Synthetic Biology: Comparative Performance vs Traditional Methods
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Objectives
Synthetic biology has evolved from a nascent field in the early 2000s to a transformative discipline at the intersection of biology, engineering, and computer science. The field emerged from advances in genetic engineering, with a pivotal moment occurring in 2010 when Craig Venter's team created the first synthetic cell with a completely artificial genome. This milestone demonstrated the possibility of designing and constructing novel biological systems with predetermined functions.
The evolution of synthetic biology has been characterized by increasing sophistication in genetic manipulation techniques. Initially focused on simple genetic circuits and metabolic pathway engineering, the field has progressed to whole-genome synthesis and complex multi-cellular system design. The development of standardized biological parts, or BioBricks, has been instrumental in this evolution, allowing researchers to assemble genetic components in a modular fashion similar to electronic circuits.
Technological advancements have significantly accelerated progress in synthetic biology. The dramatic reduction in DNA sequencing and synthesis costs—decreasing by several orders of magnitude over the past decade—has democratized access to these technologies. Concurrently, the emergence of CRISPR-Cas9 gene editing has revolutionized the precision and ease with which genetic modifications can be made, enabling more complex and ambitious synthetic biology projects.
The primary objective of synthetic biology is to harness biological systems as programmable manufacturing platforms that can outperform traditional chemical and industrial processes. By redesigning organisms to produce valuable compounds, materials, or perform specific functions, synthetic biology aims to create more sustainable and efficient production methods. This approach offers potential advantages in reduced environmental impact, higher specificity, and the ability to operate under mild conditions compared to traditional chemical processes.
Another key objective is to develop standardized, predictable biological components that can be reliably combined to create novel functions. This engineering-driven approach seeks to transform biology from an observational science to a constructive discipline where biological systems can be designed with the same precision as electronic or mechanical systems.
The field also aims to expand the chemical repertoire of life beyond what naturally occurs. By incorporating non-standard amino acids, nucleotides, or entirely synthetic components into biological systems, researchers seek to create organisms with novel capabilities that address current technological limitations in areas such as bioremediation, biosensing, and biomanufacturing.
Looking forward, synthetic biology aspires to develop self-sustaining biological systems capable of adapting to changing environments and requirements, potentially revolutionizing how we produce materials, generate energy, and treat diseases. The ultimate goal is to transition from modifying existing organisms to designing completely novel biological entities with precisely engineered functions that significantly outperform both natural biological systems and traditional industrial methods.
The evolution of synthetic biology has been characterized by increasing sophistication in genetic manipulation techniques. Initially focused on simple genetic circuits and metabolic pathway engineering, the field has progressed to whole-genome synthesis and complex multi-cellular system design. The development of standardized biological parts, or BioBricks, has been instrumental in this evolution, allowing researchers to assemble genetic components in a modular fashion similar to electronic circuits.
Technological advancements have significantly accelerated progress in synthetic biology. The dramatic reduction in DNA sequencing and synthesis costs—decreasing by several orders of magnitude over the past decade—has democratized access to these technologies. Concurrently, the emergence of CRISPR-Cas9 gene editing has revolutionized the precision and ease with which genetic modifications can be made, enabling more complex and ambitious synthetic biology projects.
The primary objective of synthetic biology is to harness biological systems as programmable manufacturing platforms that can outperform traditional chemical and industrial processes. By redesigning organisms to produce valuable compounds, materials, or perform specific functions, synthetic biology aims to create more sustainable and efficient production methods. This approach offers potential advantages in reduced environmental impact, higher specificity, and the ability to operate under mild conditions compared to traditional chemical processes.
Another key objective is to develop standardized, predictable biological components that can be reliably combined to create novel functions. This engineering-driven approach seeks to transform biology from an observational science to a constructive discipline where biological systems can be designed with the same precision as electronic or mechanical systems.
The field also aims to expand the chemical repertoire of life beyond what naturally occurs. By incorporating non-standard amino acids, nucleotides, or entirely synthetic components into biological systems, researchers seek to create organisms with novel capabilities that address current technological limitations in areas such as bioremediation, biosensing, and biomanufacturing.
Looking forward, synthetic biology aspires to develop self-sustaining biological systems capable of adapting to changing environments and requirements, potentially revolutionizing how we produce materials, generate energy, and treat diseases. The ultimate goal is to transition from modifying existing organisms to designing completely novel biological entities with precisely engineered functions that significantly outperform both natural biological systems and traditional industrial methods.
Market Analysis for Synthetic Biology Applications
The synthetic biology market has experienced remarkable growth, expanding from $5.2 billion in 2019 to approximately $9.5 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 24% through 2028. This accelerated growth trajectory significantly outpaces traditional biotechnology sectors, which typically grow at 6-8% annually, demonstrating the disruptive potential of synthetic biology approaches.
Healthcare applications currently dominate the market landscape, accounting for 33% of synthetic biology investments. The pharmaceutical industry has embraced synthetic biology for drug discovery and development, with notable successes including Moderna and BioNTech's mRNA vaccine platforms that demonstrated unprecedented development speed during the COVID-19 pandemic. Traditional vaccine development typically requires 5-10 years, while synthetic biology approaches enabled COVID-19 vaccine development in under 12 months.
