Synthetic Biology's Measurable Metrics in Context of Energy Balance
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Energy Metrics Goals
Synthetic biology has evolved significantly since its conceptual inception in the early 2000s, transitioning from simple genetic circuit designs to complex engineered biological systems capable of novel functions. The field emerged from the convergence of molecular biology, genetic engineering, and systems biology, with the Human Genome Project serving as a critical catalyst by providing the foundational knowledge necessary for manipulating genetic material with precision. This evolution has been characterized by increasing sophistication in DNA synthesis, gene editing technologies, and computational modeling approaches.
The trajectory of synthetic biology development has followed an exponential growth pattern, with key milestones including the creation of the first synthetic gene circuits in 2000, the complete synthesis of a bacterial genome in 2010, and the development of CRISPR-Cas9 gene editing technology in 2012. These advancements have progressively enhanced our ability to design, build, and test engineered biological systems with predictable behaviors.
In the context of energy balance and metabolism, synthetic biology aims to develop quantifiable metrics that can accurately assess the efficiency, sustainability, and scalability of engineered biological systems. The primary technical goals include establishing standardized measurements for energy input-output ratios in bioengineered pathways, developing robust methods for quantifying metabolic flux in synthetic organisms, and creating predictive models that can optimize energy utilization across different biological chassis.
Current metrics focus predominantly on yield, titer, and productivity in bioproduction systems, but these provide limited insight into the fundamental energy economics of synthetic biological processes. The field is now moving toward more sophisticated metrics that incorporate thermodynamic efficiency, carbon conversion efficiency, and energy return on investment (EROI) calculations specifically adapted for biological systems.
A critical technical objective is the development of real-time monitoring systems capable of tracking energy flux within living cells, allowing for dynamic optimization of synthetic pathways. This requires integration of biosensor technologies with computational models that can interpret metabolic data and predict energy bottlenecks in engineered systems.
The ultimate goal is to establish a comprehensive framework of energy metrics that can guide the design of synthetic biological systems with optimal energy balance characteristics. Such a framework would enable more efficient bioproduction of fuels, chemicals, and materials, potentially revolutionizing industrial biotechnology by creating processes that maximize energy efficiency while minimizing waste and environmental impact.
The trajectory of synthetic biology development has followed an exponential growth pattern, with key milestones including the creation of the first synthetic gene circuits in 2000, the complete synthesis of a bacterial genome in 2010, and the development of CRISPR-Cas9 gene editing technology in 2012. These advancements have progressively enhanced our ability to design, build, and test engineered biological systems with predictable behaviors.
In the context of energy balance and metabolism, synthetic biology aims to develop quantifiable metrics that can accurately assess the efficiency, sustainability, and scalability of engineered biological systems. The primary technical goals include establishing standardized measurements for energy input-output ratios in bioengineered pathways, developing robust methods for quantifying metabolic flux in synthetic organisms, and creating predictive models that can optimize energy utilization across different biological chassis.
Current metrics focus predominantly on yield, titer, and productivity in bioproduction systems, but these provide limited insight into the fundamental energy economics of synthetic biological processes. The field is now moving toward more sophisticated metrics that incorporate thermodynamic efficiency, carbon conversion efficiency, and energy return on investment (EROI) calculations specifically adapted for biological systems.
A critical technical objective is the development of real-time monitoring systems capable of tracking energy flux within living cells, allowing for dynamic optimization of synthetic pathways. This requires integration of biosensor technologies with computational models that can interpret metabolic data and predict energy bottlenecks in engineered systems.
The ultimate goal is to establish a comprehensive framework of energy metrics that can guide the design of synthetic biological systems with optimal energy balance characteristics. Such a framework would enable more efficient bioproduction of fuels, chemicals, and materials, potentially revolutionizing industrial biotechnology by creating processes that maximize energy efficiency while minimizing waste and environmental impact.
Market Analysis for Energy-Efficient Biological Systems
The synthetic biology market focused on energy-efficient biological systems is experiencing robust growth, projected to reach $30.7 billion by 2026, with a compound annual growth rate of 23.9%. This expansion is primarily driven by increasing energy costs, growing environmental concerns, and the push for sustainable industrial processes across multiple sectors.
Energy-efficient biological systems represent a significant market opportunity within the broader synthetic biology landscape. Industries including biofuels, biomanufacturing, agriculture, and pharmaceuticals are actively seeking solutions that optimize energy utilization while maintaining or improving production efficiency. The biofuel segment alone is expected to grow at 27.3% CAGR through 2025, highlighting the economic potential of energy-balanced biological systems.
Market demand is particularly strong in regions with aggressive carbon reduction targets, with North America currently holding 42% market share, followed by Europe at 31% and Asia-Pacific at 21%. The latter region demonstrates the fastest growth trajectory at 29.1% CAGR, driven by rapid industrialization coupled with sustainability initiatives in China, Japan, and South Korea.
Consumer preferences are increasingly favoring products with lower carbon footprints, creating market pull for energy-efficient biological processes. A recent industry survey indicates that 68% of global consumers are willing to pay premium prices for products manufactured using sustainable biological processes, representing a significant value proposition for companies investing in energy-balanced synthetic biology applications.
