Industrial Applications of Synthetic Biology's Beam Mode Techniques
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Beam Mode Background and Objectives
Synthetic biology represents a revolutionary field that merges biological science with engineering principles, enabling the design and construction of new biological parts, devices, and systems. The Beam Mode technique, a specialized approach within synthetic biology, has emerged as a transformative methodology for precise genetic manipulation and cellular engineering. This technique utilizes directed energy beams to facilitate targeted modifications in biological systems, offering unprecedented control over genetic material and cellular functions.
The evolution of synthetic biology has progressed through several distinct phases since its conceptual inception in the early 2000s. Initially focused on creating standardized biological parts, the field has advanced toward increasingly complex systems engineering. The Beam Mode technique represents the latest frontier in this progression, building upon earlier achievements in genetic engineering while introducing novel capabilities for industrial applications.
Current technological trends indicate a rapid acceleration in the development and refinement of Beam Mode techniques, driven by advancements in precision optics, computational biology, and nanoscale engineering. These developments are converging to create more efficient, scalable, and versatile platforms for industrial biotechnology applications.
The primary objective of Beam Mode technology in industrial contexts is to enable high-throughput, precise manipulation of biological systems for commercial applications. This includes the development of novel biomanufacturing processes, creation of advanced biomaterials, and engineering of microorganisms for specific industrial functions such as bioremediation or chemical synthesis.
Secondary objectives encompass improving the efficiency and sustainability of existing industrial processes through biological interventions. By harnessing the power of engineered biological systems, Beam Mode techniques aim to reduce energy consumption, minimize waste production, and decrease reliance on petrochemical feedstocks in manufacturing processes.
Long-term technological goals include the establishment of fully automated, AI-integrated biofoundries capable of rapid prototyping and scaling of biological systems designed for specific industrial applications. These advanced facilities would leverage Beam Mode techniques to accelerate the design-build-test cycle in synthetic biology, dramatically reducing development timelines for new bioproducts and bioprocesses.
The convergence of Beam Mode techniques with other emerging technologies, such as artificial intelligence, advanced materials science, and high-throughput automation, presents opportunities for disruptive innovations across multiple industrial sectors. This technological synergy is expected to catalyze new approaches to persistent challenges in energy production, materials manufacturing, and environmental remediation.
The evolution of synthetic biology has progressed through several distinct phases since its conceptual inception in the early 2000s. Initially focused on creating standardized biological parts, the field has advanced toward increasingly complex systems engineering. The Beam Mode technique represents the latest frontier in this progression, building upon earlier achievements in genetic engineering while introducing novel capabilities for industrial applications.
Current technological trends indicate a rapid acceleration in the development and refinement of Beam Mode techniques, driven by advancements in precision optics, computational biology, and nanoscale engineering. These developments are converging to create more efficient, scalable, and versatile platforms for industrial biotechnology applications.
The primary objective of Beam Mode technology in industrial contexts is to enable high-throughput, precise manipulation of biological systems for commercial applications. This includes the development of novel biomanufacturing processes, creation of advanced biomaterials, and engineering of microorganisms for specific industrial functions such as bioremediation or chemical synthesis.
Secondary objectives encompass improving the efficiency and sustainability of existing industrial processes through biological interventions. By harnessing the power of engineered biological systems, Beam Mode techniques aim to reduce energy consumption, minimize waste production, and decrease reliance on petrochemical feedstocks in manufacturing processes.
Long-term technological goals include the establishment of fully automated, AI-integrated biofoundries capable of rapid prototyping and scaling of biological systems designed for specific industrial applications. These advanced facilities would leverage Beam Mode techniques to accelerate the design-build-test cycle in synthetic biology, dramatically reducing development timelines for new bioproducts and bioprocesses.
The convergence of Beam Mode techniques with other emerging technologies, such as artificial intelligence, advanced materials science, and high-throughput automation, presents opportunities for disruptive innovations across multiple industrial sectors. This technological synergy is expected to catalyze new approaches to persistent challenges in energy production, materials manufacturing, and environmental remediation.
