Patent Implications for Synthetic Biology Material Developments
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Patent Landscape and Objectives
Synthetic biology represents a transformative field at the intersection of biology, engineering, and materials science, with significant implications for intellectual property landscapes globally. The evolution of this field has witnessed remarkable growth since the early 2000s, with patent filings increasing exponentially as researchers and companies seek to protect novel biological systems, pathways, and materials developed through synthetic approaches.
The historical trajectory of synthetic biology patents reveals distinct phases of development. Initially focused on basic genetic circuit components and simple biological systems, the field has progressively expanded to encompass complex engineered organisms, novel biomaterials, and sophisticated biological manufacturing processes. This evolution reflects the maturation of synthetic biology from a primarily academic pursuit to a commercially viable technology platform with diverse applications.
Current patent trends indicate concentrated activity in several key areas: engineered microorganisms for bioproduction, CRISPR-based genome editing tools, synthetic biological circuits, and novel biomaterials with programmable properties. The geographical distribution of patent activity shows dominance by the United States, followed by China and Europe, with emerging contributions from Japan, South Korea, and Singapore.
The primary objectives of this technical assessment are multifaceted. First, to comprehensively map the existing patent landscape in synthetic biology materials development, identifying key players, technological clusters, and emerging trends. Second, to analyze potential freedom-to-operate challenges for new entrants and identify strategic white spaces for innovation. Third, to evaluate the strength and breadth of foundational patents that may impact future developments in the field.
Additionally, this assessment aims to identify potential regulatory considerations that may influence patent strategy, particularly given the evolving nature of regulations surrounding genetically modified organisms and biosafety. The intersection of patent law with other forms of intellectual property protection, such as trade secrets and biological materials deposits, presents unique challenges that warrant careful consideration.
Understanding this complex landscape is crucial for strategic decision-making in research and development investments, partnership opportunities, and commercialization pathways. As synthetic biology continues to advance toward creating novel materials with unprecedented properties, navigating the patent landscape effectively will be essential for maintaining competitive advantage and fostering innovation in this rapidly evolving field.
The historical trajectory of synthetic biology patents reveals distinct phases of development. Initially focused on basic genetic circuit components and simple biological systems, the field has progressively expanded to encompass complex engineered organisms, novel biomaterials, and sophisticated biological manufacturing processes. This evolution reflects the maturation of synthetic biology from a primarily academic pursuit to a commercially viable technology platform with diverse applications.
Current patent trends indicate concentrated activity in several key areas: engineered microorganisms for bioproduction, CRISPR-based genome editing tools, synthetic biological circuits, and novel biomaterials with programmable properties. The geographical distribution of patent activity shows dominance by the United States, followed by China and Europe, with emerging contributions from Japan, South Korea, and Singapore.
The primary objectives of this technical assessment are multifaceted. First, to comprehensively map the existing patent landscape in synthetic biology materials development, identifying key players, technological clusters, and emerging trends. Second, to analyze potential freedom-to-operate challenges for new entrants and identify strategic white spaces for innovation. Third, to evaluate the strength and breadth of foundational patents that may impact future developments in the field.
Additionally, this assessment aims to identify potential regulatory considerations that may influence patent strategy, particularly given the evolving nature of regulations surrounding genetically modified organisms and biosafety. The intersection of patent law with other forms of intellectual property protection, such as trade secrets and biological materials deposits, presents unique challenges that warrant careful consideration.
Understanding this complex landscape is crucial for strategic decision-making in research and development investments, partnership opportunities, and commercialization pathways. As synthetic biology continues to advance toward creating novel materials with unprecedented properties, navigating the patent landscape effectively will be essential for maintaining competitive advantage and fostering innovation in this rapidly evolving field.
Market Analysis for Synthetic Biology Materials
The synthetic biology materials market is experiencing unprecedented growth, driven by advancements in genetic engineering, metabolic engineering, and systems biology. Current market valuations place the global synthetic biology market at approximately $9.5 billion in 2022, with projections indicating growth to reach $30.7 billion by 2027, representing a compound annual growth rate (CAGR) of 26.5%. Materials development represents a significant portion of this market, with applications spanning pharmaceuticals, agriculture, industrial chemicals, and consumer products.
