How Synthetic Biology Impacts Industrial Microstructure Development
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Industrial Objectives
Synthetic biology has evolved significantly since its conceptual emergence in the early 2000s, transitioning from a theoretical framework to a practical discipline with transformative industrial applications. The field originated from the convergence of molecular biology, genetic engineering, and computational sciences, with the Human Genome Project serving as a critical catalyst by providing essential genetic information that enabled more sophisticated biological engineering approaches.
The evolution of synthetic biology can be traced through several distinct phases. Initially, researchers focused on creating basic genetic circuits and standardized biological parts, exemplified by the BioBrick standard developed at MIT. This standardization approach aimed to make biological components modular and interchangeable, similar to electronic components. The second phase saw the development of more complex genetic systems and the emergence of genome synthesis technologies, enabling the creation of synthetic chromosomes and eventually synthetic cells, as demonstrated by the Venter Institute's milestone achievement of creating the first synthetic bacterial genome in 2010.
Currently, synthetic biology has entered an industrial application phase, where engineered biological systems are being deployed to address real-world challenges across multiple sectors. This transition has been facilitated by significant advancements in DNA sequencing and synthesis technologies, CRISPR-Cas9 gene editing tools, and computational modeling capabilities that allow for more precise design and prediction of biological system behaviors.
The industrial objectives of synthetic biology span diverse sectors. In healthcare, objectives include developing novel therapeutics, vaccines, and diagnostic tools through engineered biological systems. The agricultural sector aims to create crops with enhanced nutritional profiles, improved resistance to pests and diseases, and better adaptation to changing climate conditions. In materials science, synthetic biology enables the production of novel biomaterials with tailored properties for applications ranging from construction to electronics.
Energy production represents another critical objective, with synthetic biology facilitating the development of next-generation biofuels and sustainable energy solutions. Additionally, environmental remediation efforts benefit from engineered microorganisms capable of degrading pollutants or capturing carbon dioxide. The chemical industry increasingly relies on synthetic biology for the production of specialty chemicals, pharmaceuticals, and industrial enzymes through more sustainable and efficient bioprocesses.
The overarching goal of synthetic biology in industrial contexts is to transition from traditional chemical and mechanical manufacturing processes to biological systems that offer greater sustainability, efficiency, and specificity. This biological manufacturing paradigm promises reduced environmental impact, lower energy requirements, and the ability to create products with unprecedented properties and functionalities.
The evolution of synthetic biology can be traced through several distinct phases. Initially, researchers focused on creating basic genetic circuits and standardized biological parts, exemplified by the BioBrick standard developed at MIT. This standardization approach aimed to make biological components modular and interchangeable, similar to electronic components. The second phase saw the development of more complex genetic systems and the emergence of genome synthesis technologies, enabling the creation of synthetic chromosomes and eventually synthetic cells, as demonstrated by the Venter Institute's milestone achievement of creating the first synthetic bacterial genome in 2010.
Currently, synthetic biology has entered an industrial application phase, where engineered biological systems are being deployed to address real-world challenges across multiple sectors. This transition has been facilitated by significant advancements in DNA sequencing and synthesis technologies, CRISPR-Cas9 gene editing tools, and computational modeling capabilities that allow for more precise design and prediction of biological system behaviors.
The industrial objectives of synthetic biology span diverse sectors. In healthcare, objectives include developing novel therapeutics, vaccines, and diagnostic tools through engineered biological systems. The agricultural sector aims to create crops with enhanced nutritional profiles, improved resistance to pests and diseases, and better adaptation to changing climate conditions. In materials science, synthetic biology enables the production of novel biomaterials with tailored properties for applications ranging from construction to electronics.
Energy production represents another critical objective, with synthetic biology facilitating the development of next-generation biofuels and sustainable energy solutions. Additionally, environmental remediation efforts benefit from engineered microorganisms capable of degrading pollutants or capturing carbon dioxide. The chemical industry increasingly relies on synthetic biology for the production of specialty chemicals, pharmaceuticals, and industrial enzymes through more sustainable and efficient bioprocesses.