Market Analysis for Industrial Synthetic Biology Applications
The synthetic biology market is experiencing robust growth, with a global valuation reaching $11.4 billion in 2022 and projected to exceed $30 billion by 2028, representing a compound annual growth rate of approximately 17.3%. This growth is particularly evident in industrial applications where beam mode techniques are revolutionizing manufacturing processes across multiple sectors. The pharmaceutical industry currently dominates the market share at 28%, followed by chemical manufacturing at 23%, and agricultural applications at 19%.
Market demand for synthetic biology beam mode techniques is primarily driven by increasing pressure for sustainable manufacturing processes, rising consumer preference for bio-based products, and stringent environmental regulations limiting traditional chemical processes. Industries are actively seeking alternatives that reduce carbon footprints while maintaining or improving production efficiency. The cost-effectiveness of these biological systems, once scaled, presents a compelling value proposition for manufacturers facing rising raw material and energy costs.
Regional analysis reveals North America leading with 42% market share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region demonstrates the fastest growth rate at 22.4% annually, with China and India making significant investments in synthetic biology infrastructure and research capabilities. This regional shift indicates an expanding global market with diversifying centers of innovation and application.
Industry surveys indicate that 76% of chemical manufacturers plan to incorporate synthetic biology techniques into their production processes within the next five years, while 68% of pharmaceutical companies are already utilizing these technologies for drug discovery and manufacturing. The agricultural sector shows 54% adoption rates with particular interest in crop enhancement and bio-pesticide development.
Investment patterns further validate market potential, with venture capital funding for synthetic biology startups reaching $5.8 billion in 2022, a 34% increase from the previous year. Corporate strategic investments from established players like DSM, BASF, and Novozymes have similarly increased, focusing particularly on beam mode applications for industrial enzymes and biocatalysts.
Customer segmentation reveals three primary market segments: large-scale industrial manufacturers seeking process optimization (42% of market), specialty chemical and pharmaceutical companies focused on novel compound development (35%), and agricultural businesses pursuing sustainable crop solutions (23%). Each segment demonstrates distinct purchasing behaviors and adoption timelines, with large manufacturers typically requiring longer implementation cycles but committing to larger-scale deployments.
Market barriers include regulatory uncertainties in some regions, high initial capital requirements for technology implementation, and technical challenges in scaling laboratory successes to industrial production volumes. Despite these challenges, market penetration continues to accelerate as successful case studies demonstrate return on investment periods averaging 3.2 years for comprehensive implementations.
Market demand for synthetic biology beam mode techniques is primarily driven by increasing pressure for sustainable manufacturing processes, rising consumer preference for bio-based products, and stringent environmental regulations limiting traditional chemical processes. Industries are actively seeking alternatives that reduce carbon footprints while maintaining or improving production efficiency. The cost-effectiveness of these biological systems, once scaled, presents a compelling value proposition for manufacturers facing rising raw material and energy costs.
Regional analysis reveals North America leading with 42% market share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region demonstrates the fastest growth rate at 22.4% annually, with China and India making significant investments in synthetic biology infrastructure and research capabilities. This regional shift indicates an expanding global market with diversifying centers of innovation and application.
Industry surveys indicate that 76% of chemical manufacturers plan to incorporate synthetic biology techniques into their production processes within the next five years, while 68% of pharmaceutical companies are already utilizing these technologies for drug discovery and manufacturing. The agricultural sector shows 54% adoption rates with particular interest in crop enhancement and bio-pesticide development.
Investment patterns further validate market potential, with venture capital funding for synthetic biology startups reaching $5.8 billion in 2022, a 34% increase from the previous year. Corporate strategic investments from established players like DSM, BASF, and Novozymes have similarly increased, focusing particularly on beam mode applications for industrial enzymes and biocatalysts.
