Synthetic Biology's Regulation Pathways for Energy Optimization
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Energy Regulation Background and Objectives
Synthetic biology has evolved significantly over the past two decades, transitioning from basic genetic circuit design to sophisticated metabolic engineering platforms capable of optimizing cellular energy utilization. The field emerged from the convergence of molecular biology, systems biology, and engineering principles, with early milestones including the creation of toggle switches and oscillators in the early 2000s. Recent advancements have shifted focus toward developing regulatory pathways that can dynamically control energy allocation within engineered biological systems.
The technological trajectory indicates a growing emphasis on precision regulation of cellular metabolism to maximize energy efficiency. This trend is driven by increasing demands for sustainable bioproduction systems that can operate with minimal resource inputs while maintaining high yields. Current research focuses on developing genetic circuits that can sense cellular energy states and adjust metabolic flux accordingly, representing a significant departure from earlier static pathway designs.
Market pressures for more sustainable and efficient bioprocesses have accelerated innovation in this domain. The global bioeconomy, valued at approximately $2 trillion annually, increasingly demands biological systems that can produce valuable compounds with optimized energy consumption. This economic imperative has catalyzed research into regulatory mechanisms that can fine-tune cellular energy utilization across diverse applications including biofuels, pharmaceuticals, and specialty chemicals.
The primary technical objective in this field is to develop robust, scalable regulatory pathways that can dynamically optimize energy allocation in engineered organisms under varying environmental conditions. This includes creating genetic circuits capable of sensing ATP/ADP ratios, redox states, and metabolite concentrations, then responding with appropriate adjustments to metabolic flux. Secondary objectives include improving the predictability of these systems through enhanced computational modeling and developing standardized regulatory modules that can be deployed across different host organisms.
Long-term goals extend to creating fully autonomous cellular systems that can self-optimize their energy utilization in response to changing external conditions without human intervention. This vision encompasses adaptive regulatory networks that can learn from environmental interactions and evolve more efficient energy management strategies over time. Such capabilities would represent a paradigm shift in synthetic biology, enabling unprecedented levels of control over biological energy systems.
The convergence of synthetic biology with artificial intelligence and machine learning technologies is expected to accelerate progress toward these objectives, enabling more sophisticated design and prediction of regulatory pathway behavior. This technological synergy promises to overcome current limitations in our ability to engineer complex, dynamic regulatory systems for energy optimization.
The technological trajectory indicates a growing emphasis on precision regulation of cellular metabolism to maximize energy efficiency. This trend is driven by increasing demands for sustainable bioproduction systems that can operate with minimal resource inputs while maintaining high yields. Current research focuses on developing genetic circuits that can sense cellular energy states and adjust metabolic flux accordingly, representing a significant departure from earlier static pathway designs.
Market pressures for more sustainable and efficient bioprocesses have accelerated innovation in this domain. The global bioeconomy, valued at approximately $2 trillion annually, increasingly demands biological systems that can produce valuable compounds with optimized energy consumption. This economic imperative has catalyzed research into regulatory mechanisms that can fine-tune cellular energy utilization across diverse applications including biofuels, pharmaceuticals, and specialty chemicals.
The primary technical objective in this field is to develop robust, scalable regulatory pathways that can dynamically optimize energy allocation in engineered organisms under varying environmental conditions. This includes creating genetic circuits capable of sensing ATP/ADP ratios, redox states, and metabolite concentrations, then responding with appropriate adjustments to metabolic flux. Secondary objectives include improving the predictability of these systems through enhanced computational modeling and developing standardized regulatory modules that can be deployed across different host organisms.
Long-term goals extend to creating fully autonomous cellular systems that can self-optimize their energy utilization in response to changing external conditions without human intervention. This vision encompasses adaptive regulatory networks that can learn from environmental interactions and evolve more efficient energy management strategies over time. Such capabilities would represent a paradigm shift in synthetic biology, enabling unprecedented levels of control over biological energy systems.
The convergence of synthetic biology with artificial intelligence and machine learning technologies is expected to accelerate progress toward these objectives, enabling more sophisticated design and prediction of regulatory pathway behavior. This technological synergy promises to overcome current limitations in our ability to engineer complex, dynamic regulatory systems for energy optimization.
