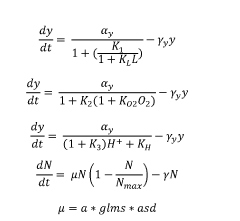Synthetic Biology and Patent Regulations Impact
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Research Objectives
Synthetic biology has evolved significantly since its conceptual emergence in the 1970s, transitioning from theoretical frameworks to practical applications that merge biology with engineering principles. The field formally coalesced in the early 2000s when researchers at MIT and other institutions established foundational methodologies for standardizing genetic components. This standardization approach, inspired by electronic circuit design, enabled the creation of biological parts that could be assembled into functional systems with predictable behaviors.
The evolution of synthetic biology has been accelerated by technological advancements in DNA sequencing and synthesis. The Human Genome Project's completion in 2003 provided crucial genomic knowledge, while the development of next-generation sequencing technologies dramatically reduced costs and increased throughput. Concurrently, DNA synthesis capabilities have improved exponentially, allowing researchers to construct increasingly complex genetic circuits and metabolic pathways.
CRISPR-Cas9 gene editing technology, introduced in 2012, represents a pivotal milestone that revolutionized the field by providing precise, efficient genetic modification tools. This advancement has enabled more sophisticated engineering of biological systems and expanded the scope of potential applications across medicine, agriculture, and industrial biotechnology.
The research objectives in synthetic biology currently focus on several key areas. First, enhancing the predictability and reliability of engineered biological systems remains a central challenge, requiring improved computational models and design tools. Second, developing more sophisticated genetic circuits with complex functionalities, including sensing, computing, and responding to environmental stimuli, continues to drive innovation in the field.
Additionally, researchers aim to expand the genetic code beyond natural amino acids, potentially creating novel proteins with unprecedented functions. This includes efforts to incorporate non-standard amino acids and develop entirely new biochemical pathways. The creation of minimal genomes and synthetic cells represents another frontier, with the goal of building cellular systems from first principles to better understand fundamental biological processes.
From a regulatory perspective, research objectives include establishing appropriate governance frameworks that balance innovation with safety considerations. This involves developing standards for risk assessment, containment strategies, and ethical guidelines that can adapt to rapidly evolving technologies while addressing concerns about biosecurity and environmental impact.
The convergence of synthetic biology with other emerging technologies, including artificial intelligence, nanotechnology, and advanced materials science, presents opportunities for transformative applications but also raises complex regulatory challenges regarding intellectual property protection and technology transfer across international boundaries.
The evolution of synthetic biology has been accelerated by technological advancements in DNA sequencing and synthesis. The Human Genome Project's completion in 2003 provided crucial genomic knowledge, while the development of next-generation sequencing technologies dramatically reduced costs and increased throughput. Concurrently, DNA synthesis capabilities have improved exponentially, allowing researchers to construct increasingly complex genetic circuits and metabolic pathways.
CRISPR-Cas9 gene editing technology, introduced in 2012, represents a pivotal milestone that revolutionized the field by providing precise, efficient genetic modification tools. This advancement has enabled more sophisticated engineering of biological systems and expanded the scope of potential applications across medicine, agriculture, and industrial biotechnology.
The research objectives in synthetic biology currently focus on several key areas. First, enhancing the predictability and reliability of engineered biological systems remains a central challenge, requiring improved computational models and design tools. Second, developing more sophisticated genetic circuits with complex functionalities, including sensing, computing, and responding to environmental stimuli, continues to drive innovation in the field.
Additionally, researchers aim to expand the genetic code beyond natural amino acids, potentially creating novel proteins with unprecedented functions. This includes efforts to incorporate non-standard amino acids and develop entirely new biochemical pathways. The creation of minimal genomes and synthetic cells represents another frontier, with the goal of building cellular systems from first principles to better understand fundamental biological processes.
From a regulatory perspective, research objectives include establishing appropriate governance frameworks that balance innovation with safety considerations. This involves developing standards for risk assessment, containment strategies, and ethical guidelines that can adapt to rapidly evolving technologies while addressing concerns about biosecurity and environmental impact.
The convergence of synthetic biology with other emerging technologies, including artificial intelligence, nanotechnology, and advanced materials science, presents opportunities for transformative applications but also raises complex regulatory challenges regarding intellectual property protection and technology transfer across international boundaries.
