Evaluation of Synthetic Biology's Impact on Manufacturing Techniques
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Manufacturing Goals
Synthetic biology has evolved significantly since its conceptual emergence in the early 2000s, transitioning from theoretical frameworks to practical applications that are revolutionizing manufacturing processes across multiple industries. The field represents the convergence of biology, engineering, computer science, and chemistry, enabling the design and construction of new biological parts, devices, and systems, as well as the redesign of existing natural biological systems for useful purposes.
The evolution of synthetic biology can be traced through several distinct phases. Initially, researchers focused on creating standardized biological parts and simple genetic circuits. This foundation-building phase established the BioBrick standard and Registry of Standard Biological Parts, which enabled modular approaches to biological design. The second phase saw the development of more complex genetic circuits and metabolic pathways, allowing for programmed cellular behaviors and production of valuable compounds.
Currently, synthetic biology has entered a third phase characterized by whole-genome synthesis capabilities, CRISPR-based genome editing technologies, and advanced computational tools for biological design. These developments have dramatically expanded the scope and precision of biological engineering, making industrial applications increasingly viable.
In manufacturing contexts, synthetic biology aims to transform production paradigms by harnessing biological systems as manufacturing platforms. Key goals include developing sustainable biomanufacturing processes that reduce environmental impact compared to traditional chemical manufacturing methods. This involves creating biological systems capable of producing chemicals, materials, and pharmaceuticals with greater efficiency, specificity, and reduced waste generation.
Another critical objective is establishing scalable and standardized biological manufacturing platforms that can reliably produce consistent products at industrial scales. This requires overcoming challenges in process control, organism stability, and production optimization. The field also seeks to develop "cell factories" - engineered organisms optimized for specific manufacturing tasks with predictable performance characteristics.
Looking forward, synthetic biology aims to enable distributed manufacturing capabilities, where production can occur closer to points of use rather than in centralized facilities. This could revolutionize supply chains and reduce transportation-related environmental impacts. Additionally, there is significant interest in developing responsive manufacturing systems that can adapt to changing conditions or requirements without extensive reconfiguration.
The convergence of synthetic biology with other advanced technologies, including artificial intelligence, automation, and advanced materials science, represents a frontier with tremendous potential to transform manufacturing paradigms fundamentally. These integrated approaches may eventually enable manufacturing systems with unprecedented flexibility, sustainability, and capabilities beyond what traditional methods can achieve.
The evolution of synthetic biology can be traced through several distinct phases. Initially, researchers focused on creating standardized biological parts and simple genetic circuits. This foundation-building phase established the BioBrick standard and Registry of Standard Biological Parts, which enabled modular approaches to biological design. The second phase saw the development of more complex genetic circuits and metabolic pathways, allowing for programmed cellular behaviors and production of valuable compounds.
Currently, synthetic biology has entered a third phase characterized by whole-genome synthesis capabilities, CRISPR-based genome editing technologies, and advanced computational tools for biological design. These developments have dramatically expanded the scope and precision of biological engineering, making industrial applications increasingly viable.
In manufacturing contexts, synthetic biology aims to transform production paradigms by harnessing biological systems as manufacturing platforms. Key goals include developing sustainable biomanufacturing processes that reduce environmental impact compared to traditional chemical manufacturing methods. This involves creating biological systems capable of producing chemicals, materials, and pharmaceuticals with greater efficiency, specificity, and reduced waste generation.
Another critical objective is establishing scalable and standardized biological manufacturing platforms that can reliably produce consistent products at industrial scales. This requires overcoming challenges in process control, organism stability, and production optimization. The field also seeks to develop "cell factories" - engineered organisms optimized for specific manufacturing tasks with predictable performance characteristics.
Looking forward, synthetic biology aims to enable distributed manufacturing capabilities, where production can occur closer to points of use rather than in centralized facilities. This could revolutionize supply chains and reduce transportation-related environmental impacts. Additionally, there is significant interest in developing responsive manufacturing systems that can adapt to changing conditions or requirements without extensive reconfiguration.