Demand for synthetic biology materials is particularly strong in the pharmaceutical sector, where engineered biological systems are revolutionizing drug discovery and production processes. The ability to design microorganisms that produce complex pharmaceutical compounds has reduced production costs by up to 50% for certain molecules while increasing production efficiency by 30-40% compared to traditional chemical synthesis methods.
In the industrial materials sector, companies are leveraging synthetic biology to develop novel biomaterials with enhanced properties. These include biodegradable plastics, advanced textiles, and construction materials with reduced environmental footprints. Market research indicates that bio-based materials could capture 25% of the conventional plastics market by 2030, representing a $45 billion opportunity.
Consumer awareness and preference for sustainable products is driving demand for synthetic biology materials in everyday products. Surveys indicate that 68% of consumers are willing to pay premium prices for products made with sustainable materials, creating significant market pull for synthetic biology innovations.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing investments in biotechnology infrastructure and favorable government policies.
Regulatory frameworks significantly impact market dynamics, with patent protection being a critical factor in commercialization strategies. Companies with strong patent portfolios command premium valuations, with an average increase of 35% in company valuation for those holding key synthetic biology patents.
Investment in the sector has seen remarkable growth, with venture capital funding exceeding $8 billion in 2021 alone. Strategic partnerships between startups and established corporations have become increasingly common, accelerating the path to market for novel synthetic biology materials and expanding potential applications across diverse industries.
Demand for synthetic biology materials is particularly strong in the pharmaceutical sector, where engineered biological systems are revolutionizing drug discovery and production processes. The ability to design microorganisms that produce complex pharmaceutical compounds has reduced production costs by up to 50% for certain molecules while increasing production efficiency by 30-40% compared to traditional chemical synthesis methods.
In the industrial materials sector, companies are leveraging synthetic biology to develop novel biomaterials with enhanced properties. These include biodegradable plastics, advanced textiles, and construction materials with reduced environmental footprints. Market research indicates that bio-based materials could capture 25% of the conventional plastics market by 2030, representing a $45 billion opportunity.
Consumer awareness and preference for sustainable products is driving demand for synthetic biology materials in everyday products. Surveys indicate that 68% of consumers are willing to pay premium prices for products made with sustainable materials, creating significant market pull for synthetic biology innovations.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 28% and Asia-Pacific at 22%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing investments in biotechnology infrastructure and favorable government policies.
Regulatory frameworks significantly impact market dynamics, with patent protection being a critical factor in commercialization strategies. Companies with strong patent portfolios command premium valuations, with an average increase of 35% in company valuation for those holding key synthetic biology patents.
Investment in the sector has seen remarkable growth, with venture capital funding exceeding $8 billion in 2021 alone. Strategic partnerships between startups and established corporations have become increasingly common, accelerating the path to market for novel synthetic biology materials and expanding potential applications across diverse industries.
Current Challenges in Synthetic Biology IP Protection
The synthetic biology field faces significant intellectual property protection challenges that have intensified as the industry matures. Traditional patent frameworks struggle to accommodate the unique characteristics of biological innovations, creating substantial barriers for researchers and companies.
One primary challenge is determining the appropriate scope of protection for synthetic biology inventions. Unlike mechanical or electrical innovations, biological systems exhibit inherent complexity and variability that make precise patent claims difficult to draft. Patent examiners and courts often lack specialized knowledge to evaluate these claims effectively, resulting in inconsistent protection standards across jurisdictions.
The boundary between naturally occurring and engineered biological materials presents another critical challenge. Following the landmark Supreme Court decision in Association for Molecular Pathology v. Myriad Genetics, naturally occurring DNA sequences remain unpatentable, while engineered sequences may qualify for protection. This distinction creates significant uncertainty for innovators working with modified natural systems or minimally altered genetic sequences.