Market Analysis for Synthetic Biology Applications
The synthetic biology market has experienced remarkable growth in recent years, with a global market valuation reaching $9.5 billion in 2021 and projections indicating expansion to $30.7 billion by 2026, representing a compound annual growth rate (CAGR) of 26.5%. This accelerated growth is primarily driven by increasing investments in research and development, growing demand for pharmaceutical and diagnostic applications, and expanding applications across multiple industries.
Healthcare and pharmaceuticals currently dominate the synthetic biology market landscape, accounting for approximately 33% of market share. The application of synthetic biology in drug discovery and development has revolutionized pharmaceutical research, enabling the creation of novel therapeutic compounds and personalized medicine approaches. Notable examples include engineered microorganisms producing insulin and other biologics, significantly reducing production costs compared to traditional methods.
Agricultural applications represent the second-largest market segment, with synthetic biology enabling the development of crops with enhanced nutritional profiles, improved resistance to pests and diseases, and increased yield potential. Companies like Bayer and Corteva Agriscience have made substantial investments in this sector, recognizing its potential to address global food security challenges.
Industrial biotechnology applications, including biofuels, biomaterials, and specialty chemicals, constitute a rapidly growing segment with a projected CAGR of 28.7% through 2026. The shift toward sustainable manufacturing processes has accelerated adoption in this sector, with companies like Amyris and Genomatica demonstrating commercial viability of bio-based alternatives to petroleum-derived products.
Regionally, North America leads the synthetic biology market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing government investments in biotechnology infrastructure and growing adoption across various industries.
Consumer awareness and acceptance remain significant market challenges, particularly regarding genetically modified organisms and biosafety concerns. Regulatory frameworks vary significantly across regions, creating market entry barriers and compliance challenges for companies operating globally. The complex patent landscape further complicates market dynamics, with overlapping intellectual property claims potentially hindering innovation and commercialization efforts.
Despite these challenges, the synthetic biology market presents substantial opportunities for growth and innovation. Emerging applications in environmental remediation, biosensing, and consumer products are expected to create new market segments, while advances in enabling technologies like CRISPR gene editing and artificial intelligence are likely to accelerate development timelines and expand the range of possible applications.
Healthcare and pharmaceuticals currently dominate the synthetic biology market landscape, accounting for approximately 33% of market share. The application of synthetic biology in drug discovery and development has revolutionized pharmaceutical research, enabling the creation of novel therapeutic compounds and personalized medicine approaches. Notable examples include engineered microorganisms producing insulin and other biologics, significantly reducing production costs compared to traditional methods.
Agricultural applications represent the second-largest market segment, with synthetic biology enabling the development of crops with enhanced nutritional profiles, improved resistance to pests and diseases, and increased yield potential. Companies like Bayer and Corteva Agriscience have made substantial investments in this sector, recognizing its potential to address global food security challenges.
Industrial biotechnology applications, including biofuels, biomaterials, and specialty chemicals, constitute a rapidly growing segment with a projected CAGR of 28.7% through 2026. The shift toward sustainable manufacturing processes has accelerated adoption in this sector, with companies like Amyris and Genomatica demonstrating commercial viability of bio-based alternatives to petroleum-derived products.
Regionally, North America leads the synthetic biology market with approximately 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing government investments in biotechnology infrastructure and growing adoption across various industries.
Consumer awareness and acceptance remain significant market challenges, particularly regarding genetically modified organisms and biosafety concerns. Regulatory frameworks vary significantly across regions, creating market entry barriers and compliance challenges for companies operating globally. The complex patent landscape further complicates market dynamics, with overlapping intellectual property claims potentially hindering innovation and commercialization efforts.
Despite these challenges, the synthetic biology market presents substantial opportunities for growth and innovation. Emerging applications in environmental remediation, biosensing, and consumer products are expected to create new market segments, while advances in enabling technologies like CRISPR gene editing and artificial intelligence are likely to accelerate development timelines and expand the range of possible applications.
Current Challenges in Synthetic Biology Development
Synthetic biology faces significant technical and regulatory challenges that impede its full potential. The field's interdisciplinary nature requires expertise across molecular biology, genetics, biochemistry, and engineering, creating substantial knowledge barriers for researchers and companies. This complexity is further compounded by the rapid evolution of techniques and tools, making it difficult for practitioners to stay current with cutting-edge methodologies.