The convergence of synthetic biology with other advanced technologies, including artificial intelligence, automation, and advanced materials science, represents a frontier with tremendous potential to transform manufacturing paradigms fundamentally. These integrated approaches may eventually enable manufacturing systems with unprecedented flexibility, sustainability, and capabilities beyond what traditional methods can achieve.
Market Demand Analysis for Bio-Manufacturing Solutions
The global bio-manufacturing market is experiencing unprecedented growth, driven by increasing demand for sustainable production methods and advanced biological solutions. Current market analyses indicate that the synthetic biology sector is projected to reach $30 billion by 2026, with bio-manufacturing applications representing approximately 40% of this value. This significant market expansion reflects the growing recognition of synthetic biology's potential to revolutionize traditional manufacturing processes across multiple industries.
Healthcare and pharmaceutical sectors currently dominate the demand landscape, with bio-manufactured therapeutics, vaccines, and diagnostic tools showing compound annual growth rates exceeding 15%. The COVID-19 pandemic has further accelerated this trend, highlighting the critical importance of rapid, scalable biological manufacturing capabilities for global health security.
Industrial biotechnology represents another major demand driver, as companies increasingly seek alternatives to petroleum-based chemical production. The market for bio-based chemicals and materials is growing at nearly 12% annually, with particular strength in biodegradable plastics, specialty enzymes, and sustainable textiles. Major chemical manufacturers have begun strategic pivots toward bio-based production platforms, recognizing both consumer demand for sustainable products and potential cost advantages through reduced dependence on volatile petroleum markets.
Agricultural applications constitute a third significant market segment, with bio-manufactured crop protection products, biofertilizers, and engineered seed treatments gaining traction. This sector's growth correlates strongly with increasing regulatory pressure on conventional agricultural chemicals and consumer preference for organic and sustainable farming practices.
Regional analysis reveals differentiated market dynamics, with North America leading in pharmaceutical applications, Europe focusing on sustainable materials and chemicals, and Asia-Pacific showing the fastest overall growth rate due to rapid industrialization coupled with sustainability initiatives. Developing economies are increasingly viewing bio-manufacturing as an opportunity to "leapfrog" traditional industrial development pathways.
Consumer goods companies represent an emerging demand center, with synthetic biology enabling novel product formulations in food, beverages, cosmetics, and household products. The "clean label" movement has accelerated interest in naturally derived ingredients that can be produced through precision fermentation and other bio-manufacturing techniques.
Market barriers include high initial capital requirements for bio-manufacturing facilities, regulatory uncertainties across different jurisdictions, and technical challenges in scaling production processes. However, these barriers are progressively diminishing as technology matures and regulatory frameworks evolve to accommodate bio-based manufacturing approaches.
Healthcare and pharmaceutical sectors currently dominate the demand landscape, with bio-manufactured therapeutics, vaccines, and diagnostic tools showing compound annual growth rates exceeding 15%. The COVID-19 pandemic has further accelerated this trend, highlighting the critical importance of rapid, scalable biological manufacturing capabilities for global health security.
Industrial biotechnology represents another major demand driver, as companies increasingly seek alternatives to petroleum-based chemical production. The market for bio-based chemicals and materials is growing at nearly 12% annually, with particular strength in biodegradable plastics, specialty enzymes, and sustainable textiles. Major chemical manufacturers have begun strategic pivots toward bio-based production platforms, recognizing both consumer demand for sustainable products and potential cost advantages through reduced dependence on volatile petroleum markets.
Agricultural applications constitute a third significant market segment, with bio-manufactured crop protection products, biofertilizers, and engineered seed treatments gaining traction. This sector's growth correlates strongly with increasing regulatory pressure on conventional agricultural chemicals and consumer preference for organic and sustainable farming practices.
Regional analysis reveals differentiated market dynamics, with North America leading in pharmaceutical applications, Europe focusing on sustainable materials and chemicals, and Asia-Pacific showing the fastest overall growth rate due to rapid industrialization coupled with sustainability initiatives. Developing economies are increasingly viewing bio-manufacturing as an opportunity to "leapfrog" traditional industrial development pathways.