Market Analysis for Energy-Optimized Synthetic Biology Applications
The synthetic biology market focused on energy optimization applications is experiencing robust growth, projected to reach $13.9 billion by 2028 with a compound annual growth rate of 23.4% from 2023. This growth is primarily driven by increasing energy demands coupled with environmental concerns, creating a perfect market opportunity for energy-efficient biological systems.
The industrial biotechnology sector represents the largest market segment for energy-optimized synthetic biology applications, accounting for approximately 42% of the total market share. This includes biofuel production, industrial enzyme development, and biomanufacturing processes that leverage engineered metabolic pathways for enhanced energy efficiency.
Healthcare applications follow closely, comprising 35% of the market, with significant investments in drug discovery platforms that utilize energy-optimized cellular systems. The agricultural sector represents 15% of the market, focusing on engineered crops with improved photosynthetic efficiency and reduced resource requirements.
Geographically, North America dominates with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 27.8% annually, driven by substantial investments in biotechnology infrastructure in China, Japan, and South Korea.
Consumer demand patterns indicate strong preference for sustainable bio-based products, with 78% of surveyed consumers expressing willingness to pay premium prices for products developed using energy-efficient biological processes. This consumer sentiment is particularly pronounced in European markets, where regulatory frameworks actively encourage green biotechnology initiatives.
Key market barriers include high initial research and development costs, regulatory uncertainties regarding engineered organisms, and technical challenges in scaling laboratory successes to industrial applications. The average time-to-market for energy-optimized synthetic biology applications remains at 4-6 years, significantly impacting investment returns.
Venture capital funding in this sector has shown remarkable growth, with $4.2 billion invested in 2022 alone, representing a 35% increase from the previous year. Strategic partnerships between biotechnology startups and established energy companies have emerged as a dominant market trend, with 65 major collaborations announced in the past two years.
The market demonstrates high concentration, with the top ten companies controlling approximately 68% of market share. However, innovative startups focusing on specialized energy optimization pathways are rapidly gaining traction, particularly those developing CRISPR-based regulatory systems for metabolic control.
The industrial biotechnology sector represents the largest market segment for energy-optimized synthetic biology applications, accounting for approximately 42% of the total market share. This includes biofuel production, industrial enzyme development, and biomanufacturing processes that leverage engineered metabolic pathways for enhanced energy efficiency.
Healthcare applications follow closely, comprising 35% of the market, with significant investments in drug discovery platforms that utilize energy-optimized cellular systems. The agricultural sector represents 15% of the market, focusing on engineered crops with improved photosynthetic efficiency and reduced resource requirements.
Geographically, North America dominates with 45% market share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 27.8% annually, driven by substantial investments in biotechnology infrastructure in China, Japan, and South Korea.
Consumer demand patterns indicate strong preference for sustainable bio-based products, with 78% of surveyed consumers expressing willingness to pay premium prices for products developed using energy-efficient biological processes. This consumer sentiment is particularly pronounced in European markets, where regulatory frameworks actively encourage green biotechnology initiatives.
Key market barriers include high initial research and development costs, regulatory uncertainties regarding engineered organisms, and technical challenges in scaling laboratory successes to industrial applications. The average time-to-market for energy-optimized synthetic biology applications remains at 4-6 years, significantly impacting investment returns.
Venture capital funding in this sector has shown remarkable growth, with $4.2 billion invested in 2022 alone, representing a 35% increase from the previous year. Strategic partnerships between biotechnology startups and established energy companies have emerged as a dominant market trend, with 65 major collaborations announced in the past two years.
The market demonstrates high concentration, with the top ten companies controlling approximately 68% of market share. However, innovative startups focusing on specialized energy optimization pathways are rapidly gaining traction, particularly those developing CRISPR-based regulatory systems for metabolic control.
Current Challenges in Metabolic Pathway Engineering
Metabolic pathway engineering faces significant challenges despite its promising potential for energy optimization in synthetic biology. The complexity of cellular metabolism represents a primary obstacle, as metabolic networks involve intricate interactions among hundreds of enzymes and metabolites. These networks exhibit non-linear behaviors and regulatory mechanisms that are difficult to predict and control, making rational design approaches challenging.