Standardization remains a critical obstacle in synthetic biology development. Despite efforts to establish common biological parts and protocols, the field still lacks universally accepted standards for characterizing genetic components, data reporting, and experimental procedures. This absence of standardization hinders reproducibility and slows collaborative progress across research institutions and industry partners.
Biosafety and biosecurity concerns present persistent challenges that demand careful consideration. As synthetic biology capabilities advance, the potential for accidental release of engineered organisms or deliberate misuse of the technology increases. Current containment strategies and risk assessment frameworks struggle to keep pace with technological advancements, creating regulatory uncertainties that can delay innovation and commercialization.
The patent landscape surrounding synthetic biology has become increasingly complex and contentious. Overlapping intellectual property claims on fundamental biological components and processes create legal ambiguities that discourage investment and collaboration. The question of whether naturally derived genetic sequences should be patentable continues to generate significant debate among stakeholders, with different jurisdictions adopting varying approaches to these issues.
Ethical considerations further complicate synthetic biology development. Public perception and acceptance of genetically modified organisms remain mixed, with concerns about "playing god" or creating "unnatural" life forms influencing regulatory decisions and market adoption. The lack of harmonized international regulations creates additional challenges for companies operating across multiple jurisdictions, as they must navigate inconsistent requirements and approval processes.
Resource limitations also constrain synthetic biology advancement. The field requires sophisticated laboratory infrastructure, expensive reagents, and specialized equipment that may be inaccessible to researchers in resource-limited settings. This creates inequitable development opportunities and potentially widens the technological divide between wealthy and developing nations.
Data management presents another significant challenge. The exponential growth in biological data generation has outpaced the development of computational tools and infrastructure needed to effectively analyze and utilize this information. Integrating diverse datasets across different biological scales and experimental platforms remains difficult, limiting the predictive power of models used in synthetic biology design.
Standardization remains a critical obstacle in synthetic biology development. Despite efforts to establish common biological parts and protocols, the field still lacks universally accepted standards for characterizing genetic components, data reporting, and experimental procedures. This absence of standardization hinders reproducibility and slows collaborative progress across research institutions and industry partners.
Biosafety and biosecurity concerns present persistent challenges that demand careful consideration. As synthetic biology capabilities advance, the potential for accidental release of engineered organisms or deliberate misuse of the technology increases. Current containment strategies and risk assessment frameworks struggle to keep pace with technological advancements, creating regulatory uncertainties that can delay innovation and commercialization.
The patent landscape surrounding synthetic biology has become increasingly complex and contentious. Overlapping intellectual property claims on fundamental biological components and processes create legal ambiguities that discourage investment and collaboration. The question of whether naturally derived genetic sequences should be patentable continues to generate significant debate among stakeholders, with different jurisdictions adopting varying approaches to these issues.
Ethical considerations further complicate synthetic biology development. Public perception and acceptance of genetically modified organisms remain mixed, with concerns about "playing god" or creating "unnatural" life forms influencing regulatory decisions and market adoption. The lack of harmonized international regulations creates additional challenges for companies operating across multiple jurisdictions, as they must navigate inconsistent requirements and approval processes.
Resource limitations also constrain synthetic biology advancement. The field requires sophisticated laboratory infrastructure, expensive reagents, and specialized equipment that may be inaccessible to researchers in resource-limited settings. This creates inequitable development opportunities and potentially widens the technological divide between wealthy and developing nations.
Data management presents another significant challenge. The exponential growth in biological data generation has outpaced the development of computational tools and infrastructure needed to effectively analyze and utilize this information. Integrating diverse datasets across different biological scales and experimental platforms remains difficult, limiting the predictive power of models used in synthetic biology design.