Patent thickets have emerged as synthetic biology matures, with fundamental techniques and genetic components becoming encumbered by overlapping intellectual property rights. These dense networks of patents create navigation challenges for new entrants and increase transaction costs through complex licensing negotiations. The BioBrick Foundation's attempt to establish an open-source framework represents one response to this challenge, though its adoption remains limited in commercial contexts.
International harmonization of synthetic biology IP protection remains elusive. Significant differences exist between major jurisdictions like the United States, Europe, and Asia regarding the patentability of biological materials. These disparities create regulatory arbitrage opportunities but also generate uncertainty for global development and commercialization strategies.
The rapid pace of innovation in synthetic biology often outstrips the patent system's processing capabilities. Patent examination backlogs can extend to several years, during which technological advances may render the original innovation obsolete or significantly altered. This temporal mismatch particularly affects startups and academic institutions with limited resources to maintain extended patent prosecution efforts.
Disclosure requirements present additional complications, as comprehensive documentation of biological inventions requires extensive data that may exceed traditional patent disclosure norms. The tension between sufficient disclosure for patent validity and protection of valuable know-how creates strategic dilemmas for innovators seeking to maximize their competitive advantage while securing legal protection.
One primary challenge is determining the appropriate scope of protection for synthetic biology inventions. Unlike mechanical or electrical innovations, biological systems exhibit inherent complexity and variability that make precise patent claims difficult to draft. Patent examiners and courts often lack specialized knowledge to evaluate these claims effectively, resulting in inconsistent protection standards across jurisdictions.
The boundary between naturally occurring and engineered biological materials presents another critical challenge. Following the landmark Supreme Court decision in Association for Molecular Pathology v. Myriad Genetics, naturally occurring DNA sequences remain unpatentable, while engineered sequences may qualify for protection. This distinction creates significant uncertainty for innovators working with modified natural systems or minimally altered genetic sequences.
Patent thickets have emerged as synthetic biology matures, with fundamental techniques and genetic components becoming encumbered by overlapping intellectual property rights. These dense networks of patents create navigation challenges for new entrants and increase transaction costs through complex licensing negotiations. The BioBrick Foundation's attempt to establish an open-source framework represents one response to this challenge, though its adoption remains limited in commercial contexts.
International harmonization of synthetic biology IP protection remains elusive. Significant differences exist between major jurisdictions like the United States, Europe, and Asia regarding the patentability of biological materials. These disparities create regulatory arbitrage opportunities but also generate uncertainty for global development and commercialization strategies.
The rapid pace of innovation in synthetic biology often outstrips the patent system's processing capabilities. Patent examination backlogs can extend to several years, during which technological advances may render the original innovation obsolete or significantly altered. This temporal mismatch particularly affects startups and academic institutions with limited resources to maintain extended patent prosecution efforts.
Disclosure requirements present additional complications, as comprehensive documentation of biological inventions requires extensive data that may exceed traditional patent disclosure norms. The tension between sufficient disclosure for patent validity and protection of valuable know-how creates strategic dilemmas for innovators seeking to maximize their competitive advantage while securing legal protection.
Existing Patent Strategies for Synthetic Materials
01 Patentability of synthetic biological materials
Synthetic biology materials present unique challenges for patent protection due to their nature as engineered biological systems. These materials, which combine genetic elements in novel ways, raise questions about what constitutes patentable subject matter. The implications include considerations of novelty, non-obviousness, and utility requirements specific to biological inventions. Patent strategies must address the distinction between naturally occurring and synthetically created biological materials to ensure adequate protection.- Patentability of synthetic biological materials: Synthetic biology materials present unique challenges for patent protection due to their nature as engineered biological systems. These materials, which combine elements of living organisms with novel functionalities, raise questions about what constitutes patentable subject matter. The patentability considerations include novelty, non-obviousness, and utility requirements specific to biological inventions. Legal frameworks are evolving to address these engineered biological systems that blur the line between natural and artificial constructs.