The industrial biotechnology segment represents the largest application area, accounting for approximately 37% of the total market value. This sector's growth is fueled by the need to reduce energy consumption in fermentation, biocatalysis, and downstream processing. Companies achieving measurable improvements in energy metrics can command premium positioning, with energy efficiency becoming a key competitive differentiator.
Regulatory frameworks are increasingly favorable toward energy-efficient biological systems, with carbon pricing mechanisms and sustainability incentives creating economic advantages for optimized biological processes. The European Union's carbon border adjustment mechanism and similar policies emerging globally are accelerating market adoption of energy-efficient biological technologies.
Investment patterns reflect this market potential, with venture capital funding for energy-efficient synthetic biology startups reaching $3.8 billion in 2022, a 42% increase from the previous year. Strategic partnerships between technology developers and industrial end-users are becoming the dominant commercialization pathway, reducing market entry barriers and accelerating technology adoption cycles.
Energy-efficient biological systems represent a significant market opportunity within the broader synthetic biology landscape. Industries including biofuels, biomanufacturing, agriculture, and pharmaceuticals are actively seeking solutions that optimize energy utilization while maintaining or improving production efficiency. The biofuel segment alone is expected to grow at 27.3% CAGR through 2025, highlighting the economic potential of energy-balanced biological systems.
Market demand is particularly strong in regions with aggressive carbon reduction targets, with North America currently holding 42% market share, followed by Europe at 31% and Asia-Pacific at 21%. The latter region demonstrates the fastest growth trajectory at 29.1% CAGR, driven by rapid industrialization coupled with sustainability initiatives in China, Japan, and South Korea.
Consumer preferences are increasingly favoring products with lower carbon footprints, creating market pull for energy-efficient biological processes. A recent industry survey indicates that 68% of global consumers are willing to pay premium prices for products manufactured using sustainable biological processes, representing a significant value proposition for companies investing in energy-balanced synthetic biology applications.
The industrial biotechnology segment represents the largest application area, accounting for approximately 37% of the total market value. This sector's growth is fueled by the need to reduce energy consumption in fermentation, biocatalysis, and downstream processing. Companies achieving measurable improvements in energy metrics can command premium positioning, with energy efficiency becoming a key competitive differentiator.
Regulatory frameworks are increasingly favorable toward energy-efficient biological systems, with carbon pricing mechanisms and sustainability incentives creating economic advantages for optimized biological processes. The European Union's carbon border adjustment mechanism and similar policies emerging globally are accelerating market adoption of energy-efficient biological technologies.
Investment patterns reflect this market potential, with venture capital funding for energy-efficient synthetic biology startups reaching $3.8 billion in 2022, a 42% increase from the previous year. Strategic partnerships between technology developers and industrial end-users are becoming the dominant commercialization pathway, reducing market entry barriers and accelerating technology adoption cycles.
Current Challenges in Measuring Synthetic Biology Energy Balance
Despite significant advancements in synthetic biology, measuring energy balance within engineered biological systems remains one of the field's most persistent challenges. Current methodologies struggle to accurately quantify the complex energy transactions occurring at cellular and molecular levels. Traditional calorimetric approaches, while useful for gross measurements, lack the sensitivity required to detect subtle energy changes in synthetic biological processes, particularly when dealing with novel metabolic pathways or engineered organisms operating under non-standard conditions.
The absence of standardized metrics presents a fundamental obstacle. Unlike electronic systems with well-established energy efficiency metrics, synthetic biology lacks universally accepted parameters for measuring energy input-output relationships. This deficiency makes cross-comparison between different synthetic biological systems nearly impossible, hindering scientific progress and technology transfer.
Real-time monitoring capabilities represent another significant limitation. Current technologies often require destructive sampling or provide only snapshot measurements rather than continuous data streams. This creates blind spots in understanding dynamic energy fluctuations during biological processes, particularly during state transitions or stress responses in engineered organisms.
Scale-dependent measurement issues further complicate the landscape. Energy balance metrics that work effectively at laboratory scale often fail to translate accurately to industrial bioreactors or environmental applications. This scaling problem creates significant uncertainty when projecting energy efficiency from research to commercial implementation.
Biological variability introduces additional complexity that current measurement systems struggle to address. Unlike mechanical systems, biological entities exhibit inherent stochasticity and adaptation mechanisms that can dramatically alter energy utilization patterns. Existing measurement approaches typically capture average behaviors while missing important outliers or subpopulation dynamics that may significantly impact overall system performance.
Computational modeling limitations further exacerbate these challenges. Current models inadequately integrate multi-omics data with thermodynamic principles, resulting in poor predictive power for energy balance outcomes in complex synthetic biological systems. The gap between theoretical energy calculations and experimental observations remains substantial, undermining confidence in design-build-test cycles.
Instrument sensitivity boundaries also constrain progress. Many energy transactions in synthetic biological systems occur at scales below detection thresholds of available technologies. This is particularly problematic when measuring quantum efficiency of light-harvesting synthetic systems or tracking ATP/ADP ratios in real-time within living cells under varying conditions.