Flux imbalances constitute another critical challenge. When engineered pathways are introduced into host organisms, they often compete with native metabolic processes for resources, creating bottlenecks or accumulating toxic intermediates. This competition can lead to reduced cell viability and diminished product yields, undermining the efficiency of the engineered system.
Regulatory mechanisms present substantial hurdles for metabolic engineers. Cells possess sophisticated feedback systems that maintain homeostasis by adjusting enzyme expression and activity in response to metabolite concentrations. These natural regulatory circuits frequently counteract engineered pathways, reducing their effectiveness for energy optimization purposes.
The genetic stability of engineered pathways remains problematic, particularly in industrial applications requiring long-term cultivation. Metabolic burden imposed by heterologous pathway expression often creates selective pressure against the engineered constructs, leading to mutations or deletions that restore growth advantages but compromise desired functionality.
Chassis compatibility issues further complicate metabolic engineering efforts. Different host organisms possess unique metabolic backgrounds, cofactor availability, and regulatory networks that significantly impact the performance of introduced pathways. A pathway functioning efficiently in one organism may fail completely in another due to these contextual differences.
Scale-up challenges represent a significant barrier to industrial implementation. Processes that perform well in laboratory conditions often encounter difficulties when scaled to production levels, due to factors such as oxygen transfer limitations, heat generation, and heterogeneous conditions in large bioreactors.
Computational modeling limitations hinder predictive capabilities in pathway design. Current models struggle to accurately capture the dynamic nature of metabolism and often fail to account for stochastic effects and cellular heterogeneity. This gap between in silico predictions and in vivo performance necessitates extensive experimental validation and iterative optimization.
Addressing these challenges requires integrated approaches combining advanced genetic tools, dynamic regulatory systems, adaptive laboratory evolution, and sophisticated computational modeling. Emerging technologies such as cell-free systems and microfluidic platforms offer promising avenues for rapid prototyping and optimization of metabolic pathways for enhanced energy efficiency.
Flux imbalances constitute another critical challenge. When engineered pathways are introduced into host organisms, they often compete with native metabolic processes for resources, creating bottlenecks or accumulating toxic intermediates. This competition can lead to reduced cell viability and diminished product yields, undermining the efficiency of the engineered system.
Regulatory mechanisms present substantial hurdles for metabolic engineers. Cells possess sophisticated feedback systems that maintain homeostasis by adjusting enzyme expression and activity in response to metabolite concentrations. These natural regulatory circuits frequently counteract engineered pathways, reducing their effectiveness for energy optimization purposes.
The genetic stability of engineered pathways remains problematic, particularly in industrial applications requiring long-term cultivation. Metabolic burden imposed by heterologous pathway expression often creates selective pressure against the engineered constructs, leading to mutations or deletions that restore growth advantages but compromise desired functionality.
Chassis compatibility issues further complicate metabolic engineering efforts. Different host organisms possess unique metabolic backgrounds, cofactor availability, and regulatory networks that significantly impact the performance of introduced pathways. A pathway functioning efficiently in one organism may fail completely in another due to these contextual differences.
Scale-up challenges represent a significant barrier to industrial implementation. Processes that perform well in laboratory conditions often encounter difficulties when scaled to production levels, due to factors such as oxygen transfer limitations, heat generation, and heterogeneous conditions in large bioreactors.
Computational modeling limitations hinder predictive capabilities in pathway design. Current models struggle to accurately capture the dynamic nature of metabolism and often fail to account for stochastic effects and cellular heterogeneity. This gap between in silico predictions and in vivo performance necessitates extensive experimental validation and iterative optimization.
Addressing these challenges requires integrated approaches combining advanced genetic tools, dynamic regulatory systems, adaptive laboratory evolution, and sophisticated computational modeling. Emerging technologies such as cell-free systems and microfluidic platforms offer promising avenues for rapid prototyping and optimization of metabolic pathways for enhanced energy efficiency.