Current Synthetic Biology Technical Solutions
01 Regulatory frameworks for synthetic biology patents
Regulatory frameworks govern the patenting of synthetic biology innovations, addressing the unique challenges posed by this emerging field. These frameworks establish guidelines for patent eligibility, examination procedures, and enforcement mechanisms specific to synthetic biology inventions. They aim to balance innovation incentives with ethical considerations and public interest, while also addressing concerns related to biosafety and biosecurity.- Regulatory frameworks for synthetic biology patents: Various regulatory frameworks exist for patents in synthetic biology, addressing the unique challenges of this interdisciplinary field. These frameworks establish guidelines for patentability criteria, disclosure requirements, and examination procedures specific to synthetic biological inventions. They aim to balance innovation protection with ethical considerations and public interest, while accommodating the rapid technological advancements in this field.
- Patentability of engineered biological systems: The patentability of engineered biological systems involves specific considerations regarding novelty, non-obviousness, and industrial applicability. These systems, which may include modified microorganisms, synthetic genetic circuits, or artificial metabolic pathways, present unique challenges for patent examination. Regulations address issues such as the distinction between naturally occurring and artificially created biological components, the scope of protection for biological inventions, and the assessment of inventive step in this rapidly evolving field.
- Ethical and biosafety considerations in synthetic biology patents: Patent regulations for synthetic biology incorporate ethical and biosafety considerations to address potential risks associated with engineered biological systems. These regulations may include requirements for risk assessment, containment measures, and environmental impact evaluations. They also address ethical concerns related to the creation of artificial life forms, genetic modification, and potential dual-use applications, establishing boundaries for what can be patented while ensuring responsible innovation.
- International harmonization of synthetic biology patent regulations: Efforts toward international harmonization of synthetic biology patent regulations aim to address the global nature of biotechnology research and commercialization. These initiatives seek to establish consistent standards for patentability, examination procedures, and enforcement across different jurisdictions. Harmonization efforts also address challenges related to cross-border technology transfer, licensing, and collaboration in synthetic biology research, while respecting national sovereignty and policy objectives.
- Intellectual property strategies for synthetic biology innovations: Intellectual property strategies for synthetic biology innovations encompass various approaches to protect and commercialize inventions in this field. These strategies may include patent portfolio development, trade secret protection, and open-source licensing models. They address the unique challenges of synthetic biology, such as the modular nature of biological parts, the role of standardization, and the balance between proprietary rights and knowledge sharing to advance the field.
02 Patentability criteria for synthetic biological systems
Specific criteria determine the patentability of synthetic biological systems, including engineered organisms, genetic circuits, and biological parts. These criteria typically assess novelty, non-obviousness, industrial applicability, and sufficient disclosure. The evaluation process considers the unique aspects of living systems, such as reproducibility and functional characterization. Standards for describing synthetic biology inventions in patent applications are also established to ensure clarity and enablement.Expand Specific Solutions03 International harmonization of synthetic biology patent regulations
Efforts to harmonize synthetic biology patent regulations across different jurisdictions aim to address the global nature of biotechnology research and commercialization. International agreements and collaborative frameworks facilitate consistent patent examination and enforcement while respecting national sovereignty. These harmonization initiatives seek to reduce regulatory barriers to innovation while ensuring appropriate oversight of synthetic biology technologies across borders.Expand Specific Solutions04 Ethical and biosafety considerations in synthetic biology patenting
Ethical and biosafety considerations play a crucial role in the regulation of synthetic biology patents. Regulatory frameworks incorporate mechanisms to assess potential environmental impacts, biosecurity risks, and ethical implications of synthetic biology inventions. Patent offices may require additional documentation regarding containment measures, risk assessments, and compliance with biosafety protocols. These considerations aim to ensure responsible innovation while preventing misuse or unintended consequences of synthetic biology technologies.Expand Specific Solutions05 Intellectual property protection strategies for synthetic biology innovations
Various intellectual property protection strategies are available for synthetic biology innovations, including utility patents, design patents, trade secrets, and regulatory exclusivities. These strategies can be tailored to different types of synthetic biology inventions, such as engineered organisms, biological parts, or production methods. Companies and research institutions develop comprehensive IP portfolios that combine different protection mechanisms to maximize the commercial value of their synthetic biology innovations while navigating regulatory requirements.Expand Specific Solutions
Leading Companies and Research Institutions
Synthetic biology is currently in a transitional phase from early development to commercial application, with the market expected to reach $30 billion by 2026. The competitive landscape features established pharmaceutical companies like Eli Lilly, Pfizer, and Sanofi investing heavily in R&D, alongside academic powerhouses such as MIT, Columbia University, and University of California driving fundamental research. Patent regulations are increasingly complex as companies like DuPont, BASF Plant Science, and Viridos advance proprietary technologies in genetic engineering. The field shows varying degrees of technical maturity, with therapeutic applications led by Epizyme and Incyte demonstrating more advanced development, while agricultural applications from Pioneer Hi-Bred face ongoing regulatory scrutiny and public acceptance challenges.