Consumer goods companies represent an emerging demand center, with synthetic biology enabling novel product formulations in food, beverages, cosmetics, and household products. The "clean label" movement has accelerated interest in naturally derived ingredients that can be produced through precision fermentation and other bio-manufacturing techniques.
Market barriers include high initial capital requirements for bio-manufacturing facilities, regulatory uncertainties across different jurisdictions, and technical challenges in scaling production processes. However, these barriers are progressively diminishing as technology matures and regulatory frameworks evolve to accommodate bio-based manufacturing approaches.
Current State and Challenges in Synthetic Biology Manufacturing
Synthetic biology has evolved significantly over the past decade, transitioning from laboratory curiosities to viable manufacturing platforms. Currently, the field encompasses diverse approaches including cell-free systems, engineered microorganisms, and hybrid biotic-abiotic systems. The global synthetic biology market reached approximately $9.5 billion in 2022 and is projected to grow at a CAGR of 24% through 2030, with manufacturing applications representing a substantial portion of this growth.
Despite impressive advancements, synthetic biology manufacturing faces several critical challenges. Scalability remains a primary concern, as processes that function efficiently in laboratory settings often encounter difficulties when scaled to industrial production levels. Metabolic burden on engineered organisms frequently results in reduced yields and genetic instability during prolonged cultivation periods, compromising manufacturing consistency and economic viability.
Standardization represents another significant hurdle. Unlike traditional manufacturing, biological systems exhibit inherent variability and context-dependency that complicate the establishment of universal standards. The BioBricks Foundation and other organizations have attempted to create standardized biological parts, but their performance often varies across different chassis organisms and environmental conditions, limiting plug-and-play capabilities essential for advanced manufacturing.
Regulatory frameworks worldwide remain fragmented and underdeveloped for synthetic biology products. This regulatory uncertainty creates significant barriers to commercialization, with companies facing unclear approval pathways and inconsistent requirements across different jurisdictions. The novelty of many synthetic biology applications further complicates regulatory assessment, as existing frameworks may not adequately address the unique characteristics of these technologies.
Technical limitations persist in several areas. Precise control over gene expression remains challenging, with current inducible systems often exhibiting leaky expression or requiring expensive inducers unsuitable for large-scale manufacturing. Metabolic engineering tools, while improving, still struggle with predicting complex pathway interactions, leading to inefficient resource allocation within engineered organisms.
Infrastructure requirements present additional challenges. Synthetic biology manufacturing often demands specialized equipment and containment facilities that require substantial capital investment. The interdisciplinary nature of the field necessitates expertise spanning molecular biology, engineering, and data science—a combination that remains scarce in the current workforce.
Emerging concerns regarding biosecurity and biocontainment have also intensified scrutiny of synthetic biology manufacturing. Preventing horizontal gene transfer, ensuring containment of engineered organisms, and addressing potential environmental impacts remain active areas of research that directly impact manufacturing implementation.
Despite impressive advancements, synthetic biology manufacturing faces several critical challenges. Scalability remains a primary concern, as processes that function efficiently in laboratory settings often encounter difficulties when scaled to industrial production levels. Metabolic burden on engineered organisms frequently results in reduced yields and genetic instability during prolonged cultivation periods, compromising manufacturing consistency and economic viability.
Standardization represents another significant hurdle. Unlike traditional manufacturing, biological systems exhibit inherent variability and context-dependency that complicate the establishment of universal standards. The BioBricks Foundation and other organizations have attempted to create standardized biological parts, but their performance often varies across different chassis organisms and environmental conditions, limiting plug-and-play capabilities essential for advanced manufacturing.
Regulatory frameworks worldwide remain fragmented and underdeveloped for synthetic biology products. This regulatory uncertainty creates significant barriers to commercialization, with companies facing unclear approval pathways and inconsistent requirements across different jurisdictions. The novelty of many synthetic biology applications further complicates regulatory assessment, as existing frameworks may not adequately address the unique characteristics of these technologies.