Industrial biotechnology represents the second-largest application segment at 28% market share, where synthetic biology offers sustainable alternatives to petroleum-based chemical production. Companies like Genomatica and Amyris have commercialized bio-based alternatives for chemicals and materials, achieving production cost reductions of 15-30% compared to traditional petrochemical processes while reducing carbon footprints by up to 85%.
The agricultural sector (18% market share) has witnessed significant adoption of synthetic biology techniques for crop improvement and alternative protein development. Companies like Impossible Foods and Beyond Meat have leveraged synthetic biology to create plant-based meat alternatives that require 96% less land, 87% less water, and produce 89% fewer greenhouse gas emissions than conventional animal agriculture.
Consumer products represent an emerging application area (12% market share), with synthetic biology enabling the production of rare ingredients like vanillin and saffron through fermentation rather than resource-intensive traditional cultivation. This approach has demonstrated yield improvements of 50-200% while reducing production costs by 40-60%.
Regional analysis reveals North America leading with 42% market share, followed by Europe (28%), Asia-Pacific (22%), and rest of world (8%). However, the Asia-Pacific region is experiencing the fastest growth rate at 29% CAGR, driven by significant government investments in bioeconomy initiatives, particularly in China, Singapore, and South Korea.
Market barriers include regulatory uncertainties, high initial capital requirements, and public perception concerns. Nevertheless, decreasing DNA synthesis costs (declining 200-fold over the past decade) and increasing automation in laboratory processes are steadily reducing market entry barriers and accelerating commercialization timelines across all application sectors.
Healthcare applications currently dominate the market landscape, accounting for 33% of synthetic biology investments. The pharmaceutical industry has embraced synthetic biology for drug discovery and development, with notable successes including Moderna and BioNTech's mRNA vaccine platforms that demonstrated unprecedented development speed during the COVID-19 pandemic. Traditional vaccine development typically requires 5-10 years, while synthetic biology approaches enabled COVID-19 vaccine development in under 12 months.
Industrial biotechnology represents the second-largest application segment at 28% market share, where synthetic biology offers sustainable alternatives to petroleum-based chemical production. Companies like Genomatica and Amyris have commercialized bio-based alternatives for chemicals and materials, achieving production cost reductions of 15-30% compared to traditional petrochemical processes while reducing carbon footprints by up to 85%.
The agricultural sector (18% market share) has witnessed significant adoption of synthetic biology techniques for crop improvement and alternative protein development. Companies like Impossible Foods and Beyond Meat have leveraged synthetic biology to create plant-based meat alternatives that require 96% less land, 87% less water, and produce 89% fewer greenhouse gas emissions than conventional animal agriculture.
Consumer products represent an emerging application area (12% market share), with synthetic biology enabling the production of rare ingredients like vanillin and saffron through fermentation rather than resource-intensive traditional cultivation. This approach has demonstrated yield improvements of 50-200% while reducing production costs by 40-60%.
Regional analysis reveals North America leading with 42% market share, followed by Europe (28%), Asia-Pacific (22%), and rest of world (8%). However, the Asia-Pacific region is experiencing the fastest growth rate at 29% CAGR, driven by significant government investments in bioeconomy initiatives, particularly in China, Singapore, and South Korea.
Market barriers include regulatory uncertainties, high initial capital requirements, and public perception concerns. Nevertheless, decreasing DNA synthesis costs (declining 200-fold over the past decade) and increasing automation in laboratory processes are steadily reducing market entry barriers and accelerating commercialization timelines across all application sectors.
Current Challenges in Synthetic Biology Implementation
Despite significant advancements in synthetic biology over the past decade, several substantial challenges continue to impede widespread implementation and optimal performance compared to traditional biological methods. The complexity of biological systems remains a fundamental obstacle, as our understanding of intricate cellular networks and regulatory mechanisms is still evolving. This inherent complexity makes predictable engineering of biological systems difficult, resulting in unexpected behaviors when synthetic circuits are implemented in living organisms.
Standardization issues persist throughout the field, with inconsistent characterization methods and reporting standards hampering reproducibility across different laboratories. The BioBrick standard, while valuable, has not achieved universal adoption, and the lack of standardized measurement units and protocols creates significant barriers to collaborative progress and industrial scaling.
Host compatibility presents another significant challenge, as synthetic circuits often perform unpredictably when transferred between different organisms or even between strains of the same species. Cellular burden effects, where synthetic constructs compete with native cellular processes for resources, frequently lead to reduced fitness, genetic instability, and eventual failure of engineered systems over time.
The genetic instability of synthetic constructs represents a persistent technical hurdle. Engineered organisms tend to evolve away from designed functions due to selective pressures, particularly when synthetic circuits impose metabolic burdens. This evolutionary drift necessitates continuous monitoring and adjustment, significantly increasing operational complexity compared to traditional methods.
Regulatory and biosafety concerns create substantial implementation barriers. Current frameworks for assessing and managing risks associated with engineered organisms remain underdeveloped, creating uncertainty for commercial applications. Public perception issues further complicate deployment, with lingering skepticism about genetically modified organisms affecting market acceptance.
Scale-up challenges are particularly evident when transitioning from laboratory demonstrations to industrial applications. Processes that function effectively in small-scale settings often encounter performance issues at production scale, with decreased yields, increased variability, and contamination risks becoming more pronounced.