- Intellectual property strategies for synthetic biology innovations: Developing effective intellectual property strategies is crucial for protecting synthetic biology innovations. This includes determining the optimal protection method (patents, trade secrets, or a combination), establishing clear ownership of biological materials, and navigating cross-licensing agreements. Strategic considerations must account for the rapidly evolving nature of synthetic biology technology and the global variations in biotechnology patent laws. Comprehensive IP strategies help maximize commercial value while ensuring freedom to operate in this complex field.
- Regulatory and ethical implications of synthetic biology patents: Synthetic biology patents raise significant regulatory and ethical considerations that impact their implementation and enforcement. These include biosafety concerns, potential environmental impacts, and questions about the appropriate scope of monopoly rights for fundamental biological building blocks. Regulatory frameworks must balance innovation incentives with public health and environmental safety. Ethical considerations include questions of access to essential technologies, benefit-sharing with source communities, and the moral implications of creating and patenting novel biological entities.
- Manufacturing processes for synthetic biological materials: Patent protection for manufacturing processes of synthetic biological materials covers novel methods for producing engineered organisms, biopolymers, and other biological constructs. These processes may include specialized fermentation techniques, bioreactor designs, purification methods, and quality control procedures specific to synthetic biology applications. Process patents are particularly valuable in the synthetic biology field as they can provide protection even when the end product might be difficult to patent. Manufacturing innovations focus on scalability, reproducibility, and cost-effectiveness of producing synthetic biological materials.
- Computational tools and methods for synthetic biology design: Computational tools and methods play a crucial role in synthetic biology innovation and are increasingly the subject of patent protection. These include software for genetic circuit design, predictive modeling of biological systems, automated DNA assembly planning, and machine learning approaches for optimizing biological functions. Patent protection in this area covers novel algorithms, user interfaces, and integrated systems that enable the efficient design and testing of synthetic biological materials. These computational innovations accelerate the development cycle and improve the predictability of engineered biological systems.
02 Intellectual property frameworks for engineered biological systems
Specialized intellectual property frameworks are emerging to address the unique aspects of engineered biological systems. These frameworks consider the modular nature of synthetic biology, where standardized biological parts can be combined to create novel functions. The implications include developing appropriate claim strategies that protect both individual components and their novel combinations. Such frameworks must balance innovation protection with concerns about creating monopolies over fundamental biological building blocks.Expand Specific Solutions03 Cross-border regulatory considerations for synthetic biology patents
Synthetic biology materials face varying regulatory landscapes across different jurisdictions, affecting patent strategy and enforcement. These differences impact how claims should be structured to ensure global protection. The implications include navigating different standards for patentability of biological materials, ethical considerations that vary by country, and compliance with international treaties governing genetic resources. Patent applicants must develop strategies that account for these jurisdictional differences to maximize protection worldwide.Expand Specific Solutions04 Material composition and manufacturing process protection
Protection strategies for synthetic biology innovations often involve claims covering both the novel biological materials themselves and the processes used to create them. This dual approach strengthens overall patent protection by addressing both product and method aspects. The implications include developing comprehensive claim strategies that protect the composition of matter, methods of production, and applications of synthetic biological materials. This approach helps prevent competitors from designing around patents through alternative manufacturing methods.Expand Specific Solutions05 AI integration in synthetic biology patent landscape
The integration of artificial intelligence with synthetic biology creates new patent implications at this technological intersection. AI tools are increasingly used to design, optimize, and predict the behavior of synthetic biological systems. The implications include questions about inventorship when AI contributes to biological innovations, patentability of AI-designed biological materials, and how to properly disclose AI-assisted inventions. This convergence requires new approaches to patent drafting and prosecution that address both biological and computational aspects.Expand Specific Solutions
Key Industry Players and Patent Portfolios
The synthetic biology materials patent landscape is currently in a growth phase, with significant market expansion expected as technologies mature. Academic institutions like MIT, Northwestern University, and Harvard College are leading fundamental research, while companies such as Regeneron Pharmaceuticals, Bristol Myers Squibb, and Abbott Laboratories are commercializing applications. The market is characterized by a blend of established pharmaceutical players and specialized biotech firms like ZymoGenetics and Epizyme. Research universities hold substantial intellectual property portfolios, creating a competitive environment where cross-sector collaborations are increasingly common. The technology is approaching commercial viability in several sectors, though regulatory frameworks for synthetic biology materials remain in development, creating both challenges and strategic opportunities for market participants.