The overarching goal of synthetic biology in industrial contexts is to transition from traditional chemical and mechanical manufacturing processes to biological systems that offer greater sustainability, efficiency, and specificity. This biological manufacturing paradigm promises reduced environmental impact, lower energy requirements, and the ability to create products with unprecedented properties and functionalities.
Market Demand Analysis for Bio-Engineered Microstructures
The global market for bio-engineered microstructures is experiencing unprecedented growth, driven by advancements in synthetic biology and increasing demand across multiple industries. Current market valuations indicate that the synthetic biology market reached approximately 9.5 billion USD in 2021, with bio-engineered microstructures representing a significant and rapidly expanding segment. Industry forecasts project a compound annual growth rate exceeding 20% for the next decade, positioning this sector among the fastest-growing biotechnology applications.
Healthcare and pharmaceutical industries demonstrate the strongest demand for bio-engineered microstructures, particularly for drug delivery systems, tissue engineering, and diagnostic applications. The precision medicine movement has accelerated this trend, with microstructures enabling targeted therapies that minimize side effects while maximizing therapeutic outcomes. Market research indicates that over 30% of new drug development programs now incorporate some form of bio-engineered microstructure technology.
The materials science sector represents another substantial market, where bio-engineered microstructures are revolutionizing the development of advanced materials with unprecedented properties. Industries ranging from construction to aerospace are seeking bio-inspired solutions that offer enhanced strength-to-weight ratios, self-healing capabilities, and environmental sustainability. This segment is growing at approximately 25% annually, outpacing traditional materials development approaches.
Environmental applications constitute an emerging but rapidly expanding market segment. Bio-engineered microstructures designed for environmental remediation, pollution detection, and sustainable resource recovery are gaining significant traction as regulatory pressures for environmental protection intensify globally. Market analysis reveals that government initiatives and corporate sustainability commitments are driving annual investments exceeding 2 billion USD in this application area.
Consumer goods industries are increasingly incorporating bio-engineered microstructures into product development pipelines. From cosmetics with enhanced delivery systems to food products with improved nutritional profiles and extended shelf life, these applications represent a market segment valued at approximately 3 billion USD with projected annual growth of 18%.
Regional market analysis indicates that North America currently leads in market share, followed closely by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the highest growth rate, driven by substantial government investments in biotechnology infrastructure and favorable regulatory environments in countries like China, Singapore, and South Korea.
Market barriers include high development costs, regulatory uncertainties, and public perception challenges. Nevertheless, decreasing production costs through technological improvements and economies of scale are gradually reducing these barriers. Industry surveys indicate that over 65% of major chemical and materials companies have established dedicated research programs focused on bio-engineered microstructures, signaling strong confidence in future market expansion.
Healthcare and pharmaceutical industries demonstrate the strongest demand for bio-engineered microstructures, particularly for drug delivery systems, tissue engineering, and diagnostic applications. The precision medicine movement has accelerated this trend, with microstructures enabling targeted therapies that minimize side effects while maximizing therapeutic outcomes. Market research indicates that over 30% of new drug development programs now incorporate some form of bio-engineered microstructure technology.
The materials science sector represents another substantial market, where bio-engineered microstructures are revolutionizing the development of advanced materials with unprecedented properties. Industries ranging from construction to aerospace are seeking bio-inspired solutions that offer enhanced strength-to-weight ratios, self-healing capabilities, and environmental sustainability. This segment is growing at approximately 25% annually, outpacing traditional materials development approaches.
Environmental applications constitute an emerging but rapidly expanding market segment. Bio-engineered microstructures designed for environmental remediation, pollution detection, and sustainable resource recovery are gaining significant traction as regulatory pressures for environmental protection intensify globally. Market analysis reveals that government initiatives and corporate sustainability commitments are driving annual investments exceeding 2 billion USD in this application area.
Consumer goods industries are increasingly incorporating bio-engineered microstructures into product development pipelines. From cosmetics with enhanced delivery systems to food products with improved nutritional profiles and extended shelf life, these applications represent a market segment valued at approximately 3 billion USD with projected annual growth of 18%.