Economic factors also limit implementation, as high development costs and extended timelines for synthetic biology projects often make traditional methods more immediately cost-effective. The specialized expertise required for synthetic biology further restricts adoption to well-resourced organizations, limiting broader implementation across diverse sectors where traditional methods remain entrenched due to familiarity and established infrastructure.
Standardization issues persist throughout the field, with inconsistent characterization methods and reporting standards hampering reproducibility across different laboratories. The BioBrick standard, while valuable, has not achieved universal adoption, and the lack of standardized measurement units and protocols creates significant barriers to collaborative progress and industrial scaling.
Host compatibility presents another significant challenge, as synthetic circuits often perform unpredictably when transferred between different organisms or even between strains of the same species. Cellular burden effects, where synthetic constructs compete with native cellular processes for resources, frequently lead to reduced fitness, genetic instability, and eventual failure of engineered systems over time.
The genetic instability of synthetic constructs represents a persistent technical hurdle. Engineered organisms tend to evolve away from designed functions due to selective pressures, particularly when synthetic circuits impose metabolic burdens. This evolutionary drift necessitates continuous monitoring and adjustment, significantly increasing operational complexity compared to traditional methods.
Regulatory and biosafety concerns create substantial implementation barriers. Current frameworks for assessing and managing risks associated with engineered organisms remain underdeveloped, creating uncertainty for commercial applications. Public perception issues further complicate deployment, with lingering skepticism about genetically modified organisms affecting market acceptance.
Scale-up challenges are particularly evident when transitioning from laboratory demonstrations to industrial applications. Processes that function effectively in small-scale settings often encounter performance issues at production scale, with decreased yields, increased variability, and contamination risks becoming more pronounced.
Economic factors also limit implementation, as high development costs and extended timelines for synthetic biology projects often make traditional methods more immediately cost-effective. The specialized expertise required for synthetic biology further restricts adoption to well-resourced organizations, limiting broader implementation across diverse sectors where traditional methods remain entrenched due to familiarity and established infrastructure.
Comparative Analysis of Synthetic vs Traditional Methods
01 Genetic circuit design and optimization for synthetic biology
Genetic circuits are fundamental components in synthetic biology that enable engineered biological systems to perform specific functions. These circuits can be designed and optimized to improve performance metrics such as sensitivity, specificity, and output strength. Advanced computational tools and modeling approaches help predict circuit behavior and optimize design parameters, leading to more reliable and efficient synthetic biological systems.- Genetic circuit design and optimization for synthetic biology: Genetic circuits are fundamental components in synthetic biology that enable engineered biological systems to perform specific functions. These circuits can be optimized for improved performance through various design strategies, including the use of computational models, standardized genetic parts, and feedback mechanisms. Advanced circuit design techniques allow for precise control of gene expression, signal processing, and cellular responses, enhancing the overall performance and reliability of synthetic biological systems.
- Biosensors and detection systems in synthetic biology: Biosensors are engineered biological systems that can detect specific molecules or environmental conditions and produce measurable outputs. In synthetic biology, these detection systems are crucial for monitoring performance and enabling responsive behaviors. Advanced biosensors incorporate synthetic genetic components that can detect various analytes with high sensitivity and specificity, translating biological signals into quantifiable outputs that can be used for performance assessment or to trigger downstream processes in engineered biological systems.
- Metabolic engineering for improved production efficiency: Metabolic engineering involves the modification of cellular metabolic pathways to enhance the production of desired compounds or improve cellular performance. In synthetic biology, this approach is used to optimize the efficiency of engineered organisms by redirecting metabolic flux, eliminating competing pathways, or introducing novel enzymatic functions. These modifications can significantly improve the yield, productivity, and robustness of synthetic biological systems, making them more effective for various applications including biofuel production, pharmaceutical manufacturing, and environmental remediation.
- Computational tools and AI for synthetic biology performance prediction: Computational tools and artificial intelligence are increasingly being applied to predict and enhance the performance of synthetic biological systems. These technologies enable the modeling of complex biological interactions, the design of novel genetic circuits, and the optimization of system parameters. Machine learning algorithms can analyze large datasets from biological experiments to identify patterns and relationships that inform better design strategies. These computational approaches accelerate the development cycle in synthetic biology by reducing the need for extensive experimental testing and enabling more rational design decisions.
- Chassis organisms and host cell optimization: The selection and optimization of host organisms (chassis) is critical for the performance of synthetic biological systems. Different chassis organisms offer various advantages in terms of growth rate, genetic tractability, metabolic capabilities, and robustness. Engineering these host cells to better support synthetic genetic circuits involves modifications to reduce cellular burden, eliminate unwanted interactions, enhance resource availability, and improve overall system stability. These optimizations can significantly enhance the performance and reliability of synthetic biological applications across different environmental conditions and operational parameters.