Massachusetts Institute of Technology
Technical Solution: MIT has established a sophisticated patent portfolio in synthetic biology materials through its Synthetic Biology Center and Broad Institute collaborations. Their technical approach centers on standardized biological parts ("BioBricks") with well-defined intellectual property frameworks that balance open innovation with commercial protection. MIT researchers have developed the Registry of Standard Biological Parts, creating a foundation for modular synthetic biology while carefully patenting key innovations. Their patent strategy includes protection for computational design tools that predict biological material properties, engineered microorganisms that produce novel biomaterials, and cell-free protein synthesis systems. MIT has pioneered responsible licensing models that include research exemptions and humanitarian use provisions, particularly for applications in developing countries. They've also developed specialized material transfer agreements for synthetic biology components that facilitate research while preserving commercial rights for breakthrough applications.
Strengths: Leader in standardization of biological parts with clear IP frameworks; strong computational tools for designing synthetic biological materials; balanced approach to open innovation and proprietary protection. Weaknesses: Potential conflicts between open-source philosophy and commercial interests; complex co-ownership arrangements with partner institutions may complicate licensing.
The Regents of the University of California
Technical Solution: UC system has developed an extensive patent portfolio covering synthetic biology materials across multiple campuses, with UC Berkeley and UCSF leading significant innovations. Their approach includes strategic patenting of foundational technologies like CRISPR-Cas9 gene editing systems specifically optimized for synthetic biology applications, with particular attention to manufacturing processes for synthetic biological materials. UC researchers have pioneered methods for creating cell-free protein synthesis systems with extended shelf life and stability, protected through a comprehensive patent strategy. The UC system has implemented a sophisticated patent management approach that includes establishing the Innovative Genomics Institute to coordinate IP development across institutions. Their licensing strategy incorporates tiered structures based on application fields, with different terms for therapeutic, agricultural, and industrial applications of synthetic biology materials. UC has also developed specialized material transfer agreements specifically designed for synthetic biological components to facilitate research while maintaining commercial potential.
Strengths: Extensive patent portfolio covering fundamental synthetic biology technologies; sophisticated IP management across multiple research centers; strong position in CRISPR applications for synthetic biology. Weaknesses: Ongoing patent disputes with other institutions over foundational technologies; complex bureaucracy may slow commercialization processes compared to more nimble organizations.
Critical IP Innovations in Synthetic Biology
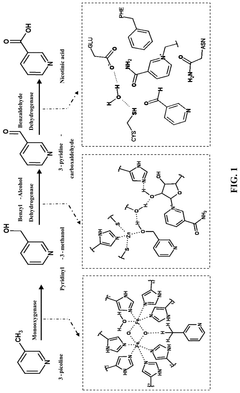
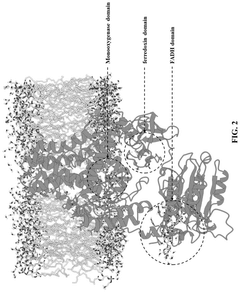
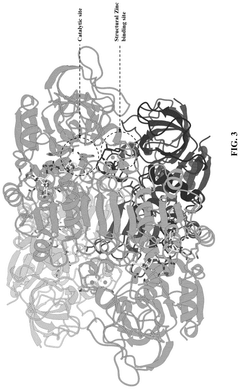
Synthetic biology approach to synthesize nicotinic acid from 3-picoline
PatentPendingUS20250101476A1
Innovation
- The development of a biosynthetic method using microbial biotransformation, involving the integration of monooxygenase, electron transfer component, benzyl alcohol dehydrogenase, and benzaldehyde dehydrogenase enzymes, optimized through gene isolation, enzyme engineering, and structural insights, to achieve a high conversion rate of 90% or higher from 3-picoline to nicotinic acid.