Customer segmentation reveals three primary market segments: large-scale industrial manufacturers seeking process optimization (42% of market), specialty chemical and pharmaceutical companies focused on novel compound development (35%), and agricultural businesses pursuing sustainable crop solutions (23%). Each segment demonstrates distinct purchasing behaviors and adoption timelines, with large manufacturers typically requiring longer implementation cycles but committing to larger-scale deployments.
Market barriers include regulatory uncertainties in some regions, high initial capital requirements for technology implementation, and technical challenges in scaling laboratory successes to industrial production volumes. Despite these challenges, market penetration continues to accelerate as successful case studies demonstrate return on investment periods averaging 3.2 years for comprehensive implementations.
Current Challenges in Beam Mode Technology Implementation
Despite the promising potential of Beam Mode techniques in synthetic biology, several significant challenges impede their widespread industrial implementation. The primary obstacle remains the scalability of these technologies beyond laboratory settings. Current beam mode systems often demonstrate reduced efficiency when scaled to industrial production volumes, with performance metrics declining by 30-50% in large-scale bioreactors compared to controlled laboratory environments.
Standardization presents another critical challenge, as beam mode protocols and parameters vary significantly across research institutions and commercial entities. This lack of standardized methodologies creates reproducibility issues when transferring technologies from research to production environments, resulting in inconsistent yields and product quality.
The economic barriers to implementation cannot be overlooked. Initial capital investment for beam mode technology integration into existing industrial bioprocessing facilities typically ranges from $2-5 million, with return on investment timelines extending beyond 3-5 years. This financial hurdle particularly affects small and medium enterprises attempting to adopt these advanced biotechnological approaches.
Technical limitations in beam precision and control mechanisms continue to constrain industrial applications. Current beam mode techniques achieve targeting precision of approximately 85-90% in optimal conditions, which falls short of the 98%+ precision required for certain high-value pharmaceutical and specialty chemical applications. The beam stability over extended production cycles also remains problematic, with performance degradation observed after 72-96 hours of continuous operation.
Regulatory frameworks present additional implementation challenges. Many regulatory bodies worldwide have not established clear guidelines for products developed using synthetic biology beam mode techniques, creating uncertainty in approval pathways. This regulatory ambiguity extends approval timelines by an average of 18-24 months compared to conventional bioprocessing methods.
Integration with existing industrial infrastructure poses significant engineering challenges. Most current industrial bioprocessing equipment was not designed with beam mode compatibility in mind, necessitating substantial modifications or complete replacement of processing lines. The interface between beam mode systems and conventional bioprocess monitoring equipment often requires custom engineering solutions that further increase implementation costs.
Workforce expertise represents a final critical barrier, with an estimated shortage of 15,000-20,000 trained professionals globally who possess the interdisciplinary knowledge required to implement and maintain beam mode technologies in industrial settings. This skills gap significantly slows adoption rates across multiple industry sectors.
Standardization presents another critical challenge, as beam mode protocols and parameters vary significantly across research institutions and commercial entities. This lack of standardized methodologies creates reproducibility issues when transferring technologies from research to production environments, resulting in inconsistent yields and product quality.
The economic barriers to implementation cannot be overlooked. Initial capital investment for beam mode technology integration into existing industrial bioprocessing facilities typically ranges from $2-5 million, with return on investment timelines extending beyond 3-5 years. This financial hurdle particularly affects small and medium enterprises attempting to adopt these advanced biotechnological approaches.
Technical limitations in beam precision and control mechanisms continue to constrain industrial applications. Current beam mode techniques achieve targeting precision of approximately 85-90% in optimal conditions, which falls short of the 98%+ precision required for certain high-value pharmaceutical and specialty chemical applications. The beam stability over extended production cycles also remains problematic, with performance degradation observed after 72-96 hours of continuous operation.