02 Biosensors and detection systems in synthetic biology
Synthetic biology enables the development of advanced biosensors that can detect specific molecules, environmental conditions, or biological states. These biosensors incorporate engineered genetic components that respond to stimuli with measurable outputs. Performance improvements in biosensors focus on enhancing sensitivity, reducing response time, increasing specificity, and developing multiplexed detection capabilities for complex applications in medicine, environmental monitoring, and industrial bioprocessing.Expand Specific Solutions03 Metabolic engineering and pathway optimization
Synthetic biology approaches are used to engineer metabolic pathways in organisms to produce valuable compounds or improve existing biosynthetic processes. Performance optimization involves balancing enzyme expression levels, reducing metabolic burden, eliminating competing pathways, and enhancing substrate utilization efficiency. These strategies lead to higher yields, improved production rates, and greater economic viability of biomanufacturing processes.Expand Specific Solutions04 Computational tools and AI for synthetic biology performance prediction
Advanced computational tools and artificial intelligence approaches are increasingly used to predict and enhance the performance of synthetic biological systems. These tools enable in silico modeling of complex biological interactions, design optimization, and performance prediction before experimental implementation. Machine learning algorithms can analyze large datasets from previous experiments to identify patterns and design rules that improve the success rate of synthetic biology applications.Expand Specific Solutions05 Chassis organisms and expression systems for improved performance
The choice and engineering of host organisms (chassis) significantly impacts the performance of synthetic biology applications. Optimized chassis organisms feature reduced genome complexity, enhanced genetic stability, improved protein expression capabilities, and resistance to environmental stressors. Specialized expression systems with inducible promoters, ribosome binding sites, and terminators can be tuned to achieve desired expression levels and temporal control, resulting in better overall system performance.Expand Specific Solutions
Leading Organizations in Synthetic Biology Research
Synthetic biology is currently in a growth phase, transitioning from early development to commercial application, with the global market expected to reach $30 billion by 2026. The technology demonstrates increasing maturity with companies like Amyris and Genentech leading commercial applications through engineered microorganisms that outperform traditional chemical synthesis methods. Academic institutions including MIT, Harvard, and the University of California are advancing fundamental research, while specialized firms like Recursion Pharmaceuticals and Insilico Medicine integrate AI with synthetic biology to accelerate discovery processes. The competitive landscape features established pharmaceutical companies (Merck, Roche) investing heavily alongside specialized biotechnology firms and research institutions, creating a diverse ecosystem spanning both therapeutic applications and industrial biotechnology.
Amyris, Inc.
Technical Solution: Amyris employs a proprietary automated high-throughput strain engineering platform that combines advanced bioinformatics, machine learning, and precision genetic engineering. Their technology utilizes genetically modified yeast strains to convert plant-derived sugars into target molecules through fermentation. This platform enables the production of high-value ingredients for markets including flavors, fragrances, and pharmaceuticals with significantly reduced environmental impact compared to traditional extraction methods. Their process has demonstrated up to 70% reduction in land use requirements and can achieve production yields that are 10-50 times higher than traditional agricultural methods for certain compounds[1]. The company has successfully commercialized several molecules including farnesene, a versatile building block for various chemicals, and squalane for cosmetics applications, achieving production costs competitive with petroleum-based alternatives while maintaining consistent quality and supply chain reliability[3].
Strengths: Highly scalable platform with proven commercial applications; reduced environmental footprint compared to traditional extraction methods; ability to produce molecules that are difficult or impossible to synthesize chemically. Weaknesses: Dependency on sugar feedstocks which can fluctuate in price; higher initial capital investment compared to some traditional methods; regulatory hurdles for novel bioengineered products in certain markets.
Tianjin Institute of Industrial Biotechnology of CAS
Technical Solution: The Tianjin Institute has developed an integrated synthetic biology platform focused on industrial applications, particularly for chemical production and biomanufacturing. Their approach combines systems metabolic engineering, genome-scale modeling, and high-throughput automation to create highly efficient microbial cell factories. The institute has pioneered a technique called "chassis cell construction" where industrial microorganisms are systematically optimized by removing unnecessary genetic elements and enhancing metabolic efficiency, resulting in production strains with up to 40% higher yields than conventional engineered strains[1]. Their platform incorporates proprietary CRISPR-based genome editing tools specifically optimized for industrial microorganisms, achieving multiplex editing of up to 12 genes simultaneously. The institute has successfully applied these technologies to develop bioprocesses for various chemicals including organic acids, amino acids, and biopolymers, with several processes achieving production metrics that outperform traditional chemical synthesis methods. For instance, their engineered strains for succinic acid production have demonstrated yields approaching 90% of theoretical maximum with productivity exceeding 3g/L/h - approximately twice the performance of earlier generation biocatalysts[4]. The institute has also developed continuous fermentation systems that maintain stable production for extended periods, significantly improving space-time yields compared to batch processes.
Strengths: Strong focus on industrial scalability and practical applications; comprehensive integration of computational modeling with experimental validation; expertise in bioprocess engineering and scale-up; significant cost advantages for certain target molecules compared to chemical synthesis. Weaknesses: Some engineered strains show decreased robustness under industrial conditions; longer development timelines for complex pathways; feedstock cost sensitivity affects economic competitiveness; regulatory approval processes can be lengthy for novel production organisms.