Regulatory Considerations for Synthetic Biology Materials
The regulatory landscape for synthetic biology materials presents a complex and evolving framework that significantly impacts innovation and commercialization. Current regulations governing synthetic biology materials vary substantially across jurisdictions, creating challenges for global development and market access. In the United States, oversight is distributed among multiple agencies including the FDA, EPA, and USDA, each applying different regulatory frameworks depending on the intended use and characteristics of the synthetic biology product.
Risk assessment methodologies for synthetic biology materials require special consideration due to their novel properties and potential interactions with natural systems. Regulatory bodies increasingly demand comprehensive safety data that addresses both direct human health impacts and broader ecological considerations. This includes evaluation of horizontal gene transfer risks, ecosystem disruption potential, and long-term evolutionary implications of engineered biological systems.
International harmonization efforts remain in nascent stages, with significant divergence between permissive and restrictive regulatory approaches. The European Union generally applies the precautionary principle, requiring extensive safety demonstrations before market approval, while countries like Singapore and parts of South America have developed more innovation-friendly regulatory frameworks specifically tailored to synthetic biology advancements.
Intellectual property protection intersects critically with regulatory compliance, as patent strategies must account for regulatory limitations and requirements. Companies must navigate both domains simultaneously, as regulatory classifications can impact patentability and enforcement options. This dual-track consideration has led to the emergence of specialized regulatory affairs teams within synthetic biology ventures.
Public perception and stakeholder engagement have become essential components of the regulatory process. Regulatory bodies increasingly incorporate public consultation mechanisms, recognizing that social acceptance represents a de facto regulatory hurdle for synthetic biology materials. Industry self-regulation through consortia and voluntary standards has emerged as a complementary approach to formal government oversight.
Emerging regulatory trends include adaptive licensing pathways that allow for staged market entry with ongoing monitoring requirements, tiered regulatory approaches based on risk classification, and increased focus on traceability systems throughout product lifecycles. These developments reflect regulatory systems attempting to balance innovation facilitation with appropriate safeguards for novel biological technologies.
Compliance strategies for synthetic biology developers must therefore be forward-looking, anticipating regulatory evolution while actively engaging with policymakers to shape reasonable oversight frameworks. The most successful market entrants demonstrate proactive regulatory engagement, transparent safety assessment protocols, and clear communication about risk management approaches.
Risk assessment methodologies for synthetic biology materials require special consideration due to their novel properties and potential interactions with natural systems. Regulatory bodies increasingly demand comprehensive safety data that addresses both direct human health impacts and broader ecological considerations. This includes evaluation of horizontal gene transfer risks, ecosystem disruption potential, and long-term evolutionary implications of engineered biological systems.
International harmonization efforts remain in nascent stages, with significant divergence between permissive and restrictive regulatory approaches. The European Union generally applies the precautionary principle, requiring extensive safety demonstrations before market approval, while countries like Singapore and parts of South America have developed more innovation-friendly regulatory frameworks specifically tailored to synthetic biology advancements.
Intellectual property protection intersects critically with regulatory compliance, as patent strategies must account for regulatory limitations and requirements. Companies must navigate both domains simultaneously, as regulatory classifications can impact patentability and enforcement options. This dual-track consideration has led to the emergence of specialized regulatory affairs teams within synthetic biology ventures.
Public perception and stakeholder engagement have become essential components of the regulatory process. Regulatory bodies increasingly incorporate public consultation mechanisms, recognizing that social acceptance represents a de facto regulatory hurdle for synthetic biology materials. Industry self-regulation through consortia and voluntary standards has emerged as a complementary approach to formal government oversight.
Emerging regulatory trends include adaptive licensing pathways that allow for staged market entry with ongoing monitoring requirements, tiered regulatory approaches based on risk classification, and increased focus on traceability systems throughout product lifecycles. These developments reflect regulatory systems attempting to balance innovation facilitation with appropriate safeguards for novel biological technologies.