Technical limitations persist in several areas. Precise control over gene expression remains challenging, with current inducible systems often exhibiting leaky expression or requiring expensive inducers unsuitable for large-scale manufacturing. Metabolic engineering tools, while improving, still struggle with predicting complex pathway interactions, leading to inefficient resource allocation within engineered organisms.
Infrastructure requirements present additional challenges. Synthetic biology manufacturing often demands specialized equipment and containment facilities that require substantial capital investment. The interdisciplinary nature of the field necessitates expertise spanning molecular biology, engineering, and data science—a combination that remains scarce in the current workforce.
Emerging concerns regarding biosecurity and biocontainment have also intensified scrutiny of synthetic biology manufacturing. Preventing horizontal gene transfer, ensuring containment of engineered organisms, and addressing potential environmental impacts remain active areas of research that directly impact manufacturing implementation.
Current Synthetic Biology Manufacturing Methodologies
01 Genetic engineering and DNA manipulation techniques
Advanced genetic engineering methods for synthetic biology manufacturing, including DNA synthesis, gene editing, and recombinant DNA technology. These techniques allow for the precise manipulation of genetic material to create novel biological systems with desired functions. The methods enable the design and construction of artificial genetic circuits, metabolic pathways, and engineered organisms for various applications in biotechnology and pharmaceutical production.- Genetic engineering and DNA synthesis techniques: Advanced genetic engineering methods for synthetic biology manufacturing, including DNA synthesis, gene editing, and genome assembly. These techniques enable the precise manipulation of genetic material to create novel biological systems with desired functions. The methods involve designing and constructing artificial genetic sequences that can be inserted into host organisms to produce specific proteins or metabolites.
- Bioreactor systems and fermentation processes: Specialized bioreactor systems and fermentation processes designed for synthetic biology applications. These manufacturing techniques focus on optimizing growth conditions, nutrient delivery, and process control to maximize the production of desired compounds by engineered organisms. The systems include continuous fermentation, fed-batch processes, and automated bioreactors that enable scalable production of biologics and other compounds.
- Cell-free synthesis platforms: Cell-free synthesis platforms that utilize biological machinery outside of living cells to produce proteins and other biomolecules. These systems extract cellular components necessary for protein synthesis while eliminating cellular barriers, allowing for rapid prototyping and production of difficult-to-express proteins. The technique provides greater control over reaction conditions and eliminates concerns related to cell viability and growth.
- Automated and high-throughput manufacturing systems: Automated and high-throughput systems for synthetic biology manufacturing that integrate robotics, microfluidics, and computational design. These technologies enable rapid testing of multiple designs, parallel processing, and increased reproducibility in bioengineering processes. The systems incorporate machine learning algorithms to optimize production parameters and reduce development time for new biological products.
- Biosensors and quality control methods: Biosensors and quality control methods specifically developed for synthetic biology manufacturing processes. These techniques monitor production parameters, detect contaminants, and verify product quality in real-time. The methods include genetic circuit-based biosensors that can detect metabolites, environmental conditions, or process deviations, allowing for adaptive control of manufacturing processes and ensuring consistent product quality.