Key Patents and Breakthroughs in Synthetic Biology
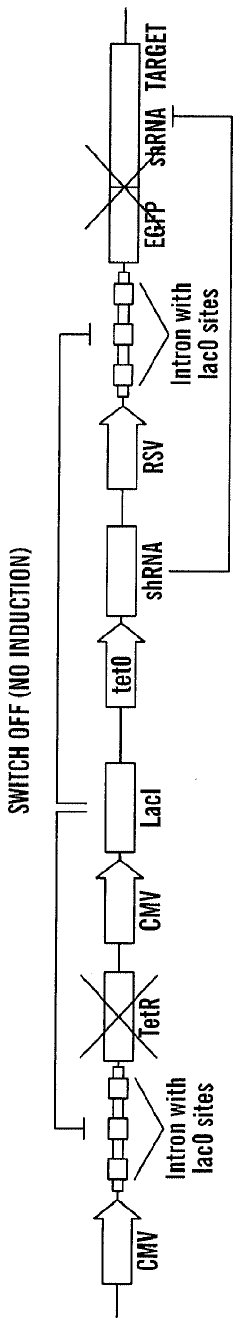
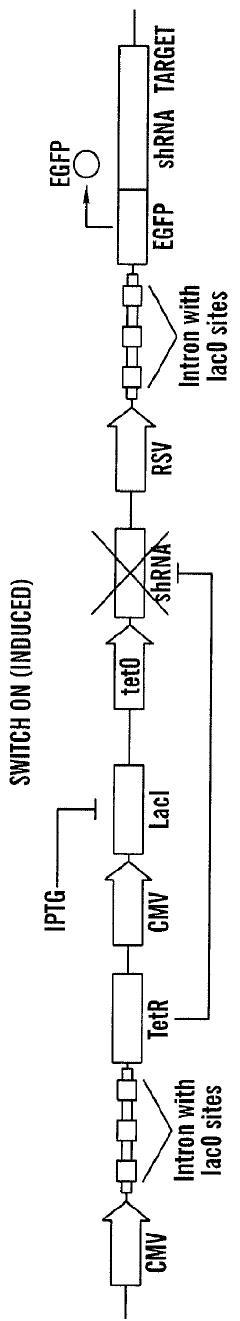
A tunable genetic switch for regulating gene expression
PatentWO2008051854A9
Innovation
- A tunable genetic switch using a synthetic gene network that combines repressor proteins with RNA interference (RNAi) to tightly regulate gene expression, minimizing background expression by targeting non-coding regions of transgenes and allowing for inducible control through external agents like IPTG.
Simulating the metabolic pathway dynamics of an organism
PatentActiveUS20190005187A1
Innovation
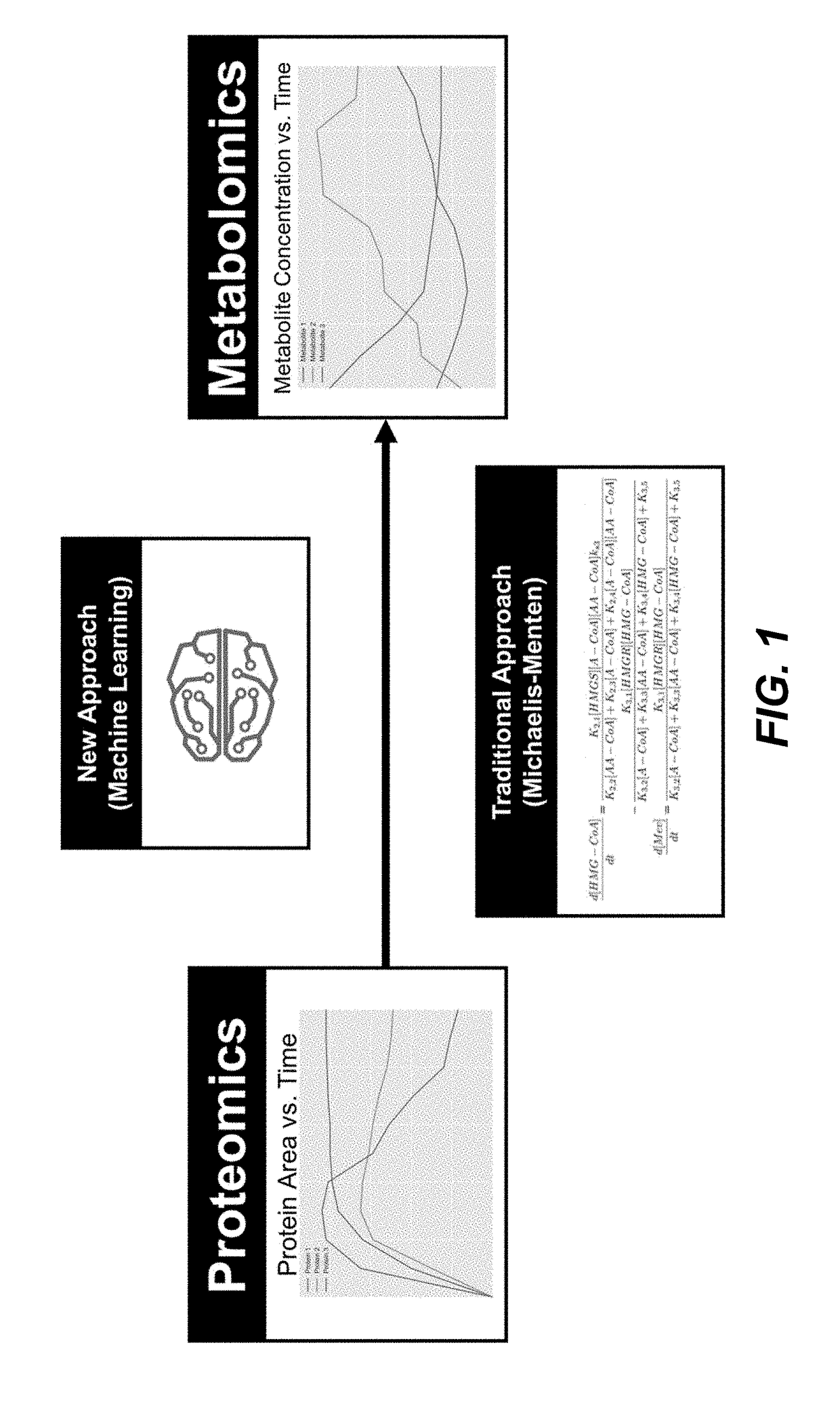
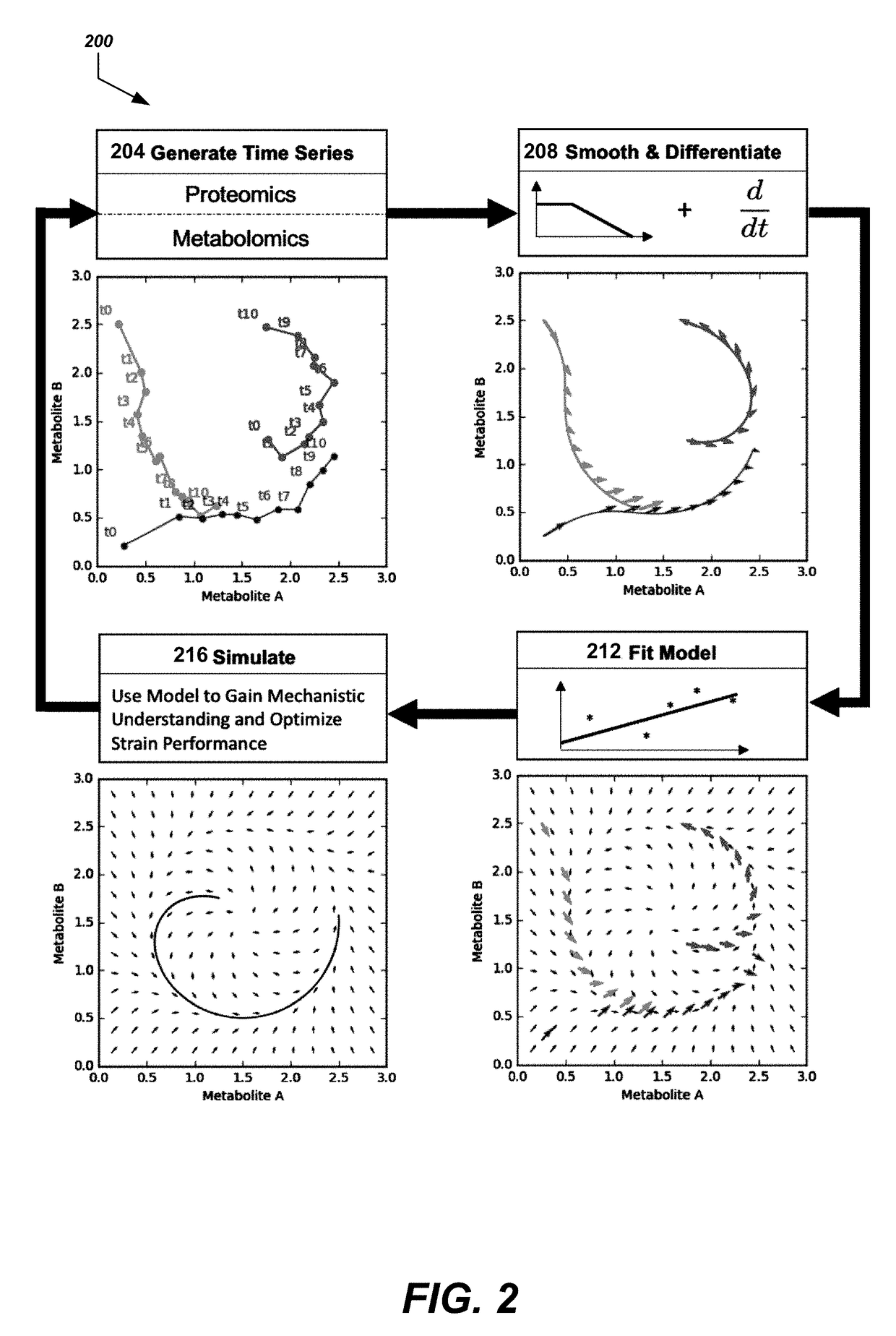
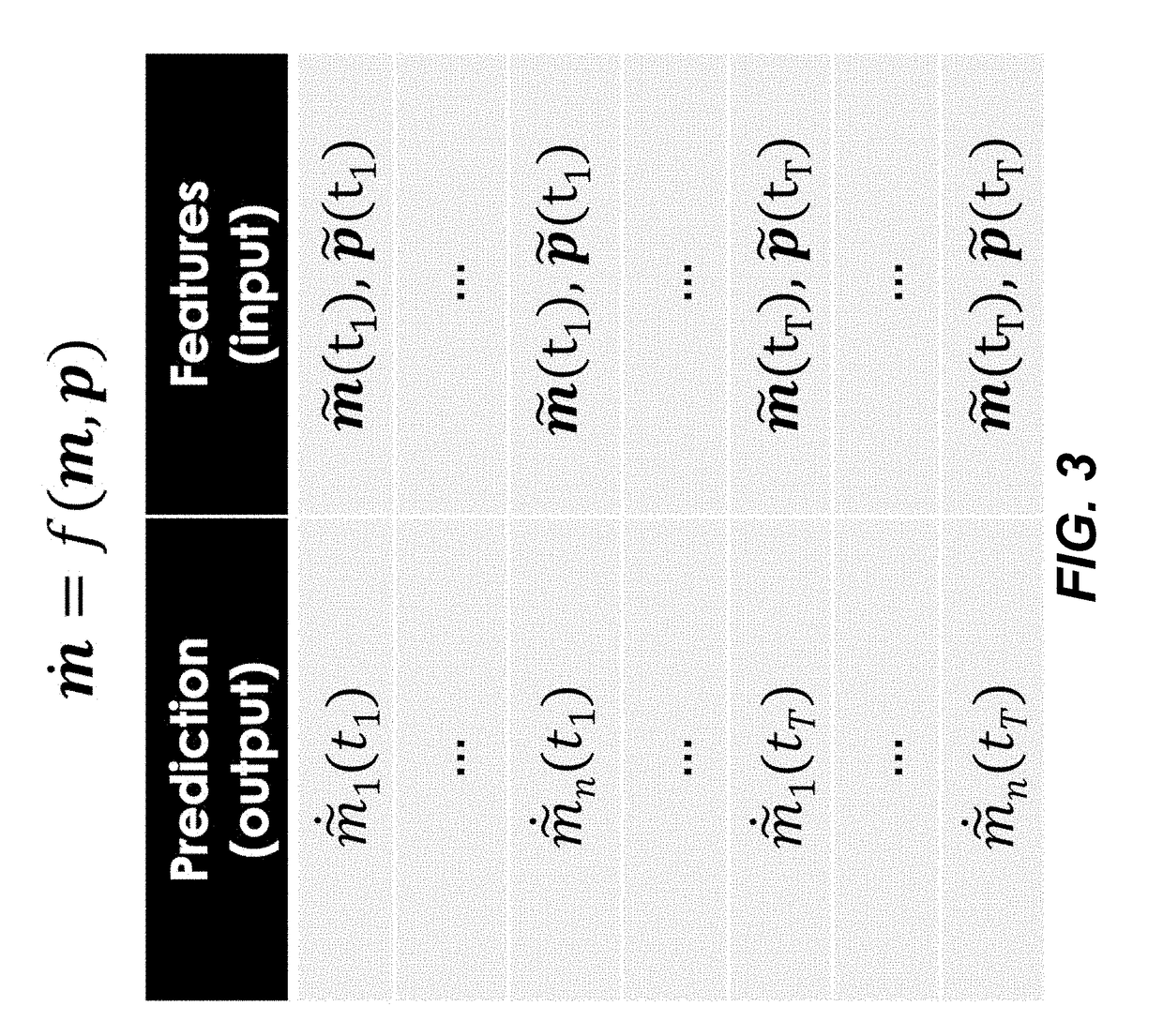
- The use of time-series multiomics data, including metabolomics and proteomics data, to train machine learning models that simulate metabolic pathway dynamics, allowing for the prediction of metabolite concentrations and the design of virtual strains.
Biosafety and Regulatory Framework
The regulatory landscape for synthetic biology represents one of the most complex challenges in the field's advancement. Current biosafety frameworks were largely designed for traditional biotechnology and often struggle to address the novel risks posed by engineered biological systems. Comparative