Regional market analysis indicates that North America currently leads in market share, followed closely by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the highest growth rate, driven by substantial government investments in biotechnology infrastructure and favorable regulatory environments in countries like China, Singapore, and South Korea.
Market barriers include high development costs, regulatory uncertainties, and public perception challenges. Nevertheless, decreasing production costs through technological improvements and economies of scale are gradually reducing these barriers. Industry surveys indicate that over 65% of major chemical and materials companies have established dedicated research programs focused on bio-engineered microstructures, signaling strong confidence in future market expansion.
Current Capabilities and Barriers in Synthetic Biology
Synthetic biology has evolved significantly over the past decade, establishing itself as a transformative field that combines biological science with engineering principles. Current capabilities in synthetic biology include advanced DNA synthesis technologies that allow for the creation of custom genetic sequences with increasing accuracy and decreasing costs. High-throughput screening methods have enabled researchers to rapidly test thousands of genetic variants, accelerating the discovery process for industrial applications. Additionally, computational tools for genetic circuit design have become more sophisticated, allowing for predictive modeling of biological systems before physical implementation.
The development of standardized biological parts, often referred to as BioBricks, has facilitated modular approaches to genetic engineering, enabling more efficient design and assembly of complex biological systems. CRISPR-Cas9 and other gene editing technologies have revolutionized the precision with which genetic modifications can be made, opening new possibilities for industrial applications. Metabolic engineering capabilities have advanced to the point where entire pathways can be optimized or transplanted between organisms to produce valuable compounds.
Despite these advancements, significant barriers remain in the field. Scale-up challenges persist when transitioning from laboratory success to industrial production, with many engineered organisms performing inconsistently in large-scale bioreactors. Biological unpredictability continues to hamper efforts, as living systems often behave in ways that current models cannot fully predict, leading to unexpected outcomes in engineered systems.
Regulatory frameworks worldwide have not kept pace with technological advancements, creating uncertainty for companies investing in synthetic biology applications. This regulatory lag has particularly affected areas such as biosecurity and intellectual property protection. Public perception and acceptance issues also present significant barriers, with concerns about safety, ethics, and environmental impact influencing market adoption of synthetic biology products.
Technical limitations in measurement and characterization tools restrict our ability to fully understand and control biological systems at the molecular level. The complexity of cellular environments makes it difficult to predict how engineered genetic circuits will function when implemented. Furthermore, the interdisciplinary nature of synthetic biology requires expertise across multiple fields, creating workforce development challenges for industries seeking to implement these technologies.
Economic barriers also exist, with high initial investment costs for research and development, uncertain return on investment timelines, and competition from established chemical processes that benefit from decades of optimization and infrastructure development. These factors collectively shape the current landscape of synthetic biology's capabilities and limitations in industrial microstructure development.
The development of standardized biological parts, often referred to as BioBricks, has facilitated modular approaches to genetic engineering, enabling more efficient design and assembly of complex biological systems. CRISPR-Cas9 and other gene editing technologies have revolutionized the precision with which genetic modifications can be made, opening new possibilities for industrial applications. Metabolic engineering capabilities have advanced to the point where entire pathways can be optimized or transplanted between organisms to produce valuable compounds.
Despite these advancements, significant barriers remain in the field. Scale-up challenges persist when transitioning from laboratory success to industrial production, with many engineered organisms performing inconsistently in large-scale bioreactors. Biological unpredictability continues to hamper efforts, as living systems often behave in ways that current models cannot fully predict, leading to unexpected outcomes in engineered systems.
Regulatory frameworks worldwide have not kept pace with technological advancements, creating uncertainty for companies investing in synthetic biology applications. This regulatory lag has particularly affected areas such as biosecurity and intellectual property protection. Public perception and acceptance issues also present significant barriers, with concerns about safety, ethics, and environmental impact influencing market adoption of synthetic biology products.
Technical limitations in measurement and characterization tools restrict our ability to fully understand and control biological systems at the molecular level. The complexity of cellular environments makes it difficult to predict how engineered genetic circuits will function when implemented. Furthermore, the interdisciplinary nature of synthetic biology requires expertise across multiple fields, creating workforce development challenges for industries seeking to implement these technologies.