02 Cell-free synthetic biology systems
Manufacturing techniques that utilize cell-free systems for biological production, bypassing the limitations of whole-cell approaches. These systems extract and purify cellular machinery for protein synthesis and metabolic reactions outside of living cells. Cell-free platforms offer advantages including rapid prototyping, simplified process control, and the ability to produce compounds that might be toxic to living cells, enabling more efficient and scalable biomanufacturing processes.Expand Specific Solutions03 Automated biofoundry platforms
Integrated automation systems for high-throughput synthetic biology manufacturing, combining robotics, machine learning, and standardized workflows. These biofoundry platforms enable rapid design-build-test-learn cycles for biological systems, increasing efficiency and reproducibility in bioengineering. The technology incorporates liquid handling robots, automated analytical equipment, and computational tools to streamline the development of engineered biological products and processes.Expand Specific Solutions04 Microbial fermentation and bioprocess engineering
Advanced fermentation techniques using engineered microorganisms for the production of chemicals, materials, and pharmaceuticals. These manufacturing methods leverage synthetic biology to optimize microbial strains for improved yield, selectivity, and robustness in industrial bioprocesses. The approaches include continuous fermentation systems, precision bioreactor control, and metabolic engineering strategies to enhance the efficiency of converting renewable feedstocks into valuable products.Expand Specific Solutions05 Biosensors and feedback control systems
Integration of biosensing technologies with synthetic biology manufacturing to enable real-time monitoring and control of bioprocesses. These systems incorporate engineered genetic circuits that respond to specific environmental conditions or metabolic states, allowing for dynamic regulation of biological production. The technology enables adaptive manufacturing processes that can automatically adjust to changing conditions, improving consistency, yield, and quality of biologically produced compounds.Expand Specific Solutions
Key Industry Players and Competitive Landscape
Synthetic biology's impact on manufacturing is evolving rapidly, currently transitioning from early-stage development to commercial application. The market is projected to reach $30 billion by 2026, growing at 24% CAGR, driven by biopharmaceuticals, sustainable chemicals, and materials production. Leading companies demonstrate varying levels of technological maturity: Amyris has established commercial-scale fermentation platforms, while Inscripta and Cellectis are advancing genome engineering tools. Academic powerhouses like MIT, Johns Hopkins, and Nanyang Technological University collaborate with industry leaders including Agilent Technologies and Microsoft to bridge research-application gaps. Chinese institutions such as Tianjin University and the Academy of Military Medical Sciences are rapidly expanding capabilities, indicating the global competitive landscape is diversifying beyond traditional Western dominance.
Amyris, Inc.
Technical Solution: Amyris has developed a proprietary synthetic biology platform that leverages engineered microorganisms to convert plant-sourced sugars into high-value molecules. Their manufacturing technique employs advanced fermentation processes where genetically modified yeast strains produce targeted compounds that traditionally would require complex chemical synthesis or extraction from rare natural sources. The company has successfully commercialized this approach for producing sustainable alternatives to petroleum-derived products, cosmetic ingredients, and pharmaceutical precursors. Their platform includes proprietary computational tools for strain design, high-throughput screening capabilities, and automated lab processes that accelerate development cycles from concept to commercial production[1][3]. Amyris has notably achieved industrial-scale production of artemisinic acid (a precursor to antimalarial drugs) and various sustainable hydrocarbon molecules, demonstrating yields that make these bio-based alternatives economically competitive with conventional manufacturing methods.
Strengths: Highly optimized fermentation processes with demonstrated commercial viability; proprietary strain engineering platform enables rapid development of new biomanufacturing pathways; reduced environmental footprint compared to traditional chemical manufacturing. Weaknesses: Dependence on agricultural feedstocks subject to price volatility; scaling challenges for certain complex molecules; higher production costs compared to some petroleum-based alternatives for certain applications.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered transformative synthetic biology manufacturing techniques through its Synthetic Biology Center and related research groups. Their approach integrates advanced genetic circuit design with novel biofabrication methods to create programmable biological systems for manufacturing applications. MIT researchers have developed standardized genetic parts and assembly methods (BioBricks) that enable modular construction of synthetic biological systems with predictable functions. Their manufacturing platform incorporates CRISPR-based genome editing tools for precise genetic modifications and cell-free protein synthesis systems that bypass limitations of living cells[2][5]. MIT has demonstrated applications in biopharmaceutical production, sustainable materials manufacturing, and biosensing technologies. Their research includes innovative bioreactor designs that optimize production conditions and yield for engineered biological systems, as well as computational tools that model and predict biological system performance under manufacturing conditions. MIT's approach emphasizes scalability and reproducibility to bridge the gap between laboratory demonstrations and industrial implementation.