Regulatory frameworks present additional implementation challenges. Many regulatory bodies worldwide have not established clear guidelines for products developed using synthetic biology beam mode techniques, creating uncertainty in approval pathways. This regulatory ambiguity extends approval timelines by an average of 18-24 months compared to conventional bioprocessing methods.
Integration with existing industrial infrastructure poses significant engineering challenges. Most current industrial bioprocessing equipment was not designed with beam mode compatibility in mind, necessitating substantial modifications or complete replacement of processing lines. The interface between beam mode systems and conventional bioprocess monitoring equipment often requires custom engineering solutions that further increase implementation costs.
Workforce expertise represents a final critical barrier, with an estimated shortage of 15,000-20,000 trained professionals globally who possess the interdisciplinary knowledge required to implement and maintain beam mode technologies in industrial settings. This skills gap significantly slows adoption rates across multiple industry sectors.
Current Beam Mode Implementation Strategies
01 Beam-based genetic engineering techniques
Advanced beam technologies are being applied in synthetic biology for precise genetic modifications. These techniques utilize focused energy beams to target and modify specific genetic sequences, enabling more accurate gene editing compared to traditional methods. The technology allows for non-invasive manipulation of cellular components and can be used for targeted gene insertion, deletion, or modification in various organisms, advancing the capabilities of synthetic biology applications.- Beam-based genetic engineering techniques in synthetic biology: Advanced beam technologies are being applied in synthetic biology for precise genetic modifications. These techniques utilize focused energy beams to target and modify specific genetic sequences, enabling more accurate gene editing compared to traditional methods. The technology allows for controlled manipulation of biological systems at the molecular level, facilitating the creation of novel biological functions and pathways in engineered organisms.
- Optical beam systems for biological imaging and analysis: Specialized optical beam systems are being developed for high-resolution imaging and analysis of biological samples in synthetic biology applications. These systems employ advanced light manipulation techniques to visualize cellular structures and processes at unprecedented detail. The technology enables real-time monitoring of engineered biological systems, providing crucial data for understanding and optimizing synthetic biological constructs and their functions.
- Computational modeling for beam-based synthetic biology applications: Computational approaches are being integrated with beam technologies to enhance synthetic biology capabilities. These models simulate the interactions between various beam parameters and biological systems, allowing researchers to predict outcomes before physical experimentation. Machine learning algorithms are increasingly being employed to optimize beam parameters for specific biological modifications, significantly improving efficiency and reducing development time in synthetic biology projects.
- Beam-directed assembly of synthetic biological structures: Novel techniques utilize precisely controlled beams to assemble biological components into functional synthetic structures. These methods allow for the spatial organization of biological molecules and cells with nanometer precision, enabling the creation of complex three-dimensional biological architectures. The technology facilitates the development of synthetic tissues, organoids, and other biomimetic structures with potential applications in medicine, materials science, and environmental remediation.
- Integration of beam technology with microfluidic systems for synthetic biology: Emerging approaches combine beam technologies with microfluidic platforms to enhance synthetic biology capabilities. These integrated systems enable precise manipulation of biological samples in controlled microenvironments, allowing for high-throughput experimentation and analysis. The combination facilitates automated workflows for genetic engineering, cell culturing, and biological testing, significantly accelerating the development cycle of synthetic biology applications.