Economic barriers also exist, with high initial investment costs for research and development, uncertain return on investment timelines, and competition from established chemical processes that benefit from decades of optimization and infrastructure development. These factors collectively shape the current landscape of synthetic biology's capabilities and limitations in industrial microstructure development.
Established Methodologies for Microstructure Engineering
01 Engineered microorganisms for synthetic biology applications
Engineered microorganisms are developed using synthetic biology techniques to create novel biological functions. These microorganisms can be designed with specific genetic circuits and metabolic pathways to produce valuable compounds, perform bioremediation, or serve as biosensors. The microstructure of these engineered cells can be optimized to enhance their performance in various applications including pharmaceutical production, environmental cleanup, and sustainable manufacturing.- Engineered microorganisms for synthetic biology applications: Engineered microorganisms are developed using synthetic biology techniques to create novel biological systems with specific functions. These microorganisms can be designed with custom genetic circuits and metabolic pathways to produce valuable compounds, perform bioremediation, or serve as biosensors. The microstructure of these engineered organisms is optimized to enhance their performance and stability in various environmental conditions.
- Microfluidic systems for synthetic biology: Microfluidic platforms provide controlled environments for synthetic biology experiments and applications. These systems enable precise manipulation of cells and biomolecules at microscale, facilitating high-throughput screening, directed evolution, and cell-free synthetic biology. The microstructures in these devices are designed to control fluid flow, create concentration gradients, and provide compartmentalization for biological reactions.
- Biomimetic microstructures in synthetic biology: Biomimetic approaches in synthetic biology involve creating artificial structures that mimic natural biological systems. These include synthetic cells, artificial tissues, and biomimetic membranes with controlled microstructures. By replicating the microstructural features of natural biological systems, researchers can develop improved platforms for drug delivery, tissue engineering, and biosensing applications.
- Nanofabrication techniques for synthetic biology microstructures: Advanced nanofabrication methods are employed to create precise microstructures for synthetic biology applications. These techniques include lithography, 3D printing, self-assembly, and template-directed synthesis to produce structures with controlled geometry, porosity, and surface properties. The resulting microstructures serve as scaffolds for cell growth, platforms for biosensors, and components for synthetic biological systems.
- Computational design of synthetic biology microstructures: Computational tools and algorithms are used to design and optimize microstructures for synthetic biology applications. These approaches include modeling of genetic circuits, simulation of cellular behavior, and prediction of protein-protein interactions. Machine learning and artificial intelligence techniques are increasingly applied to improve the design of synthetic biological systems and their associated microstructures.
02 Microfluidic systems for synthetic biology
Microfluidic platforms provide controlled environments for synthetic biology experiments at microscale. These systems enable precise manipulation of cells, reagents, and conditions necessary for engineering biological systems. The microstructures within these platforms facilitate high-throughput screening, cell sorting, and analysis of synthetic biological constructs. Advanced microfluidic devices incorporate features such as droplet generation, gradient formation, and integrated sensors to support synthetic biology research and applications.Expand Specific Solutions03 Biomimetic synthetic microstructures
Biomimetic approaches in synthetic biology involve creating artificial structures that mimic natural biological systems. These synthetic microstructures can replicate functions of cellular components such as membranes, organelles, or cytoskeletal elements. By incorporating principles from nature, researchers develop synthetic cells, artificial tissues, and biomimetic materials with controlled properties. These structures serve as platforms for studying biological processes and developing new biotechnological applications.Expand Specific Solutions04 Computational modeling for synthetic biology microstructures
Computational tools and modeling approaches are essential for designing and predicting the behavior of synthetic biology microstructures. These methods enable in silico testing of genetic circuits, metabolic pathways, and cellular architectures before experimental implementation. Advanced algorithms and machine learning techniques help optimize the design of synthetic biological systems by simulating their behavior under various conditions. Computational approaches also facilitate the analysis of large datasets generated from synthetic biology experiments.Expand Specific Solutions05 Nanoscale components for synthetic biology