Strengths: World-leading expertise in genetic circuit design and standardization; strong integration of computational modeling with experimental validation; innovative approaches to overcome traditional limitations of biological manufacturing. Weaknesses: Some technologies remain at early research stages requiring further development for industrial deployment; complexity of engineered biological systems can lead to unpredictable behaviors in scaled manufacturing environments.
Core Patents and Innovations in Bio-Manufacturing
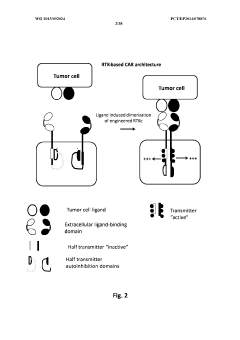
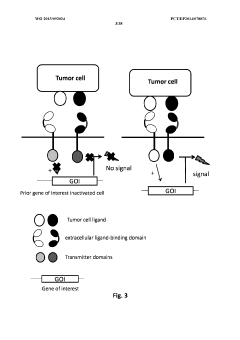
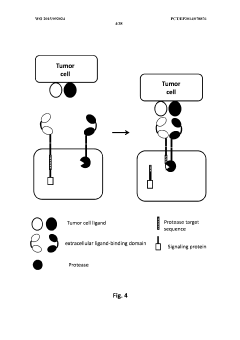
Method of engineering multi-input signal sensitive t cell for immunotherapy
PatentWO2015092024A2
Innovation
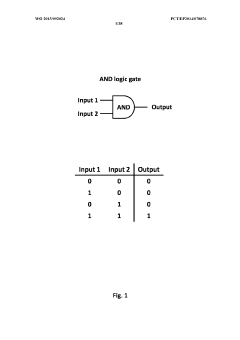
- Engineering immune cells with a logic 'AND GATE' system where activation requires the combination of at least two input signals, utilizing modular AND gate design in synthetic biology principles to ensure safe and specific activation of immune cells only when both signals are present, preventing activation by single antigen binding.
Synthetic promoters generated based on genomic DNA sequences
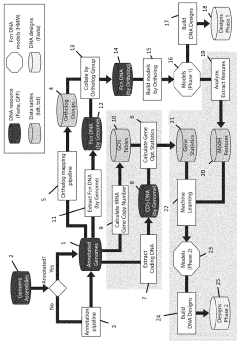
PatentWO2023135151A1
Innovation
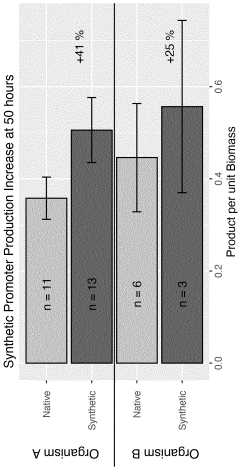
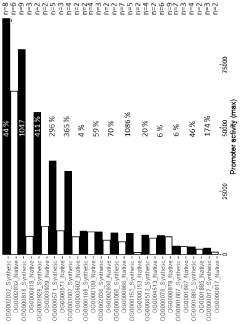
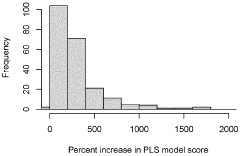
- A computer-implemented method that uses gene sequences from multiple closely related species to identify homolog groups, calculate gene statistics, and build hidden Markov models to generate synthetic promoters and terminators that are scalable, efficient, and adaptable, optimizing DNA contexts for improved gene expression.
Regulatory Framework and Biosafety Considerations
The regulatory landscape surrounding synthetic biology in manufacturing is evolving rapidly as technologies advance beyond existing frameworks. Current regulations primarily operate within three interconnected domains: biosafety protocols, intellectual property rights, and product approval pathways. The United States FDA has established the Coordinated Framework for Regulation of Biotechnology, which distributes oversight across multiple agencies including the EPA and USDA, creating a complex regulatory environment that manufacturers must navigate. Similarly, the European Union employs the precautionary principle through directives like 2001/18/EC, imposing stringent risk assessment requirements before market authorization.