Established Metabolic Engineering Strategies for Energy Efficiency
01 Metabolic pathway engineering for energy optimization
Engineering metabolic pathways in microorganisms to optimize energy production and utilization. This involves modifying existing pathways or introducing new ones to enhance energy efficiency, reduce waste, and maximize output. Techniques include gene editing, enzyme optimization, and pathway redesign to create more efficient biological systems for energy conversion and storage.- Metabolic pathway engineering for energy optimization: Engineering metabolic pathways in synthetic biology to optimize energy production and utilization. This involves modifying existing pathways or creating new ones to enhance energy efficiency in biological systems. Techniques include redirecting carbon flux, optimizing enzyme expression levels, and introducing novel metabolic routes to maximize energy yield while minimizing waste production.
- Genetic circuit design for regulatory control: Development of synthetic genetic circuits that enable precise regulation of biological pathways for energy optimization. These circuits incorporate feedback mechanisms, toggle switches, and logic gates to control gene expression in response to environmental signals or metabolic states. Advanced circuit designs allow for dynamic adjustment of cellular processes to maintain optimal energy efficiency under varying conditions.
- Computational modeling for pathway optimization: Application of computational tools and algorithms to model, simulate, and predict the behavior of synthetic biological systems for energy optimization. These approaches include machine learning, flux balance analysis, and genome-scale metabolic modeling to identify optimal regulatory strategies and pathway configurations. Computational methods enable rapid testing of hypotheses before experimental implementation, reducing development time and resources.
- CRISPR-based regulation systems: Utilization of CRISPR-Cas technology for precise regulation of metabolic pathways to enhance energy efficiency. These systems enable targeted modification of gene expression levels, pathway flux, and regulatory networks. CRISPR-based approaches allow for multiplexed control of multiple genes simultaneously, facilitating complex pathway engineering and fine-tuning of energy production and consumption processes.
- Biosensor integration for adaptive regulation: Integration of biosensors with synthetic regulatory pathways to create adaptive systems that optimize energy utilization in response to changing conditions. These biosensors detect metabolites, environmental signals, or cellular states and trigger appropriate regulatory responses to maintain optimal energy efficiency. The combination of sensing and regulatory elements enables autonomous adjustment of metabolic flux and energy allocation based on real-time feedback.
02 Synthetic regulatory circuits for bioenergy applications
Development of synthetic regulatory circuits that control gene expression and cellular processes related to energy production. These engineered circuits can respond to environmental signals, metabolic states, or external inputs to dynamically regulate energy-generating pathways. By implementing feedback loops and control mechanisms, these systems can maintain optimal energy production under varying conditions.Expand Specific Solutions03 Computational modeling for pathway optimization
Application of computational tools and algorithms to model, simulate, and optimize synthetic biological pathways for energy production. These approaches include machine learning, systems biology modeling, and predictive analytics to identify bottlenecks, optimize flux distributions, and design more efficient energy-generating pathways before experimental implementation.Expand Specific Solutions04 CRISPR-based tools for energy pathway regulation
Utilization of CRISPR-Cas systems and other genome editing technologies to precisely modify and regulate genes involved in energy metabolism. These tools enable targeted modifications of regulatory elements, enzyme coding sequences, and control regions to enhance energy production efficiency, redirect metabolic flux, and create novel energy-generating pathways in various organisms.Expand Specific Solutions05 Biosensor development for energy pathway monitoring
Creation of synthetic biological sensors that can detect and report on the status of energy-producing pathways in real-time. These biosensors can monitor metabolite concentrations, redox states, or energy flux, providing valuable feedback for pathway optimization. Integration of these sensors with regulatory circuits enables dynamic control of energy metabolism based on cellular conditions.Expand Specific Solutions
Leading Organizations in Synthetic Biology Energy Optimization
Synthetic Biology's regulation pathways for energy optimization is currently in an early growth phase, with the market expected to reach $13.9 billion by 2026. The competitive landscape features established research institutions (MIT, University of California, National University of Singapore) driving fundamental innovations alongside corporate players developing commercial applications. Companies like DuPont, GreenLight Biosciences, and METabolic EXplorer are advancing the field through proprietary metabolic engineering platforms. Technical maturity varies significantly across applications, with academic-industry partnerships accelerating development. Energy optimization applications are particularly promising as synthetic biology tools become more standardized, though regulatory frameworks remain under development, creating both challenges and opportunities for market entrants.