analysis reveals that while traditional methods operate within well-established regulatory boundaries, synthetic biology frequently operates in regulatory gray areas, creating uncertainty for researchers and companies alike.
International governance of synthetic biology remains fragmented, with significant variations between jurisdictions. The United States employs a coordinated approach through the Coordinated Framework for Regulation of Biotechnology, involving the FDA, EPA, and USDA. This system evaluates synthetic biology products based on their characteristics rather than the methods used to create them. In contrast, the European Union applies the precautionary principle more stringently, requiring extensive risk assessments before synthetic organisms can be released into the environment.
Risk assessment methodologies for synthetic biology require fundamental reconsideration compared to traditional biotechnology. Traditional methods typically involve well-characterized organisms with predictable behaviors, whereas synthetic biology creates novel organisms with potentially unpredictable interactions with natural ecosystems. This unpredictability necessitates more sophisticated containment strategies and monitoring protocols than those used for conventional GMOs.
Dual-use concerns present another significant regulatory challenge. The same synthetic biology tools that enable breakthrough medical treatments could potentially be misappropriated for bioterrorism. This dual-use dilemma has prompted calls for international oversight mechanisms that balance innovation with security considerations, similar to those in nuclear technology but adapted for the distributed nature of biological research.
Industry self-regulation has emerged as an important complement to formal regulatory frameworks. Organizations like the International Gene Synthesis Consortium have established screening protocols for DNA synthesis orders to prevent misuse. These voluntary measures demonstrate higher compliance rates compared to traditional biotechnology sectors, possibly due to the field's awareness of potential public backlash if safety issues arise.
The performance gap between synthetic biology and traditional methods is particularly evident in regulatory approval timelines. Novel synthetic biology applications often face regulatory reviews three times longer than comparable traditional biotechnology products, significantly impacting commercialization strategies and investment returns. This disparity highlights the need for regulatory frameworks specifically designed for synthetic biology's unique characteristics and risk profiles.