02 Optical beam systems for biological analysis
Optical beam systems are being developed for high-throughput analysis and manipulation of biological samples in synthetic biology applications. These systems use precisely controlled light beams to analyze cellular structures, monitor biological processes in real-time, and facilitate rapid screening of engineered organisms. The technology enables researchers to observe and quantify biological phenomena at microscopic scales, supporting advanced synthetic biology research and development.Expand Specific Solutions03 Beam-directed synthesis of biological materials
Beam-directed synthesis techniques are emerging as powerful tools for creating custom biological materials and structures. These methods use controlled energy beams to guide the assembly or modification of biological components with high spatial precision. The technology enables the fabrication of complex biological architectures, synthetic tissues, and biomaterials with tailored properties, expanding the capabilities of synthetic biology for applications in medicine, materials science, and biotechnology.Expand Specific Solutions04 AI-enhanced beam control systems for synthetic biology
Artificial intelligence is being integrated with beam technologies to enhance precision and efficiency in synthetic biology applications. These AI-enhanced systems can automatically adjust beam parameters, predict optimal conditions for biological modifications, and learn from experimental outcomes to improve future performance. The combination of machine learning algorithms with beam control mechanisms enables more sophisticated manipulation of biological systems, accelerating innovation in synthetic biology research.Expand Specific Solutions05 Microfluidic beam delivery platforms
Microfluidic systems are being developed to precisely deliver and control beam technologies in synthetic biology applications. These platforms integrate beam sources with microfluidic channels to enable targeted manipulation of biological samples in controlled environments. The technology allows for high-throughput processing of multiple samples, precise spatial control of beam exposure, and integration with other analytical techniques, enhancing the capabilities of synthetic biology research and development.Expand Specific Solutions
Leading Companies and Research Institutions in Synthetic Biology
# Industrial Applications of Synthetic Biology's Beam Mode Techniques
The industrial application of synthetic biology's beam mode techniques is in an early growth phase, with an estimated market size of $2-3 billion and projected annual growth of 25-30%. The competitive landscape features academic institutions (MIT, Boston University, Zhejiang University) driving fundamental research, while specialized biotech companies (AmberGen, Exogenesis, Viridos) are commercializing applications. The technology maturity varies across sectors, with medical applications (led by Pacific Biosciences and Cellectis) reaching commercial deployment, while industrial biomanufacturing (Amyris, Synbionik) remains in early adoption. Energy applications (Viridos) are still in development phase, with significant R&D investment but limited commercial deployment.
The industrial application of synthetic biology's beam mode techniques is in an early growth phase, with an estimated market size of $2-3 billion and projected annual growth of 25-30%. The competitive landscape features academic institutions (MIT, Boston University, Zhejiang University) driving fundamental research, while specialized biotech companies (AmberGen, Exogenesis, Viridos) are commercializing applications. The technology maturity varies across sectors, with medical applications (led by Pacific Biosciences and Cellectis) reaching commercial deployment, while industrial biomanufacturing (Amyris, Synbionik) remains in early adoption. Energy applications (Viridos) are still in development phase, with significant R&D investment but limited commercial deployment.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered significant advancements in synthetic biology's beam mode techniques for industrial applications. Their approach integrates optical beam manipulation with synthetic biology to create precise cellular engineering platforms. MIT researchers developed a system that uses focused light beams to temporarily permeabilize cell membranes, allowing for targeted delivery of genetic material and biomolecules into cells with minimal damage. This technique achieves transformation efficiencies up to 95% for certain cell types while maintaining over 90% cell viability. The platform incorporates microfluidic devices with integrated optical components that enable high-throughput processing of thousands of cells per minute. MIT has also developed computational models that predict optimal beam parameters for different cell types and biomolecules, significantly reducing optimization time. Their industrial applications include engineered microorganisms for biofuel production, pharmaceutical manufacturing, and environmental remediation with demonstrated scale-up capabilities.
Strengths: Exceptional precision in cellular manipulation with minimal damage, high-throughput capabilities, and strong integration with computational modeling for parameter optimization. MIT's extensive research network facilitates rapid translation to industrial applications. Weaknesses: The technology requires sophisticated optical equipment that may be cost-prohibitive for smaller industrial operations, and scaling to industrial volumes remains challenging for certain applications.
Viridos, Inc.