The Regents of the University of California
Technical Solution: The University of California system has established multiple centers of excellence in synthetic biology, particularly at UC Berkeley, UCSF, and UC San Diego. Their research encompasses foundational technologies including CRISPR-Cas9 genome editing systems, which have revolutionized precision genetic engineering capabilities. UC researchers have developed synthetic gene circuits with applications in biosensing, bioproduction, and therapeutics. Their work includes engineered microbial consortia that perform complex functions through intercellular communication networks. The UC system holds key patents related to CRISPR technology, which has sparked significant patent disputes with other institutions. Their research also addresses biosafety mechanisms such as genetic containment strategies and kill switches for engineered organisms. UC researchers have been actively involved in discussions about responsible innovation in synthetic biology, contributing to both technical advances and policy frameworks for this emerging field.
Strengths: Pioneering research in genome editing technologies; extensive patent portfolio covering fundamental synthetic biology tools; strong interdisciplinary collaboration across multiple campuses. Weaknesses: Complex patent litigation environment, particularly around CRISPR technologies; challenges in technology transfer and commercialization processes; potential conflicts between open science values and commercial interests.
Massachusetts Institute of Technology
Technical Solution: MIT has developed foundational synthetic biology technologies through its Synthetic Biology Center and Broad Institute. Their research encompasses the design of genetic circuits, CRISPR-based genome editing tools, and standardized biological parts (BioBricks). MIT researchers have pioneered the concept of "biological computers" using engineered genetic switches and logic gates that can process information within living cells. Their patent portfolio includes technologies for cell-free protein synthesis systems, engineered ribosomes, and synthetic gene networks with applications in biosensing, biomanufacturing, and therapeutics. MIT has been instrumental in establishing the Registry of Standard Biological Parts and developing the Synthetic Biology Open Language (SBOL) for standardized documentation of genetic designs. Their work addresses both technical innovations and regulatory frameworks, with researchers actively participating in policy discussions regarding biosafety, biosecurity, and intellectual property in synthetic biology.
Strengths: World-leading fundamental research capabilities; strong interdisciplinary approach combining engineering, biology, and computational design; extensive collaboration network with industry and other academic institutions. Weaknesses: Focus sometimes tilts toward basic research rather than commercial applications; complex IP landscape with multiple stakeholders; potential challenges in translating academic innovations to market-ready technologies.
Key Patents and Innovations Analysis
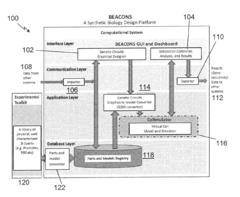
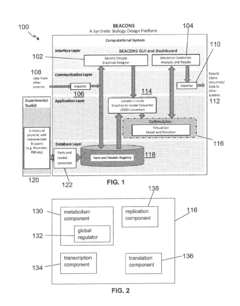
Systems and methods for synthetic biology design and host cell simulation
PatentInactiveUS20170147742A1
Innovation
- A computational system that includes a model conversion component to generate genetic circuit output data based on RNA polymerase, ribosome, and rRNA concentrations, and a host cell simulation component to simulate amino acid and nucleotide production, transcription, translation, and replication, providing a comprehensive model for predicting host cell physiological states.