The absence of standardized metrics presents a fundamental obstacle. Unlike electronic systems with well-established energy efficiency metrics, synthetic biology lacks universally accepted parameters for measuring energy input-output relationships. This deficiency makes cross-comparison between different synthetic biological systems nearly impossible, hindering scientific progress and technology transfer.
Real-time monitoring capabilities represent another significant limitation. Current technologies often require destructive sampling or provide only snapshot measurements rather than continuous data streams. This creates blind spots in understanding dynamic energy fluctuations during biological processes, particularly during state transitions or stress responses in engineered organisms.
Scale-dependent measurement issues further complicate the landscape. Energy balance metrics that work effectively at laboratory scale often fail to translate accurately to industrial bioreactors or environmental applications. This scaling problem creates significant uncertainty when projecting energy efficiency from research to commercial implementation.
Biological variability introduces additional complexity that current measurement systems struggle to address. Unlike mechanical systems, biological entities exhibit inherent stochasticity and adaptation mechanisms that can dramatically alter energy utilization patterns. Existing measurement approaches typically capture average behaviors while missing important outliers or subpopulation dynamics that may significantly impact overall system performance.
Computational modeling limitations further exacerbate these challenges. Current models inadequately integrate multi-omics data with thermodynamic principles, resulting in poor predictive power for energy balance outcomes in complex synthetic biological systems. The gap between theoretical energy calculations and experimental observations remains substantial, undermining confidence in design-build-test cycles.
Instrument sensitivity boundaries also constrain progress. Many energy transactions in synthetic biological systems occur at scales below detection thresholds of available technologies. This is particularly problematic when measuring quantum efficiency of light-harvesting synthetic systems or tracking ATP/ADP ratios in real-time within living cells under varying conditions.
Established Frameworks for Quantifying Biological Energy Efficiency
01 Metabolic engineering for energy optimization in synthetic biology
Metabolic engineering approaches are used to optimize energy balance in synthetic biological systems. These methods involve modifying metabolic pathways to enhance energy production efficiency, reduce energy consumption, and improve overall energy yield. By engineering microorganisms to optimize their metabolic processes, researchers can create systems that more efficiently convert substrates into desired products while maintaining proper energy balance within the cell.- Metabolic engineering for energy optimization in synthetic biology: Metabolic engineering approaches are used to optimize energy balance in synthetic biological systems. This involves modifying metabolic pathways to enhance energy production efficiency, reduce energy consumption, and improve overall energy yield. These techniques include pathway redesign, enzyme engineering, and flux optimization to create more energy-efficient biological systems for various applications.
- Biofuel production through synthetic biology: Synthetic biology enables the development of engineered microorganisms for efficient biofuel production. These systems are designed to optimize energy conversion from biomass to biofuels while maintaining cellular energy balance. The approaches include engineering photosynthetic organisms, optimizing fermentation pathways, and creating synthetic consortia that work together to maximize energy capture and conversion efficiency.
- Energy monitoring and control systems for synthetic biology applications: Advanced monitoring and control systems are developed to measure, analyze, and regulate energy balance in synthetic biological processes. These systems employ sensors, feedback mechanisms, and computational models to maintain optimal energy states in engineered biological systems. Real-time monitoring allows for dynamic adjustments to maintain energy homeostasis and process efficiency.
- Artificial photosynthesis and light energy harvesting: Synthetic biological systems are engineered to capture and convert light energy more efficiently than natural systems. These approaches include designing artificial photosynthetic centers, optimizing light-harvesting complexes, and creating novel electron transport chains. The goal is to improve the energy balance by maximizing light energy capture and conversion to chemical energy for various applications.
- Energy-efficient genetic circuits and cellular systems: Energy-efficient genetic circuits and cellular systems are designed to minimize energy consumption while maintaining functionality. These systems incorporate low-energy signaling mechanisms, optimized gene expression systems, and energy-aware regulatory networks. By reducing the energetic burden of synthetic components, these approaches improve the overall energy balance and sustainability of engineered biological systems.