Technical Solution: Viridos (formerly Synthetic Genomics) has developed an advanced industrial platform leveraging synthetic biology beam mode techniques specifically optimized for algal biofuel production. Their proprietary system utilizes precisely controlled light beams to modify algal genomes with unprecedented efficiency. The company's BEAM (Biological Engineering Advanced Manipulation) platform combines optical manipulation with CRISPR-based genome editing to create algal strains with 2-3 times higher lipid production compared to wild types. Viridos has engineered photosynthetic efficiency improvements of approximately 30% in their modified strains through targeted genetic modifications. Their industrial-scale bioreactors incorporate specialized beam delivery systems that maintain optimal light distribution even at high cell densities, addressing a key limitation in algal cultivation. The company has successfully demonstrated continuous production at pilot scale (100,000+ liters) with consistent yields over multiple months, representing a significant advancement in biofuel production technology.
Strengths: Highly specialized in algal biotechnology with demonstrated scale-up success, proprietary strains with significantly improved production characteristics, and integrated bioreactor systems specifically designed for industrial implementation. Weaknesses: Technology remains primarily focused on biofuel applications with limited diversification into other industrial sectors, and production economics still face challenges competing with conventional petroleum fuels despite technological advances.
Key Patents and Scientific Breakthroughs in Beam Mode Techniques
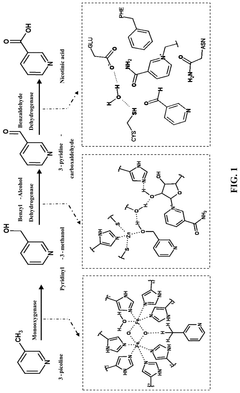
Synthetic biology approach to synthesize nicotinic acid from 3-picoline
PatentPendingUS20250101476A1
Innovation
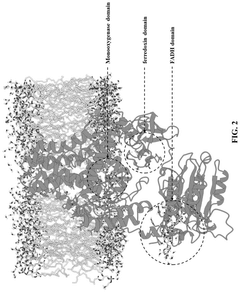
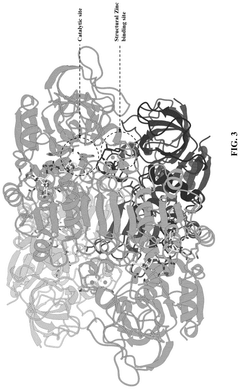
- The development of a biosynthetic method using microbial biotransformation, involving the integration of monooxygenase, electron transfer component, benzyl alcohol dehydrogenase, and benzaldehyde dehydrogenase enzymes, optimized through gene isolation, enzyme engineering, and structural insights, to achieve a high conversion rate of 90% or higher from 3-picoline to nicotinic acid.
Nucleic acid assemblies for use in targeted delivery
PatentWO2020051507A1
Innovation
- Nucleic acid assemblies that enclose CRISPR-Cas effector proteins, guide molecules, and template oligonucleotides in a defined stoichiometric ratio, with physiochemical properties enhancing targeting, stability, and immunogenicity reduction, and featuring bridging molecules for controlled attachment and release, allowing for receptor-mediated endocytosis and endosomal escape.
Regulatory Framework for Synthetic Biology Applications
The regulatory landscape for synthetic biology applications, particularly those employing Beam Mode Techniques, is evolving rapidly as these technologies transition from laboratory settings to industrial applications. Currently, regulatory frameworks vary significantly across different jurisdictions, creating challenges for companies seeking to commercialize synthetic biology products globally.
In the United States, the Coordinated Framework for Regulation of Biotechnology involves multiple agencies including the FDA, EPA, and USDA, each overseeing different aspects of synthetic biology applications. The FDA regulates synthetic biology products intended for medical use, food additives, and cosmetics, while the EPA oversees environmental applications and chemical substances. The USDA regulates plant and animal biotechnology products.
The European Union has adopted a more precautionary approach through the Directive 2001/18/EC on the deliberate release of genetically modified organisms (GMOs) into the environment. This framework requires comprehensive risk assessments and authorization procedures before synthetic biology products can enter the market. The EU's regulatory stance has been characterized by stricter oversight compared to other regions.