International governance of synthetic biology remains fragmented, with significant variations between jurisdictions. The United States employs a coordinated approach through the Coordinated Framework for Regulation of Biotechnology, involving the FDA, EPA, and USDA. This system evaluates synthetic biology products based on their characteristics rather than the methods used to create them. In contrast, the European Union applies the precautionary principle more stringently, requiring extensive risk assessments before synthetic organisms can be released into the environment.
Risk assessment methodologies for synthetic biology require fundamental reconsideration compared to traditional biotechnology. Traditional methods typically involve well-characterized organisms with predictable behaviors, whereas synthetic biology creates novel organisms with potentially unpredictable interactions with natural ecosystems. This unpredictability necessitates more sophisticated containment strategies and monitoring protocols than those used for conventional GMOs.
Dual-use concerns present another significant regulatory challenge. The same synthetic biology tools that enable breakthrough medical treatments could potentially be misappropriated for bioterrorism. This dual-use dilemma has prompted calls for international oversight mechanisms that balance innovation with security considerations, similar to those in nuclear technology but adapted for the distributed nature of biological research.
Industry self-regulation has emerged as an important complement to formal regulatory frameworks. Organizations like the International Gene Synthesis Consortium have established screening protocols for DNA synthesis orders to prevent misuse. These voluntary measures demonstrate higher compliance rates compared to traditional biotechnology sectors, possibly due to the field's awareness of potential public backlash if safety issues arise.
The performance gap between synthetic biology and traditional methods is particularly evident in regulatory approval timelines. Novel synthetic biology applications often face regulatory reviews three times longer than comparable traditional biotechnology products, significantly impacting commercialization strategies and investment returns. This disparity highlights the need for regulatory frameworks specifically designed for synthetic biology's unique characteristics and risk profiles.
Economic Impact Assessment
Synthetic biology represents a paradigm shift in how we engineer biological systems, with significant economic implications across multiple sectors. When comparing the economic impact of synthetic biology to traditional methods, several key metrics demonstrate its transformative potential. Production costs for bio-manufactured compounds typically show a 40-60% reduction compared to conventional chemical synthesis, primarily due to decreased energy requirements and simpler purification processes. For instance, artemisinic acid production through synthetic biology pathways has reduced manufacturing costs by approximately 50%, making antimalarial treatments more accessible in developing regions.
The scalability economics of synthetic biology also present compelling advantages. While traditional fermentation and chemical synthesis methods often face diminishing returns at scale, synthetic biology platforms demonstrate improved economic efficiency with increasing production volumes. This is evidenced by the biofuels sector, where synthetic biology approaches have decreased production costs from $10/gallon to under $3/gallon over the past decade, approaching cost parity with fossil fuels in certain markets.
Market entry timelines represent another critical economic dimension. Synthetic biology accelerates product development cycles by an average of 18-24 months compared to traditional methods. This acceleration creates substantial first-mover advantages and extends effective patent lifetimes, significantly enhancing return on investment for R&D expenditures. The economic multiplier effect is particularly evident in the specialty chemicals sector, where synthetic biology has enabled the creation of entirely new product categories with premium pricing structures.
Resource utilization metrics further highlight synthetic biology's economic advantages. Traditional manufacturing methods typically require 2-5 times more water, energy, and raw material inputs per unit of production compared to optimized synthetic biology processes. This translates to substantial operational cost savings and reduced exposure to supply chain volatility. Additionally, waste management costs are typically reduced by 30-45% through the implementation of synthetic biology approaches.
The labor economics of synthetic biology present a mixed picture. While requiring fewer total labor hours per production unit, synthetic biology demands a more specialized workforce with higher compensation requirements. This shift creates economic opportunities in high-skill sectors while potentially reducing employment in traditional manufacturing roles. The net economic impact varies significantly by region, with developed economies generally experiencing greater economic benefits from this transition than developing economies with labor-intensive industrial bases.
The scalability economics of synthetic biology also present compelling advantages. While traditional fermentation and chemical synthesis methods often face diminishing returns at scale, synthetic biology platforms demonstrate improved economic efficiency with increasing production volumes. This is evidenced by the biofuels sector, where synthetic biology approaches have decreased production costs from $10/gallon to under $3/gallon over the past decade, approaching cost parity with fossil fuels in certain markets.
Market entry timelines represent another critical economic dimension. Synthetic biology accelerates product development cycles by an average of 18-24 months compared to traditional methods. This acceleration creates substantial first-mover advantages and extends effective patent lifetimes, significantly enhancing return on investment for R&D expenditures. The economic multiplier effect is particularly evident in the specialty chemicals sector, where synthetic biology has enabled the creation of entirely new product categories with premium pricing structures.
Resource utilization metrics further highlight synthetic biology's economic advantages. Traditional manufacturing methods typically require 2-5 times more water, energy, and raw material inputs per unit of production compared to optimized synthetic biology processes. This translates to substantial operational cost savings and reduced exposure to supply chain volatility. Additionally, waste management costs are typically reduced by 30-45% through the implementation of synthetic biology approaches.
The labor economics of synthetic biology present a mixed picture. While requiring fewer total labor hours per production unit, synthetic biology demands a more specialized workforce with higher compensation requirements. This shift creates economic opportunities in high-skill sectors while potentially reducing employment in traditional manufacturing roles. The net economic impact varies significantly by region, with developed economies generally experiencing greater economic benefits from this transition than developing economies with labor-intensive industrial bases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!