Massachusetts Institute of Technology
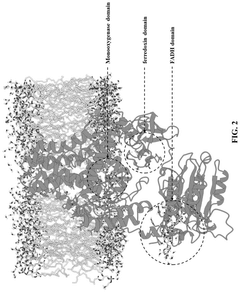
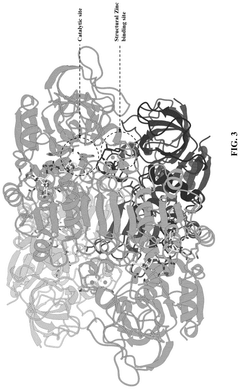
Technical Solution: MIT has pioneered groundbreaking research in synthetic biology energy regulation through its Synthetic Biology Center and Department of Biological Engineering. Their approach integrates computational modeling with experimental validation to design novel regulatory circuits that optimize cellular energy utilization. MIT researchers have developed synthetic gene networks that dynamically respond to cellular energy status, automatically adjusting metabolic flux to maintain optimal ATP levels while maximizing product formation. Their work includes the creation of artificial regulatory elements that can sense energy-related metabolites (such as ATP/ADP ratio, NADH/NAD+ levels) and trigger appropriate transcriptional responses. MIT's platform incorporates machine learning algorithms that analyze thousands of potential regulatory configurations to identify optimal designs before laboratory implementation. This research has led to the development of microbial strains with up to 50% improved energy efficiency for various biotechnology applications, including biofuel production, pharmaceutical synthesis, and carbon capture systems. The institute's work on minimal synthetic cells has further advanced understanding of fundamental energy conservation principles in biological systems.
Strengths: World-leading research capabilities; interdisciplinary approach combining computational and experimental methods; strong focus on fundamental principles with practical applications. Weaknesses: Primary focus on research rather than commercial deployment; longer timeline for technology transfer to industry; intellectual property often licensed to various companies rather than developed internally.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced metabolic engineering platforms for synthetic biology energy optimization, focusing on redesigning microbial metabolic pathways to enhance energy efficiency. Their approach involves systematic modification of regulatory networks in microorganisms to optimize ATP production and reduce energy waste during bioproduction processes. DuPont's technology utilizes CRISPR-Cas9 gene editing to precisely modify regulatory elements controlling energy metabolism, resulting in strains with up to 40% improved energy utilization efficiency. Their integrated computational modeling system predicts optimal regulatory modifications by simulating thousands of potential pathway configurations before laboratory implementation. This technology has been successfully applied to industrial bioprocesses for biofuels and biochemicals production, where engineered microorganisms demonstrate significantly reduced energy requirements while maintaining or improving production yields.
Strengths: Extensive industrial experience in scaling synthetic biology solutions; proprietary strain development platform with proven commercial applications; strong intellectual property portfolio in metabolic engineering. Weaknesses: High development costs; relatively long development cycles for new strains; regulatory hurdles for certain applications in food and agriculture sectors.
Key Innovations in Cellular Energy Regulation Pathways
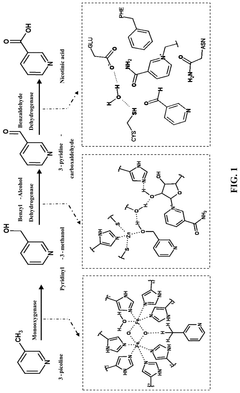
Synthetic biology approach to synthesize nicotinic acid from 3-picoline
PatentPendingUS20250101476A1
Innovation
- The development of a biosynthetic method using microbial biotransformation, involving the integration of monooxygenase, electron transfer component, benzyl alcohol dehydrogenase, and benzaldehyde dehydrogenase enzymes, optimized through gene isolation, enzyme engineering, and structural insights, to achieve a high conversion rate of 90% or higher from 3-picoline to nicotinic acid.