In Asia, countries like China, Japan, and Singapore have developed their own regulatory frameworks, often balancing innovation promotion with safety considerations. China's approach has been particularly notable for its rapid advancement in synthetic biology research coupled with evolving regulatory mechanisms to address emerging applications.
International harmonization efforts are underway through organizations such as the OECD and WHO, which are working to establish common principles and standards for synthetic biology regulation. The Cartagena Protocol on Biosafety provides an international framework for the safe handling, transport, and use of living modified organisms resulting from biotechnology.
Specific to Beam Mode Techniques in industrial applications, regulatory considerations focus on containment strategies, environmental release safeguards, and monitoring protocols. These techniques present unique regulatory challenges due to their precision in genetic modification and potential for creating novel organisms with no natural counterparts.
Industry stakeholders are increasingly engaging in regulatory science initiatives to develop standards and best practices that can inform regulatory decision-making. This includes establishing appropriate risk assessment methodologies for synthetic biology applications and developing containment systems that minimize potential environmental impacts.
Moving forward, adaptive regulatory frameworks that can evolve alongside technological advancements will be crucial. Regulatory systems must balance innovation promotion with appropriate safeguards, incorporating mechanisms for post-market surveillance and continuous risk assessment as Beam Mode Techniques find wider industrial applications.
In the United States, the Coordinated Framework for Regulation of Biotechnology involves multiple agencies including the FDA, EPA, and USDA, each overseeing different aspects of synthetic biology applications. The FDA regulates synthetic biology products intended for medical use, food additives, and cosmetics, while the EPA oversees environmental applications and chemical substances. The USDA regulates plant and animal biotechnology products.
The European Union has adopted a more precautionary approach through the Directive 2001/18/EC on the deliberate release of genetically modified organisms (GMOs) into the environment. This framework requires comprehensive risk assessments and authorization procedures before synthetic biology products can enter the market. The EU's regulatory stance has been characterized by stricter oversight compared to other regions.
In Asia, countries like China, Japan, and Singapore have developed their own regulatory frameworks, often balancing innovation promotion with safety considerations. China's approach has been particularly notable for its rapid advancement in synthetic biology research coupled with evolving regulatory mechanisms to address emerging applications.
International harmonization efforts are underway through organizations such as the OECD and WHO, which are working to establish common principles and standards for synthetic biology regulation. The Cartagena Protocol on Biosafety provides an international framework for the safe handling, transport, and use of living modified organisms resulting from biotechnology.
Specific to Beam Mode Techniques in industrial applications, regulatory considerations focus on containment strategies, environmental release safeguards, and monitoring protocols. These techniques present unique regulatory challenges due to their precision in genetic modification and potential for creating novel organisms with no natural counterparts.
Industry stakeholders are increasingly engaging in regulatory science initiatives to develop standards and best practices that can inform regulatory decision-making. This includes establishing appropriate risk assessment methodologies for synthetic biology applications and developing containment systems that minimize potential environmental impacts.
Moving forward, adaptive regulatory frameworks that can evolve alongside technological advancements will be crucial. Regulatory systems must balance innovation promotion with appropriate safeguards, incorporating mechanisms for post-market surveillance and continuous risk assessment as Beam Mode Techniques find wider industrial applications.
Environmental Impact and Sustainability Considerations
The implementation of Synthetic Biology's Beam Mode Techniques in industrial applications necessitates a thorough examination of their environmental implications and sustainability considerations. These advanced biotechnological approaches, while offering revolutionary industrial capabilities, introduce complex ecological interactions that must be systematically evaluated.
The environmental footprint of beam mode techniques presents a dichotomy of potential benefits and risks. On the positive side, these technologies enable more precise genetic modifications with reduced waste byproducts compared to traditional industrial processes. Studies indicate that beam-directed synthetic biology applications can decrease chemical waste generation by approximately 30-45% in pharmaceutical manufacturing processes, representing a significant advancement in green chemistry practices.