Biosafety considerations represent a critical dimension in synthetic biology manufacturing. The Cartagena Protocol on Biosafety, ratified by 173 countries, establishes international standards for the safe handling, transport, and use of living modified organisms. Manufacturing facilities implementing synthetic biology techniques must adhere to biosafety levels (BSL-1 through BSL-4) based on risk assessments of their biological systems. Containment strategies, including physical barriers, biological containment through engineered kill switches, and geographical isolation, are becoming standard practice in industrial applications.
Risk assessment methodologies for synthetic biology applications have evolved toward more sophisticated quantitative approaches. These include computational modeling of potential ecological impacts, experimental validation in controlled environments, and post-release monitoring protocols. The iGEM Safety Committee has developed standardized risk assessment tools specifically for synthetic biology applications that are gaining adoption across the industry.
Harmonization of international regulatory frameworks remains a significant challenge. Disparities between regions create regulatory arbitrage opportunities while potentially compromising safety standards. The OECD Working Group on Harmonisation of Regulatory Oversight in Biotechnology is attempting to address these inconsistencies by developing consensus documents and best practices for member countries.
Emerging self-governance initiatives within the synthetic biology community complement formal regulations. The International Gene Synthesis Consortium (IGSC) has established screening protocols for DNA synthesis orders to prevent misuse. Additionally, responsible innovation frameworks like RRI (Responsible Research and Innovation) are being integrated into corporate governance structures of synthetic biology manufacturers, emphasizing stakeholder engagement and ethical considerations throughout product development cycles.
Public perception and stakeholder engagement have become increasingly important components of regulatory compliance. Manufacturers are recognizing that social license to operate requires transparency about risk management practices and meaningful dialogue with communities potentially affected by synthetic biology applications.
Biosafety considerations represent a critical dimension in synthetic biology manufacturing. The Cartagena Protocol on Biosafety, ratified by 173 countries, establishes international standards for the safe handling, transport, and use of living modified organisms. Manufacturing facilities implementing synthetic biology techniques must adhere to biosafety levels (BSL-1 through BSL-4) based on risk assessments of their biological systems. Containment strategies, including physical barriers, biological containment through engineered kill switches, and geographical isolation, are becoming standard practice in industrial applications.
Risk assessment methodologies for synthetic biology applications have evolved toward more sophisticated quantitative approaches. These include computational modeling of potential ecological impacts, experimental validation in controlled environments, and post-release monitoring protocols. The iGEM Safety Committee has developed standardized risk assessment tools specifically for synthetic biology applications that are gaining adoption across the industry.
Harmonization of international regulatory frameworks remains a significant challenge. Disparities between regions create regulatory arbitrage opportunities while potentially compromising safety standards. The OECD Working Group on Harmonisation of Regulatory Oversight in Biotechnology is attempting to address these inconsistencies by developing consensus documents and best practices for member countries.
Emerging self-governance initiatives within the synthetic biology community complement formal regulations. The International Gene Synthesis Consortium (IGSC) has established screening protocols for DNA synthesis orders to prevent misuse. Additionally, responsible innovation frameworks like RRI (Responsible Research and Innovation) are being integrated into corporate governance structures of synthetic biology manufacturers, emphasizing stakeholder engagement and ethical considerations throughout product development cycles.
Public perception and stakeholder engagement have become increasingly important components of regulatory compliance. Manufacturers are recognizing that social license to operate requires transparency about risk management practices and meaningful dialogue with communities potentially affected by synthetic biology applications.
Environmental Impact and Sustainability Assessment
Synthetic biology represents a paradigm shift in manufacturing with significant environmental implications that warrant comprehensive assessment. The integration of biological systems into industrial processes offers potential advantages in resource efficiency compared to traditional chemical manufacturing. Studies indicate that bio-based production routes can reduce greenhouse gas emissions by 30-50% for certain compounds when compared to petrochemical alternatives. This efficiency stems from synthetic biology's ability to operate at ambient temperatures and pressures, substantially decreasing energy requirements.