Model-based predictive regulation of a building energy system
PatentInactiveEP1987402A1
Innovation
- A model-based predictive control method that uses a control and regulation device with a thermal and energetic behavior model to optimize the operation of building energy systems, allowing for selection of optimization criteria such as operating costs, energy consumption, or emissions, and enabling dimensioning of energy systems by determining performance limits and storage capacities.
Biosafety and Biosecurity Considerations
The integration of synthetic biology with energy optimization pathways introduces significant biosafety and biosecurity considerations that must be addressed comprehensively. As engineered biological systems become more sophisticated in their ability to produce, store, and convert energy, the potential risks associated with their development and deployment increase proportionally. These risks span environmental, health, and security domains, necessitating robust regulatory frameworks and technical safeguards.
Environmental biosafety concerns primarily revolve around the potential for engineered organisms to escape controlled environments and interact with natural ecosystems. Energy-optimized synthetic organisms might possess competitive advantages over natural counterparts, potentially disrupting ecological balances if released. Horizontal gene transfer represents another significant risk, where engineered genetic circuits for energy optimization could transfer to wild organisms with unpredictable consequences. Containment strategies, including physical barriers, genetic safeguards, and kill switches, are therefore essential components of responsible research and development.
Health-related biosafety considerations focus on potential toxicity or pathogenicity of engineered organisms or their metabolic products. Energy optimization pathways often involve the production of novel compounds or the manipulation of existing metabolic processes, which may generate unexpected byproducts with unknown health effects. Rigorous testing protocols and biocontainment measures must be implemented to mitigate these risks, particularly as applications scale from laboratory to industrial settings.
From a biosecurity perspective, the dual-use potential of synthetic biology techniques for energy optimization presents unique challenges. Knowledge, tools, and engineered organisms developed for beneficial energy applications could potentially be misappropriated for harmful purposes. This necessitates careful oversight of information sharing, technology transfer, and access to critical biological materials and equipment.
International governance frameworks for synthetic biology are still evolving, with significant variations in regulatory approaches across different jurisdictions. The development of harmonized standards specifically addressing energy optimization applications would enhance both safety and security while facilitating responsible innovation. Industry self-regulation through codes of conduct and best practices also plays a crucial role in complementing formal regulatory mechanisms.
Risk assessment methodologies for synthetic biology applications in energy optimization must continue to evolve alongside technological advancements. These should incorporate both quantitative and qualitative approaches, considering not only immediate risks but also long-term and systemic impacts. Stakeholder engagement, including public consultation, represents another essential element in developing socially acceptable risk management strategies for these emerging technologies.
Environmental biosafety concerns primarily revolve around the potential for engineered organisms to escape controlled environments and interact with natural ecosystems. Energy-optimized synthetic organisms might possess competitive advantages over natural counterparts, potentially disrupting ecological balances if released. Horizontal gene transfer represents another significant risk, where engineered genetic circuits for energy optimization could transfer to wild organisms with unpredictable consequences. Containment strategies, including physical barriers, genetic safeguards, and kill switches, are therefore essential components of responsible research and development.
Health-related biosafety considerations focus on potential toxicity or pathogenicity of engineered organisms or their metabolic products. Energy optimization pathways often involve the production of novel compounds or the manipulation of existing metabolic processes, which may generate unexpected byproducts with unknown health effects. Rigorous testing protocols and biocontainment measures must be implemented to mitigate these risks, particularly as applications scale from laboratory to industrial settings.
From a biosecurity perspective, the dual-use potential of synthetic biology techniques for energy optimization presents unique challenges. Knowledge, tools, and engineered organisms developed for beneficial energy applications could potentially be misappropriated for harmful purposes. This necessitates careful oversight of information sharing, technology transfer, and access to critical biological materials and equipment.
International governance frameworks for synthetic biology are still evolving, with significant variations in regulatory approaches across different jurisdictions. The development of harmonized standards specifically addressing energy optimization applications would enhance both safety and security while facilitating responsible innovation. Industry self-regulation through codes of conduct and best practices also plays a crucial role in complementing formal regulatory mechanisms.