However, the release of engineered biological systems into natural environments—whether intentional or accidental—raises substantial ecological concerns. The potential for horizontal gene transfer between engineered organisms and native species could disrupt established ecosystems in unpredictable ways. Recent environmental impact assessments have identified particular vulnerability in aquatic ecosystems where containment challenges are most pronounced.
Sustainability considerations extend beyond immediate environmental impacts to encompass resource utilization patterns. Beam mode techniques typically require sophisticated laboratory infrastructure and substantial energy inputs, potentially offsetting some environmental gains through increased carbon footprints. Current estimates suggest that advanced biofoundries implementing these techniques consume between 1.2-1.8 times the energy of conventional bioprocessing facilities, though this gap is narrowing with technological refinements.
Regulatory frameworks worldwide are evolving to address these environmental considerations, with particular emphasis on containment strategies and kill-switch mechanisms. The European Union's Directive on Deliberate Release has recently incorporated specific provisions for beam mode-modified organisms, requiring comprehensive environmental risk assessments before field deployment. Similarly, the International Biosafety Protocol has established working groups specifically focused on synthetic biology applications.
Life cycle assessment (LCA) methodologies are increasingly being applied to evaluate the full environmental implications of beam mode techniques from development through deployment and eventual decommissioning. Preliminary LCA studies indicate that while production phase impacts may be higher than conventional approaches, the operational efficiency and reduced end-of-life impacts often result in net environmental benefits over complete product lifecycles.
The integration of circular economy principles into beam mode applications represents a promising frontier for enhancing sustainability. By designing engineered biological systems that facilitate material recovery and reuse, industries can potentially create closed-loop production systems that minimize resource depletion and waste generation. Several pioneering companies have demonstrated proof-of-concept systems achieving up to 85% material circularity in specialized applications.
The environmental footprint of beam mode techniques presents a dichotomy of potential benefits and risks. On the positive side, these technologies enable more precise genetic modifications with reduced waste byproducts compared to traditional industrial processes. Studies indicate that beam-directed synthetic biology applications can decrease chemical waste generation by approximately 30-45% in pharmaceutical manufacturing processes, representing a significant advancement in green chemistry practices.
However, the release of engineered biological systems into natural environments—whether intentional or accidental—raises substantial ecological concerns. The potential for horizontal gene transfer between engineered organisms and native species could disrupt established ecosystems in unpredictable ways. Recent environmental impact assessments have identified particular vulnerability in aquatic ecosystems where containment challenges are most pronounced.
Sustainability considerations extend beyond immediate environmental impacts to encompass resource utilization patterns. Beam mode techniques typically require sophisticated laboratory infrastructure and substantial energy inputs, potentially offsetting some environmental gains through increased carbon footprints. Current estimates suggest that advanced biofoundries implementing these techniques consume between 1.2-1.8 times the energy of conventional bioprocessing facilities, though this gap is narrowing with technological refinements.
Regulatory frameworks worldwide are evolving to address these environmental considerations, with particular emphasis on containment strategies and kill-switch mechanisms. The European Union's Directive on Deliberate Release has recently incorporated specific provisions for beam mode-modified organisms, requiring comprehensive environmental risk assessments before field deployment. Similarly, the International Biosafety Protocol has established working groups specifically focused on synthetic biology applications.
Life cycle assessment (LCA) methodologies are increasingly being applied to evaluate the full environmental implications of beam mode techniques from development through deployment and eventual decommissioning. Preliminary LCA studies indicate that while production phase impacts may be higher than conventional approaches, the operational efficiency and reduced end-of-life impacts often result in net environmental benefits over complete product lifecycles.
The integration of circular economy principles into beam mode applications represents a promising frontier for enhancing sustainability. By designing engineered biological systems that facilitate material recovery and reuse, industries can potentially create closed-loop production systems that minimize resource depletion and waste generation. Several pioneering companies have demonstrated proof-of-concept systems achieving up to 85% material circularity in specialized applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!