Compliance strategies for synthetic biology developers must therefore be forward-looking, anticipating regulatory evolution while actively engaging with policymakers to shape reasonable oversight frameworks. The most successful market entrants demonstrate proactive regulatory engagement, transparent safety assessment protocols, and clear communication about risk management approaches.
International IP Harmonization for Synthetic Biology
The harmonization of intellectual property (IP) frameworks across international borders represents a critical challenge for the advancement of synthetic biology materials. Currently, significant disparities exist between national patent systems, creating obstacles for researchers and companies operating in multiple jurisdictions. The Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure provides some standardization, but remains insufficient for the rapidly evolving field of synthetic biology.
Major patent offices including the USPTO, EPO, and CNIPA have begun collaborative efforts to address these challenges through initiatives like the IP5, which aims to eliminate unnecessary duplication of work and improve patent quality. However, fundamental differences persist in how different jurisdictions treat living organisms and biological materials as patentable subject matter, creating uncertainty for innovators in synthetic biology.
The Nagoya Protocol on Access and Benefit Sharing further complicates the international IP landscape by establishing requirements for genetic resource utilization that intersect with patent applications. This creates additional compliance burdens for synthetic biology researchers working across borders and utilizing diverse biological resources.
Recent developments in international harmonization include the WIPO's Standing Committee on the Law of Patents discussions specifically addressing biotechnology inventions. These forums are increasingly recognizing the unique challenges posed by synthetic biology materials, which often blur traditional distinctions between naturally occurring and human-made inventions.
Industry stakeholders have proposed several models for improved harmonization, including specialized international filing pathways for biological inventions and standardized disclosure requirements for synthetic biological materials. The International Genetically Engineered Machine (iGEM) Foundation has pioneered open-source approaches through its BioBrick Public Agreement, offering an alternative model to traditional patent protection.
The economic implications of harmonization are substantial, with estimates suggesting that streamlined international IP processes could reduce R&D costs in synthetic biology by 15-20% and accelerate time-to-market by similar margins. Countries with strong biotechnology sectors, including the US, China, and EU member states, have particular incentives to lead harmonization efforts.
Looking forward, successful international IP harmonization for synthetic biology will likely require specialized protocols addressing the unique characteristics of engineered biological materials, including their self-replicating nature and potential environmental impacts. Multilateral agreements specifically tailored to synthetic biology innovations represent the most promising path toward a coherent global framework.
Major patent offices including the USPTO, EPO, and CNIPA have begun collaborative efforts to address these challenges through initiatives like the IP5, which aims to eliminate unnecessary duplication of work and improve patent quality. However, fundamental differences persist in how different jurisdictions treat living organisms and biological materials as patentable subject matter, creating uncertainty for innovators in synthetic biology.
The Nagoya Protocol on Access and Benefit Sharing further complicates the international IP landscape by establishing requirements for genetic resource utilization that intersect with patent applications. This creates additional compliance burdens for synthetic biology researchers working across borders and utilizing diverse biological resources.
Recent developments in international harmonization include the WIPO's Standing Committee on the Law of Patents discussions specifically addressing biotechnology inventions. These forums are increasingly recognizing the unique challenges posed by synthetic biology materials, which often blur traditional distinctions between naturally occurring and human-made inventions.
Industry stakeholders have proposed several models for improved harmonization, including specialized international filing pathways for biological inventions and standardized disclosure requirements for synthetic biological materials. The International Genetically Engineered Machine (iGEM) Foundation has pioneered open-source approaches through its BioBrick Public Agreement, offering an alternative model to traditional patent protection.
The economic implications of harmonization are substantial, with estimates suggesting that streamlined international IP processes could reduce R&D costs in synthetic biology by 15-20% and accelerate time-to-market by similar margins. Countries with strong biotechnology sectors, including the US, China, and EU member states, have particular incentives to lead harmonization efforts.
Looking forward, successful international IP harmonization for synthetic biology will likely require specialized protocols addressing the unique characteristics of engineered biological materials, including their self-replicating nature and potential environmental impacts. Multilateral agreements specifically tailored to synthetic biology innovations represent the most promising path toward a coherent global framework.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!