
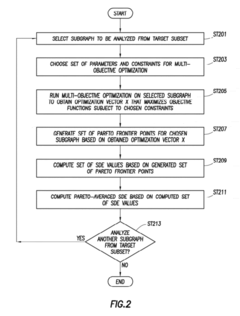
Innovation
- A method using specific dissipation energy (SDE) optimization to identify optimal biological circuit subgraphs by determining the energetic cost of various circuit configurations, allowing for the selection and design of biological circuits with minimal SDE, thereby reducing the design parameter space and enhancing efficiency.
Method and apparatus for screening of cell strains and culture conditions
PatentActiveUS20210189452A1
Innovation
- A method and apparatus that utilize a correlation model to relate culture conditions (such as temperature and pH) to metabolic reaction rates, allowing for the computation of optimal culture conditions that align with target metabolic reaction values, thereby enhancing productivity and stability of substance production.
Regulatory Compliance and Policy Implications
Synthetic biology operates at the intersection of multiple regulatory frameworks, creating a complex compliance landscape for researchers and companies. Current patent regulations were largely designed before the emergence of this field, resulting in significant challenges when applying traditional intellectual property concepts to biological innovations. The regulatory environment varies dramatically across jurisdictions, with the United States Patent and Trademark Office (USPTO) generally adopting a more permissive approach compared to the European Patent Office (EPO), which maintains stricter limitations on patenting naturally derived biological components.
The policy implications of synthetic biology patents extend beyond mere intellectual property considerations. Regulatory agencies worldwide are struggling to balance innovation promotion with ethical considerations and biosafety concerns. The FDA, EPA, and USDA in the United States have established a Coordinated Framework for the Regulation of Biotechnology, yet significant regulatory gaps remain regarding engineered biological systems that don't fit neatly into existing categories.
International harmonization efforts face substantial challenges due to divergent national interests and regulatory philosophies. The Nagoya Protocol on Access and Benefit Sharing provides some framework for genetic resource utilization but was not designed with synthetic biology specifically in mind. This creates uncertainty for companies operating globally and potentially inhibits cross-border collaboration and technology transfer.
Emerging policy trends indicate a move toward more adaptive regulatory frameworks that can accommodate the rapid pace of synthetic biology innovation. Risk-tiered approaches are gaining traction, where regulatory scrutiny scales with potential risk rather than applying uniform standards across all applications. Several jurisdictions are exploring regulatory sandboxes to allow controlled testing of synthetic biology applications while gathering data to inform permanent regulatory structures.
Compliance strategies for synthetic biology companies must be forward-looking and adaptable. This includes early engagement with regulatory authorities, participation in standards development, and implementation of robust internal governance frameworks. Companies are increasingly adopting responsible innovation principles that go beyond minimum compliance requirements to address societal concerns proactively.
The intersection of patent law and biosecurity presents particular challenges, as disclosure requirements in patent applications may potentially provide roadmaps for misuse. Policymakers are exploring mechanisms to balance innovation protection with security considerations, including potential limitations on patent disclosures for dual-use technologies and enhanced screening procedures for certain patent applications.
The policy implications of synthetic biology patents extend beyond mere intellectual property considerations. Regulatory agencies worldwide are struggling to balance innovation promotion with ethical considerations and biosafety concerns. The FDA, EPA, and USDA in the United States have established a Coordinated Framework for the Regulation of Biotechnology, yet significant regulatory gaps remain regarding engineered biological systems that don't fit neatly into existing categories.
International harmonization efforts face substantial challenges due to divergent national interests and regulatory philosophies. The Nagoya Protocol on Access and Benefit Sharing provides some framework for genetic resource utilization but was not designed with synthetic biology specifically in mind. This creates uncertainty for companies operating globally and potentially inhibits cross-border collaboration and technology transfer.
Emerging policy trends indicate a move toward more adaptive regulatory frameworks that can accommodate the rapid pace of synthetic biology innovation. Risk-tiered approaches are gaining traction, where regulatory scrutiny scales with potential risk rather than applying uniform standards across all applications. Several jurisdictions are exploring regulatory sandboxes to allow controlled testing of synthetic biology applications while gathering data to inform permanent regulatory structures.
Compliance strategies for synthetic biology companies must be forward-looking and adaptable. This includes early engagement with regulatory authorities, participation in standards development, and implementation of robust internal governance frameworks. Companies are increasingly adopting responsible innovation principles that go beyond minimum compliance requirements to address societal concerns proactively.