Risk assessment methodologies for synthetic biology applications in energy optimization must continue to evolve alongside technological advancements. These should incorporate both quantitative and qualitative approaches, considering not only immediate risks but also long-term and systemic impacts. Stakeholder engagement, including public consultation, represents another essential element in developing socially acceptable risk management strategies for these emerging technologies.
Scalability and Industrial Implementation Challenges
The scaling of synthetic biology solutions for energy optimization from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. Current pilot implementations reveal bottlenecks in maintaining consistent performance across increased production volumes. Specifically, regulatory pathways that function efficiently in small-scale laboratory environments often experience diminished effectiveness when scaled to industrial bioreactors, primarily due to metabolic burden, genetic instability, and altered cellular microenvironments.
Metabolic engineering strategies that optimize energy utilization pathways face particular scaling difficulties. The carefully balanced regulatory networks designed in laboratory strains frequently become destabilized under industrial fermentation conditions, where substrate concentrations, oxygen availability, and waste product accumulation differ substantially from controlled laboratory settings. This destabilization leads to reduced yields and inconsistent product quality, undermining economic feasibility.
Infrastructure requirements present another significant barrier to industrial implementation. The specialized equipment needed for large-scale synthetic biology operations demands substantial capital investment, while the technical expertise required for operation and maintenance creates workforce challenges. Many facilities lack the interdisciplinary teams necessary to troubleshoot complex biological-engineering interface issues that emerge during scale-up.
Regulatory frameworks worldwide remain inconsistent regarding engineered biological systems, creating uncertainty for industrial deployment. Companies must navigate complex approval processes that vary by jurisdiction, with some regions imposing stringent containment requirements that significantly increase operational costs. The absence of harmonized global standards complicates multinational implementation strategies and limits market access.
Economic factors further constrain industrial adoption. The high initial investment required for synthetic biology infrastructure, coupled with extended development timelines, creates significant financial risk. Current cost structures for many energy optimization pathways remain uncompetitive with conventional alternatives, necessitating either technological breakthroughs or supportive policy frameworks to achieve market viability.
Addressing these challenges requires integrated approaches combining biological innovation with engineering solutions. Promising strategies include the development of robust chassis organisms specifically designed for industrial conditions, modular pathway designs that maintain functionality across different scales, and advanced bioreactor systems with improved monitoring and feedback control mechanisms. Computational modeling that accurately predicts scaling effects on regulatory pathways has emerged as a critical tool for reducing empirical trial-and-error approaches.
Metabolic engineering strategies that optimize energy utilization pathways face particular scaling difficulties. The carefully balanced regulatory networks designed in laboratory strains frequently become destabilized under industrial fermentation conditions, where substrate concentrations, oxygen availability, and waste product accumulation differ substantially from controlled laboratory settings. This destabilization leads to reduced yields and inconsistent product quality, undermining economic feasibility.
Infrastructure requirements present another significant barrier to industrial implementation. The specialized equipment needed for large-scale synthetic biology operations demands substantial capital investment, while the technical expertise required for operation and maintenance creates workforce challenges. Many facilities lack the interdisciplinary teams necessary to troubleshoot complex biological-engineering interface issues that emerge during scale-up.
Regulatory frameworks worldwide remain inconsistent regarding engineered biological systems, creating uncertainty for industrial deployment. Companies must navigate complex approval processes that vary by jurisdiction, with some regions imposing stringent containment requirements that significantly increase operational costs. The absence of harmonized global standards complicates multinational implementation strategies and limits market access.
Economic factors further constrain industrial adoption. The high initial investment required for synthetic biology infrastructure, coupled with extended development timelines, creates significant financial risk. Current cost structures for many energy optimization pathways remain uncompetitive with conventional alternatives, necessitating either technological breakthroughs or supportive policy frameworks to achieve market viability.
Addressing these challenges requires integrated approaches combining biological innovation with engineering solutions. Promising strategies include the development of robust chassis organisms specifically designed for industrial conditions, modular pathway designs that maintain functionality across different scales, and advanced bioreactor systems with improved monitoring and feedback control mechanisms. Computational modeling that accurately predicts scaling effects on regulatory pathways has emerged as a critical tool for reducing empirical trial-and-error approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!