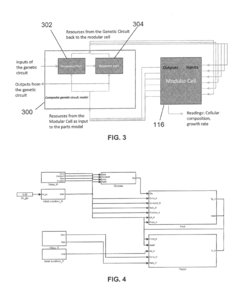
Engineered bacteria containing biosensors for precision targeting and containment
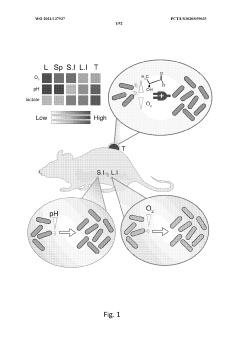
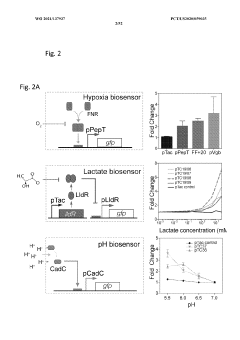
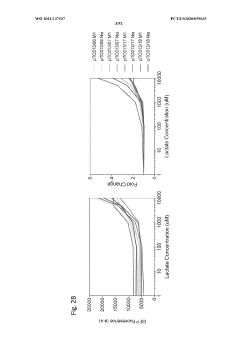
PatentWO2021137937A2
Innovation
- Engineered non-pathogenic bacteria equipped with inducible promoters responsive to specific environmental or physiological conditions, such as hypoxia, lactate, and pH, allowing for controlled expression of essential genes and containment within targeted organs or tumors.
Patent Regulation Framework for Synthetic Biology
The regulatory landscape for synthetic biology patents presents a complex framework that continues to evolve alongside technological advancements. Current patent regulations for synthetic biology operate within traditional intellectual property systems that were not specifically designed for biological innovations. In the United States, the USPTO has established guidelines following landmark cases such as Association for Molecular Pathology v. Myriad Genetics (2013) and Mayo Collaborative Services v. Prometheus Laboratories (2012), which significantly impacted the patentability of naturally occurring DNA sequences and diagnostic methods.
The European Patent Office maintains stricter standards regarding the patentability of biological materials, prohibiting patents on processes for human cloning, human germline modification, and uses of human embryos for industrial purposes under the European Biotechnology Directive (98/44/EC). Meanwhile, China has been rapidly developing its patent framework for biotechnology, with recent amendments to its Patent Law in 2020 strengthening protection for biological inventions.
International harmonization efforts through WIPO and the TRIPS Agreement provide minimum standards for intellectual property protection but leave significant discretion to national jurisdictions. This creates a fragmented global landscape where synthetic biology innovations may receive varying levels of protection across different territories, complicating international research collaboration and commercialization strategies.
Regulatory challenges specific to synthetic biology include determining the appropriate scope of patent claims for engineered biological systems, addressing concerns about patenting foundational technologies that could impede innovation, and balancing open-source principles with proprietary rights. The BioBricks Foundation's BioBrick Public Agreement represents an alternative approach, promoting standardized biological parts available for free use while allowing downstream applications to be patented.
Recent regulatory developments include the implementation of the Nagoya Protocol on Access and Benefit-sharing, which affects how genetic resources can be utilized in synthetic biology research. Additionally, regulatory bodies are increasingly considering ethical implications when evaluating patent applications for synthetic biology innovations, particularly those involving gene editing technologies like CRISPR-Cas9.
The interplay between patent regulations and biosafety frameworks adds another layer of complexity, as innovations must navigate both intellectual property protection and compliance with biosafety standards established by organizations such as the International Genetically Engineered Machine (iGEM) Foundation and national biosafety committees.
The European Patent Office maintains stricter standards regarding the patentability of biological materials, prohibiting patents on processes for human cloning, human germline modification, and uses of human embryos for industrial purposes under the European Biotechnology Directive (98/44/EC). Meanwhile, China has been rapidly developing its patent framework for biotechnology, with recent amendments to its Patent Law in 2020 strengthening protection for biological inventions.
International harmonization efforts through WIPO and the TRIPS Agreement provide minimum standards for intellectual property protection but leave significant discretion to national jurisdictions. This creates a fragmented global landscape where synthetic biology innovations may receive varying levels of protection across different territories, complicating international research collaboration and commercialization strategies.
Regulatory challenges specific to synthetic biology include determining the appropriate scope of patent claims for engineered biological systems, addressing concerns about patenting foundational technologies that could impede innovation, and balancing open-source principles with proprietary rights. The BioBricks Foundation's BioBrick Public Agreement represents an alternative approach, promoting standardized biological parts available for free use while allowing downstream applications to be patented.
Recent regulatory developments include the implementation of the Nagoya Protocol on Access and Benefit-sharing, which affects how genetic resources can be utilized in synthetic biology research. Additionally, regulatory bodies are increasingly considering ethical implications when evaluating patent applications for synthetic biology innovations, particularly those involving gene editing technologies like CRISPR-Cas9.