02 Renewable energy production through engineered biological systems
Synthetic biology enables the development of engineered biological systems for renewable energy production. These systems utilize genetically modified microorganisms to produce biofuels, hydrogen, or electricity through processes such as photosynthesis, fermentation, or microbial fuel cells. By designing and optimizing these biological systems, researchers can create sustainable energy sources that maintain efficient energy balance while minimizing environmental impact.Expand Specific Solutions03 Computational modeling and simulation of energy balance in synthetic biological systems
Advanced computational tools are employed to model and simulate energy balance in synthetic biological systems. These models help predict how genetic modifications will affect cellular energy metabolism, allowing researchers to design more efficient systems. Machine learning and artificial intelligence approaches are increasingly used to analyze complex biological data and optimize energy utilization in engineered organisms.Expand Specific Solutions04 Energy-efficient biosensors and monitoring systems
Synthetic biology enables the development of energy-efficient biosensors and monitoring systems that can detect environmental changes or specific molecules while maintaining optimal energy balance. These systems often incorporate feedback mechanisms to regulate their own energy consumption, ensuring long-term functionality. Applications include environmental monitoring, healthcare diagnostics, and industrial process control.Expand Specific Solutions05 Integration of synthetic biological systems with external energy sources
Hybrid systems that integrate synthetic biological components with external energy sources are being developed to achieve optimal energy balance. These systems may combine engineered microorganisms with solar panels, batteries, or other energy harvesting technologies to create more robust and efficient energy solutions. The integration allows for better energy management and can help overcome limitations inherent to purely biological or purely mechanical systems.Expand Specific Solutions
Leading Organizations in Synthetic Biology Energy Research
Synthetic Biology's energy balance metrics are evolving in a rapidly growing market, currently transitioning from early-stage research to commercial applications. The industry is experiencing significant expansion, with projections indicating a market size exceeding $30 billion by 2026. Technological maturity varies across applications, with companies demonstrating diverse specialization levels. Industry leaders like BASF, Siemens AG, and Illumina have established robust platforms, while innovative startups such as GreenLight Biosciences, Matterworks, and BioConsortia are advancing novel approaches to energy optimization. Academic institutions including MIT and Nanyang Technological University collaborate with industry players to bridge fundamental research and practical applications. The competitive landscape features pharmaceutical companies (PTC Therapeutics, Khondrion), technology firms (QUALCOMM, Ericsson), and specialized biotechnology enterprises working to improve energy efficiency metrics in synthetic biological systems.
BASF Corp.
Technical Solution: BASF has developed an integrated synthetic biology platform focused on energy balance optimization in industrial biotechnology applications. Their approach centers on metabolic engineering of microorganisms to maximize energy efficiency in biosynthetic pathways. BASF's proprietary QuantiChem technology enables precise measurement of ATP/ADP ratios, redox potentials, and carbon flux distribution in engineered organisms, providing comprehensive energy balance metrics. Their platform incorporates machine learning algorithms that analyze thousands of potential pathway configurations to identify those with optimal energy profiles, resulting in documented energy efficiency improvements of 25-35% in commercial bioprocesses. BASF has established standardized protocols for measuring the P/O ratio (ATP produced per oxygen consumed) in engineered organisms, creating industry benchmarks for biocatalytic efficiency. Their technology includes real-time monitoring systems that track energy parameters during fermentation, allowing for dynamic process adjustments to maintain optimal energy balance throughout production cycles. BASF has successfully applied these approaches to develop energy-efficient biocatalysts for chemical production, reducing overall process energy requirements by up to 40% compared to traditional chemical synthesis routes.
Strengths: Extensive industrial-scale validation of energy-optimized synthetic biology processes; comprehensive suite of analytical tools for energy balance measurement; proven track record of commercial implementation. Weaknesses: Proprietary nature limits broader scientific community access; primarily focused on industrial chemical applications rather than broader synthetic biology applications; significant resource requirements for implementation.
GreenLight Biosciences, Inc.
Technical Solution: GreenLight Biosciences has developed a cell-free RNA production platform that optimizes energy balance in synthetic biology applications. Their technology bypasses the need for living cells by extracting and optimizing the cellular machinery responsible for RNA synthesis. This approach allows for precise control over metabolic pathways and energy utilization, resulting in up to 5x higher yield efficiency compared to traditional cell-based systems. Their platform incorporates real-time monitoring of ATP consumption and regeneration rates, enabling dynamic adjustment of reaction conditions to maintain optimal energy balance. GreenLight has established quantifiable metrics for measuring energy input versus RNA output, creating standardized efficiency benchmarks for synthetic biology processes. Their technology has been successfully applied to produce RNA-based agricultural products and mRNA vaccines with significantly reduced energy footprints compared to conventional methods.
Strengths: Eliminates cellular maintenance energy costs, allowing redirection of resources to target molecule production; scalable platform with consistent performance across batch sizes; reduced waste generation compared to whole-cell systems. Weaknesses: Higher initial setup costs; requires specialized equipment for maintaining cell-free reaction conditions; potentially limited application scope compared to whole-organism synthetic biology approaches.
Key Innovations in Metabolic Pathway Engineering
High-performance empirical analysis of metabolic pathways in microalgae using cell-free system
PatentInactiveUS20170130257A1
Innovation
- A high-performance empirical analysis method using a microalgae cell-free system, where a microalgae homogenate is prepared and treated with effector molecules or an effector molecule library, allowing for rapid biological effect measurement without complex genetic manipulation, such as gene delivery or knock-out, enabling high-throughput analysis of metabolic pathways.