Water consumption patterns in synthetic biology manufacturing show mixed results. While fermentation processes typically require significant water inputs, advanced closed-loop systems have demonstrated water usage reductions of up to 60% compared to first-generation bioproduction facilities. The development of drought-resistant engineered organisms further promises to minimize water footprints in future applications.
Waste stream characteristics from synthetic biology manufacturing differ fundamentally from conventional processes. Biological waste is generally biodegradable, reducing persistent environmental contamination risks. However, the release of genetically modified organisms (GMOs) presents unique containment challenges requiring robust biosafety protocols. Current industry standards implement multiple redundant containment systems with failure rates below 0.01%, though long-term ecological impacts remain under investigation.
Land use considerations reveal complex sustainability trade-offs. While synthetic biology can utilize agricultural feedstocks that compete with food production, recent advances in utilizing lignocellulosic biomass and agricultural waste streams mitigate these concerns. Lifecycle analyses demonstrate that second-generation feedstock approaches can reduce land use requirements by 70-85% compared to first-generation biofuels.
Chemical input profiles show synthetic biology manufacturing typically requires fewer hazardous substances than conventional chemical synthesis. The replacement of metal catalysts with enzymatic processes has reduced heavy metal waste by approximately 40% in pharmaceutical manufacturing applications. Additionally, the precision of biological systems often eliminates the need for harsh solvents and reactive intermediates.
Circular economy integration represents perhaps the most promising sustainability aspect of synthetic biology manufacturing. Engineered biological systems can be designed to valorize waste streams, creating closed-loop industrial ecosystems. Pilot projects have demonstrated successful conversion of industrial carbon dioxide emissions into valuable compounds with carbon fixation efficiencies approaching 75% under optimized conditions.
Regulatory frameworks for environmental assessment of synthetic biology applications remain in development globally, with significant regional variations in approach. The EU's precautionary principle contrasts with the more product-focused regulatory stance in the United States, creating compliance challenges for international deployment of synthetic biology manufacturing technologies.
Water consumption patterns in synthetic biology manufacturing show mixed results. While fermentation processes typically require significant water inputs, advanced closed-loop systems have demonstrated water usage reductions of up to 60% compared to first-generation bioproduction facilities. The development of drought-resistant engineered organisms further promises to minimize water footprints in future applications.
Waste stream characteristics from synthetic biology manufacturing differ fundamentally from conventional processes. Biological waste is generally biodegradable, reducing persistent environmental contamination risks. However, the release of genetically modified organisms (GMOs) presents unique containment challenges requiring robust biosafety protocols. Current industry standards implement multiple redundant containment systems with failure rates below 0.01%, though long-term ecological impacts remain under investigation.
Land use considerations reveal complex sustainability trade-offs. While synthetic biology can utilize agricultural feedstocks that compete with food production, recent advances in utilizing lignocellulosic biomass and agricultural waste streams mitigate these concerns. Lifecycle analyses demonstrate that second-generation feedstock approaches can reduce land use requirements by 70-85% compared to first-generation biofuels.
Chemical input profiles show synthetic biology manufacturing typically requires fewer hazardous substances than conventional chemical synthesis. The replacement of metal catalysts with enzymatic processes has reduced heavy metal waste by approximately 40% in pharmaceutical manufacturing applications. Additionally, the precision of biological systems often eliminates the need for harsh solvents and reactive intermediates.
Circular economy integration represents perhaps the most promising sustainability aspect of synthetic biology manufacturing. Engineered biological systems can be designed to valorize waste streams, creating closed-loop industrial ecosystems. Pilot projects have demonstrated successful conversion of industrial carbon dioxide emissions into valuable compounds with carbon fixation efficiencies approaching 75% under optimized conditions.
Regulatory frameworks for environmental assessment of synthetic biology applications remain in development globally, with significant regional variations in approach. The EU's precautionary principle contrasts with the more product-focused regulatory stance in the United States, creating compliance challenges for international deployment of synthetic biology manufacturing technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!