The interplay between patent regulations and biosafety frameworks adds another layer of complexity, as innovations must navigate both intellectual property protection and compliance with biosafety standards established by organizations such as the International Genetically Engineered Machine (iGEM) Foundation and national biosafety committees.
Ethical and Biosafety Considerations
Synthetic biology raises profound ethical questions that extend beyond technical capabilities to fundamental considerations about human intervention in natural systems. The manipulation of genetic material to create novel organisms or modify existing ones presents unprecedented ethical challenges regarding the boundaries of human innovation. These concerns are amplified when considering the potential for unintended consequences in complex biological systems, where our understanding remains incomplete despite significant advances. Ethical frameworks must balance innovation potential against risks of disrupting ecological balances or creating organisms with unforeseen properties.
Biosafety considerations in synthetic biology operate at multiple levels, from laboratory containment to ecosystem impact assessment. Current biosafety protocols emphasize physical containment strategies, genetic safeguards, and risk assessment methodologies. However, these measures face increasing challenges as synthetic biology techniques become more accessible and powerful. The development of "kill switches" and other biological containment strategies represents an important advancement, though their reliability under diverse environmental conditions remains questionable.
The intersection of patent regulations with ethical and biosafety considerations creates additional complexity. Patent protection may incentivize responsible innovation by requiring disclosure and regulatory compliance, but may simultaneously restrict access to safety technologies or create competitive pressures that compromise safety standards. The current regulatory landscape varies significantly across jurisdictions, creating potential for regulatory arbitrage where research migrates to regions with less stringent oversight.
International governance frameworks for synthetic biology remain fragmented, with the Cartagena Protocol on Biosafety providing some guidance but lacking specific provisions for many synthetic biology applications. This regulatory gap is particularly concerning given the potential for transboundary impacts of synthetic organisms. Efforts to harmonize regulations must navigate tensions between precautionary approaches and innovation-friendly frameworks.
Public engagement represents another critical dimension of ethical governance in synthetic biology. Meaningful stakeholder participation in regulatory development can enhance legitimacy and incorporate diverse value perspectives. However, effective engagement requires addressing knowledge asymmetries and ensuring representation of marginalized communities who may bear disproportionate risks or benefits from synthetic biology applications.
Looking forward, adaptive governance approaches that evolve with technological capabilities may offer the most promising path for balancing innovation with responsible development. These frameworks must incorporate ongoing risk assessment, transparent decision-making processes, and mechanisms for responding to emerging concerns as the field advances.
Biosafety considerations in synthetic biology operate at multiple levels, from laboratory containment to ecosystem impact assessment. Current biosafety protocols emphasize physical containment strategies, genetic safeguards, and risk assessment methodologies. However, these measures face increasing challenges as synthetic biology techniques become more accessible and powerful. The development of "kill switches" and other biological containment strategies represents an important advancement, though their reliability under diverse environmental conditions remains questionable.
The intersection of patent regulations with ethical and biosafety considerations creates additional complexity. Patent protection may incentivize responsible innovation by requiring disclosure and regulatory compliance, but may simultaneously restrict access to safety technologies or create competitive pressures that compromise safety standards. The current regulatory landscape varies significantly across jurisdictions, creating potential for regulatory arbitrage where research migrates to regions with less stringent oversight.
International governance frameworks for synthetic biology remain fragmented, with the Cartagena Protocol on Biosafety providing some guidance but lacking specific provisions for many synthetic biology applications. This regulatory gap is particularly concerning given the potential for transboundary impacts of synthetic organisms. Efforts to harmonize regulations must navigate tensions between precautionary approaches and innovation-friendly frameworks.
Public engagement represents another critical dimension of ethical governance in synthetic biology. Meaningful stakeholder participation in regulatory development can enhance legitimacy and incorporate diverse value perspectives. However, effective engagement requires addressing knowledge asymmetries and ensuring representation of marginalized communities who may bear disproportionate risks or benefits from synthetic biology applications.
Looking forward, adaptive governance approaches that evolve with technological capabilities may offer the most promising path for balancing innovation with responsible development. These frameworks must incorporate ongoing risk assessment, transparent decision-making processes, and mechanisms for responding to emerging concerns as the field advances.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!