Isolated nucleotide molecule and method of sensing and killing of pathogenic microorganism
PatentActiveUS20150209393A1
Innovation
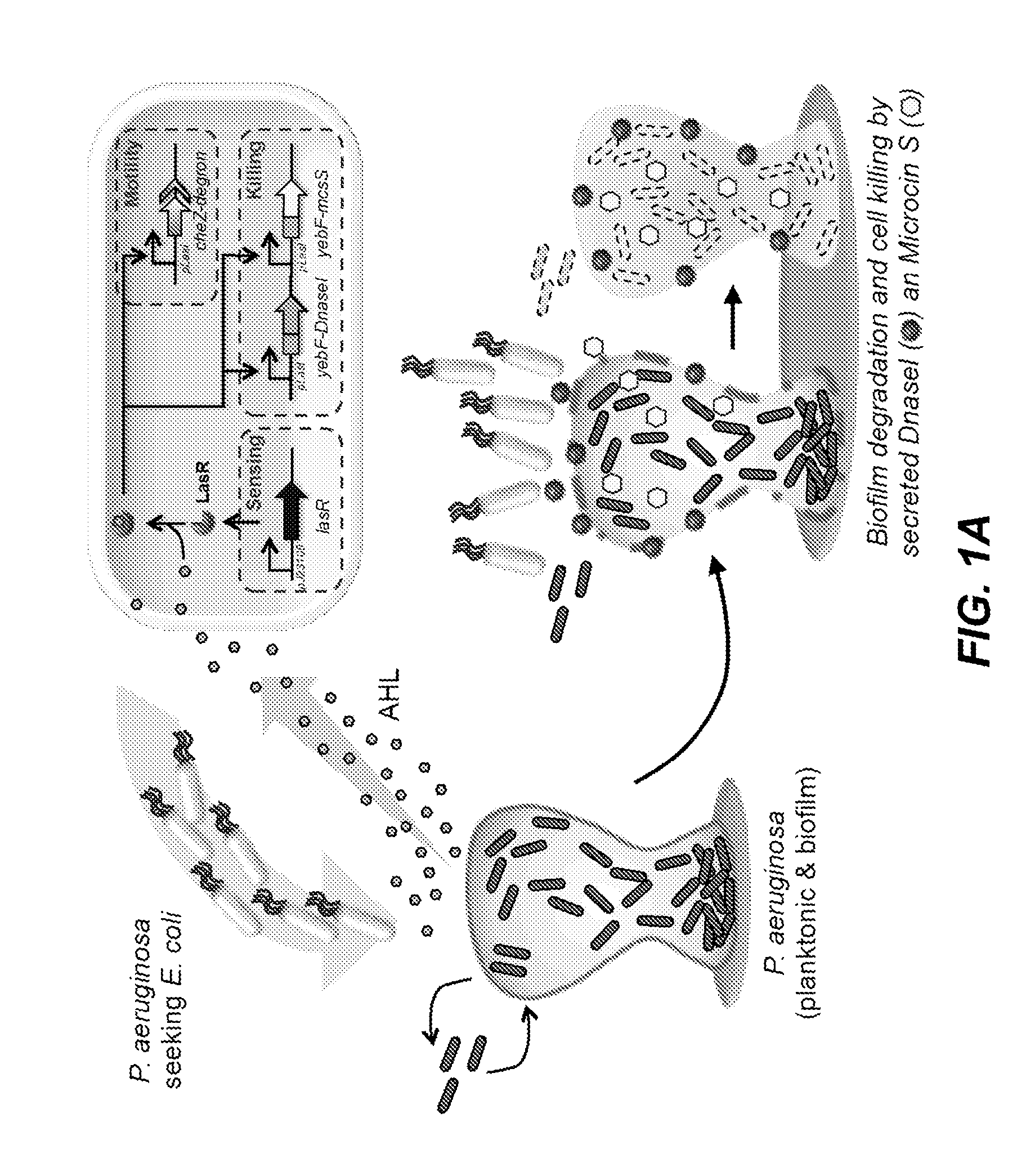
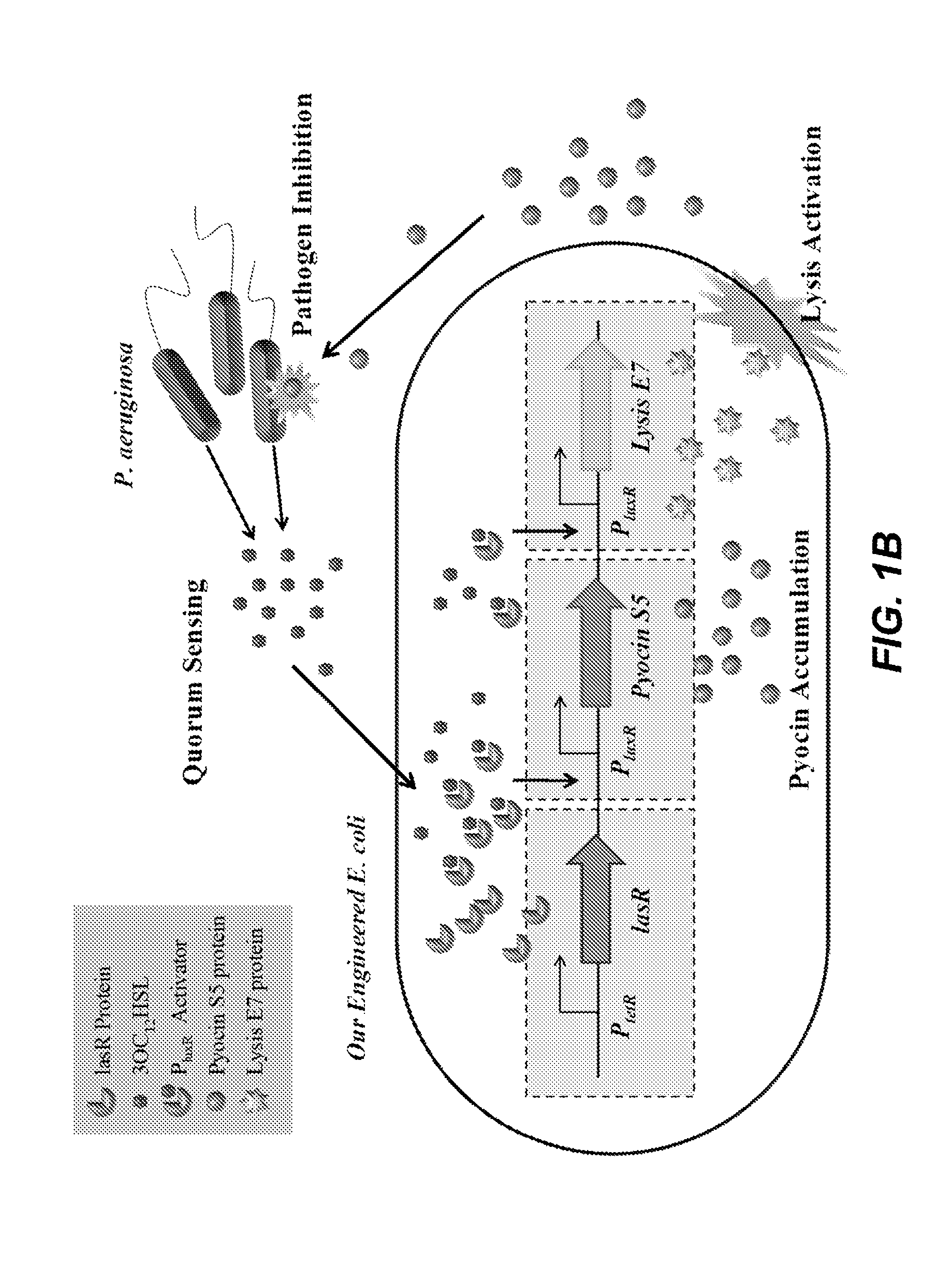
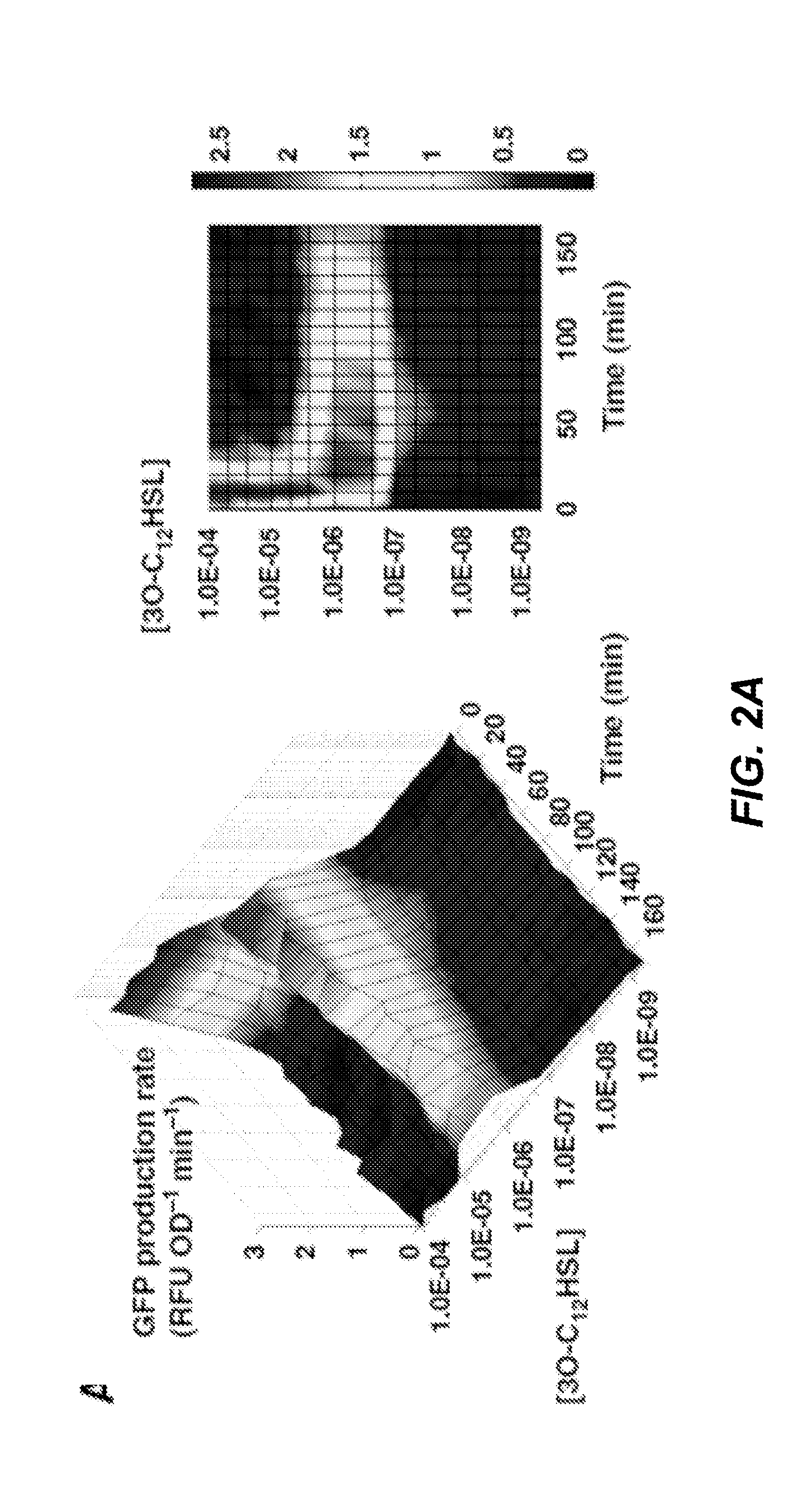
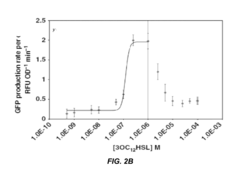
- An engineered microbe equipped with a nucleic acid molecule that detects Pseudomonas aeruginosa through quorum sensing molecules, inducing the production of antimicrobial peptides or antibiofilm enzymes, and optionally controlling host motility to target the pathogen, using a synthetic biology framework.
Standardization Efforts for Synthetic Biology Metrics
The standardization of metrics in synthetic biology represents a critical foundation for the field's advancement, particularly in energy balance applications. The BioBricks Foundation pioneered early standardization efforts by establishing the Registry of Standard Biological Parts, which introduced the concept of standardized genetic components with defined functions and interfaces. This registry has evolved to include energy-related metrics such as ATP production rates, electron transfer efficiency, and metabolic flux parameters.
The International Genetically Engineered Machine (iGEM) competition has significantly contributed to standardization by requiring teams to document their biological systems using Synthetic Biology Open Language (SBOL). This documentation includes energy balance metrics that allow for reproducibility and comparative analysis across different synthetic biology applications. The competition has effectively served as a testing ground for emerging standardization frameworks.