The intersection of patent law and biosecurity presents particular challenges, as disclosure requirements in patent applications may potentially provide roadmaps for misuse. Policymakers are exploring mechanisms to balance innovation protection with security considerations, including potential limitations on patent disclosures for dual-use technologies and enhanced screening procedures for certain patent applications.
Ethical Considerations and Biosafety Standards
The intersection of synthetic biology and ethical frameworks presents complex challenges that require careful consideration within patent regulatory systems. Synthetic biology's capacity to manipulate genetic material raises profound ethical questions about the boundaries of human intervention in natural systems. These ethical considerations must be integrated into patent regulations to ensure responsible innovation while protecting public interests.
Biosafety standards have evolved significantly in response to synthetic biology advancements. The Cartagena Protocol on Biosafety (2000) established international guidelines for the safe transfer and handling of living modified organisms. Subsequently, regulatory bodies worldwide have developed more specific frameworks addressing synthetic biology applications, including the NIH Guidelines for Research Involving Recombinant DNA Molecules in the United States and the EU Directive 2009/41/EC on contained use of genetically modified microorganisms.
Risk assessment methodologies for synthetic biology patents require multi-dimensional evaluation frameworks that consider both intended applications and potential unintended consequences. Current approaches often employ tiered assessment models that evaluate immediate biosafety concerns, medium-term ecological impacts, and long-term evolutionary implications. These assessments increasingly incorporate the concept of "responsible innovation" that balances technological advancement with precautionary principles.
Dual-use concerns represent a particularly challenging aspect of synthetic biology patent regulation. Technologies developed for beneficial applications in medicine or agriculture may potentially be repurposed for harmful uses. Patent offices globally are developing specialized review processes for applications with dual-use potential, requiring additional documentation regarding biosafety protocols and risk mitigation strategies.
The principle of transparency has emerged as a cornerstone of ethical synthetic biology patent practices. Several jurisdictions now require patent applicants to disclose comprehensive biosafety data, containment protocols, and potential ecological impacts. This transparency requirement extends to the disclosure of risk assessment methodologies and contingency plans for potential biosafety breaches.
International harmonization of biosafety standards remains an ongoing challenge in synthetic biology patent regulation. Significant variations exist between regulatory frameworks in different regions, creating potential regulatory arbitrage opportunities. Efforts by the World Intellectual Property Organization (WIPO) and the Convention on Biological Diversity are working toward establishing minimum biosafety disclosure requirements for synthetic biology patents globally, though implementation remains inconsistent.
Biosafety standards have evolved significantly in response to synthetic biology advancements. The Cartagena Protocol on Biosafety (2000) established international guidelines for the safe transfer and handling of living modified organisms. Subsequently, regulatory bodies worldwide have developed more specific frameworks addressing synthetic biology applications, including the NIH Guidelines for Research Involving Recombinant DNA Molecules in the United States and the EU Directive 2009/41/EC on contained use of genetically modified microorganisms.
Risk assessment methodologies for synthetic biology patents require multi-dimensional evaluation frameworks that consider both intended applications and potential unintended consequences. Current approaches often employ tiered assessment models that evaluate immediate biosafety concerns, medium-term ecological impacts, and long-term evolutionary implications. These assessments increasingly incorporate the concept of "responsible innovation" that balances technological advancement with precautionary principles.
Dual-use concerns represent a particularly challenging aspect of synthetic biology patent regulation. Technologies developed for beneficial applications in medicine or agriculture may potentially be repurposed for harmful uses. Patent offices globally are developing specialized review processes for applications with dual-use potential, requiring additional documentation regarding biosafety protocols and risk mitigation strategies.
The principle of transparency has emerged as a cornerstone of ethical synthetic biology patent practices. Several jurisdictions now require patent applicants to disclose comprehensive biosafety data, containment protocols, and potential ecological impacts. This transparency requirement extends to the disclosure of risk assessment methodologies and contingency plans for potential biosafety breaches.
International harmonization of biosafety standards remains an ongoing challenge in synthetic biology patent regulation. Significant variations exist between regulatory frameworks in different regions, creating potential regulatory arbitrage opportunities. Efforts by the World Intellectual Property Organization (WIPO) and the Convention on Biological Diversity are working toward establishing minimum biosafety disclosure requirements for synthetic biology patents globally, though implementation remains inconsistent.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!