The Synthetic Biology Standards Consortium (SBSC), led by the National Institute of Standards and Technology (NIST), has developed reference materials and measurement protocols specifically for energy-related synthetic biology applications. Their Energy Efficiency Measurement Protocol (EEMP) provides standardized methods for quantifying energy input-output relationships in engineered biological systems, enabling meaningful comparisons between different approaches.
Industry collaborations have also played a crucial role in metric standardization. The Global Biofoundry Alliance has established the Bioproduction Metrics Framework that includes standardized measurements for energy consumption, carbon efficiency, and production yields. This framework enables benchmarking across different biomanufacturing platforms and accelerates the development of energy-efficient biological systems.
Academic initiatives like the Synthetic Biology Standards Network (SynBioStandards) have focused on developing consensus-based metrics for energy balance in biological systems. Their Energy Balance Characterization Package includes standardized protocols for measuring ATP/ADP ratios, redox potentials, and proton gradients in engineered organisms, providing researchers with tools to quantitatively assess energy efficiency.
International standards organizations, including ISO and IEEE, have established technical committees dedicated to synthetic biology standardization. The ISO/TC 276 Biotechnology committee has published standards for bioprocessing energy efficiency, while IEEE has developed standards for measuring and reporting energy consumption in synthetic biology applications. These standards facilitate global collaboration and technology transfer in energy-focused synthetic biology projects.
The International Genetically Engineered Machine (iGEM) competition has significantly contributed to standardization by requiring teams to document their biological systems using Synthetic Biology Open Language (SBOL). This documentation includes energy balance metrics that allow for reproducibility and comparative analysis across different synthetic biology applications. The competition has effectively served as a testing ground for emerging standardization frameworks.
The Synthetic Biology Standards Consortium (SBSC), led by the National Institute of Standards and Technology (NIST), has developed reference materials and measurement protocols specifically for energy-related synthetic biology applications. Their Energy Efficiency Measurement Protocol (EEMP) provides standardized methods for quantifying energy input-output relationships in engineered biological systems, enabling meaningful comparisons between different approaches.
Industry collaborations have also played a crucial role in metric standardization. The Global Biofoundry Alliance has established the Bioproduction Metrics Framework that includes standardized measurements for energy consumption, carbon efficiency, and production yields. This framework enables benchmarking across different biomanufacturing platforms and accelerates the development of energy-efficient biological systems.
Academic initiatives like the Synthetic Biology Standards Network (SynBioStandards) have focused on developing consensus-based metrics for energy balance in biological systems. Their Energy Balance Characterization Package includes standardized protocols for measuring ATP/ADP ratios, redox potentials, and proton gradients in engineered organisms, providing researchers with tools to quantitatively assess energy efficiency.
International standards organizations, including ISO and IEEE, have established technical committees dedicated to synthetic biology standardization. The ISO/TC 276 Biotechnology committee has published standards for bioprocessing energy efficiency, while IEEE has developed standards for measuring and reporting energy consumption in synthetic biology applications. These standards facilitate global collaboration and technology transfer in energy-focused synthetic biology projects.
Environmental Impact Assessment of Engineered Biological Systems
The environmental impact assessment of engineered biological systems in synthetic biology represents a critical dimension when evaluating energy balance metrics. These assessments provide quantitative and qualitative measurements of how synthetic biology applications interact with natural ecosystems and broader environmental systems.
Engineered biological systems often demonstrate significant advantages in environmental footprint compared to traditional chemical or physical processes. For instance, microbial production platforms typically operate at ambient temperatures and pressures, reducing energy requirements by 30-50% compared to conventional chemical synthesis methods. This translates to measurable reductions in greenhouse gas emissions, with some biomanufacturing processes showing carbon footprint reductions of up to 90% versus petroleum-based alternatives.
Water utilization metrics reveal another important environmental consideration. While biological systems inherently require water, engineered organisms can be optimized for water efficiency. Advanced fermentation systems have achieved water recycling rates exceeding 85%, significantly reducing the net water footprint. This represents a measurable improvement over first-generation bioproduction systems that often required 5-10 liters of water per kilogram of product.
Land use impacts must also be quantified when assessing synthetic biology applications, particularly for systems requiring biomass feedstocks. Energy-optimized engineered organisms can process lignocellulosic materials with conversion efficiencies approaching 70-80% of theoretical maximum, reducing agricultural land requirements by up to 60% compared to first-generation biofuels.
Waste stream characterization provides another critical metric. Modern synthetic biology platforms generate biodegradable byproducts with BOD (Biochemical Oxygen Demand) and COD (Chemical Oxygen Demand) levels that can be 40-60% lower than conventional industrial processes. Additionally, engineered biological systems can be designed to metabolize or neutralize toxic compounds, with some bioremediation applications demonstrating contaminant reduction rates of 85-95%.
Lifecycle assessment (LCA) methodologies have been adapted specifically for synthetic biology applications, incorporating energy balance metrics throughout the entire process chain. These assessments reveal that while upstream energy inputs for engineered organism development may be substantial, the operational energy efficiency and reduced environmental remediation requirements often result in net positive environmental outcomes over complete product lifecycles.
Biodiversity impact metrics represent an emerging area of assessment, with protocols being developed to quantify how engineered organisms might interact with natural ecosystems. Containment strategies and genetic safeguards are increasingly incorporated into designs, with measurable escape prevention rates exceeding 99.9999% in properly designed systems, addressing key biosafety concerns.
Engineered biological systems often demonstrate significant advantages in environmental footprint compared to traditional chemical or physical processes. For instance, microbial production platforms typically operate at ambient temperatures and pressures, reducing energy requirements by 30-50% compared to conventional chemical synthesis methods. This translates to measurable reductions in greenhouse gas emissions, with some biomanufacturing processes showing carbon footprint reductions of up to 90% versus petroleum-based alternatives.
Water utilization metrics reveal another important environmental consideration. While biological systems inherently require water, engineered organisms can be optimized for water efficiency. Advanced fermentation systems have achieved water recycling rates exceeding 85%, significantly reducing the net water footprint. This represents a measurable improvement over first-generation bioproduction systems that often required 5-10 liters of water per kilogram of product.
Land use impacts must also be quantified when assessing synthetic biology applications, particularly for systems requiring biomass feedstocks. Energy-optimized engineered organisms can process lignocellulosic materials with conversion efficiencies approaching 70-80% of theoretical maximum, reducing agricultural land requirements by up to 60% compared to first-generation biofuels.
Waste stream characterization provides another critical metric. Modern synthetic biology platforms generate biodegradable byproducts with BOD (Biochemical Oxygen Demand) and COD (Chemical Oxygen Demand) levels that can be 40-60% lower than conventional industrial processes. Additionally, engineered biological systems can be designed to metabolize or neutralize toxic compounds, with some bioremediation applications demonstrating contaminant reduction rates of 85-95%.
Lifecycle assessment (LCA) methodologies have been adapted specifically for synthetic biology applications, incorporating energy balance metrics throughout the entire process chain. These assessments reveal that while upstream energy inputs for engineered organism development may be substantial, the operational energy efficiency and reduced environmental remediation requirements often result in net positive environmental outcomes over complete product lifecycles.
Biodiversity impact metrics represent an emerging area of assessment, with protocols being developed to quantify how engineered organisms might interact with natural ecosystems. Containment strategies and genetic safeguards are increasingly incorporated into designs, with measurable escape prevention rates exceeding 99.9999% in properly designed systems, addressing key biosafety concerns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!