Nanoscale components and materials are integrated into synthetic biology systems to create functional microstructures with enhanced capabilities. These include nanoparticles, nanofibers, and nanoporous materials that can be incorporated into engineered biological systems. Nanoscale components provide precise control over interactions between synthetic biological elements and their environment. Applications include targeted drug delivery, biosensing, and development of artificial cellular compartments with specific functionalities.Expand Specific Solutions
Leading Organizations in Industrial Synthetic Biology
Synthetic biology is transforming industrial microstructure development, currently positioned at the early growth stage of its industry lifecycle. The global market is expanding rapidly, projected to reach $30 billion by 2026, driven by applications in pharmaceuticals, agriculture, and renewable materials. Technical maturity varies across applications, with companies demonstrating different specialization levels. Leaders like Amyris and Zymergen have established commercial-scale production platforms, while research institutions (MIT, Johns Hopkins, Nanyang Technological University) advance fundamental capabilities. Industrial players (Cargill, TotalEnergies) are integrating synthetic biology into existing processes. Chinese entities (Tianjin Institute of Industrial Biotechnology, Sunrise Biotechnology) are rapidly closing technological gaps, creating a globally competitive landscape characterized by strategic partnerships between academic institutions and commercial enterprises.
Zymergen, Inc.
Technical Solution: Zymergen employs a proprietary platform that integrates molecular biology, automation, data science, and machine learning to design microbes for industrial applications. Their approach involves engineering microorganisms to produce novel biomaterials with specific properties that traditional chemistry cannot achieve. The company's platform enables rapid prototyping and testing of thousands of microbial strains simultaneously, accelerating the development cycle from years to months. Zymergen's technology has successfully created bio-based films, adhesives, and electronic materials with enhanced performance characteristics. Their process involves identifying genetic modifications that optimize microbe performance in industrial settings, focusing on creating sustainable alternatives to petroleum-based products[1]. The company has developed proprietary algorithms that predict which genetic changes will yield desired material properties, significantly reducing the trial-and-error typically associated with bioengineering.
Strengths: Advanced AI and machine learning integration for strain optimization; high-throughput automated testing platform; proven success in commercializing novel biomaterials. Weaknesses: High capital requirements; lengthy development timelines despite improvements; challenges in scaling production from laboratory to industrial levels; recent financial difficulties that led to restructuring.
Tianjin Institute of Industrial Biotechnology of CAS
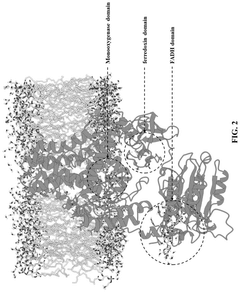
Technical Solution: The Tianjin Institute of Industrial Biotechnology (TIB) has developed comprehensive platforms for synthetic biology applications in industrial microstructure development. Their approach integrates systems and synthetic biology with industrial process engineering to create microbial cell factories for various applications. TIB has established several key technological platforms, including genome editing tools optimized for industrial microorganisms, high-throughput screening systems, and advanced bioreactor designs for process optimization. The institute has successfully engineered microbes for the production of bulk chemicals, biofuels, pharmaceuticals, and biomaterials. Their research includes developing artificial synthetic pathways that don't exist in nature, enabling the production of novel compounds with industrial applications[6]. TIB has also pioneered work in creating minimal genomes and chassis cells specifically designed for industrial applications, with reduced metabolic burden and increased production efficiency. The institute maintains extensive strain libraries and has developed proprietary computational tools for predicting gene function and optimizing metabolic pathways.
Strengths: Comprehensive research capabilities spanning fundamental science to industrial applications; strong government support and funding; extensive collaboration network with Chinese industry; integration of multiple technological platforms. Weaknesses: Potential challenges in international technology transfer and commercialization; possible gaps between laboratory research and industrial implementation; competition from well-funded private companies in Western markets; intellectual property protection concerns.
Key Patents and Breakthroughs in Biological Manufacturing
Methods of using natural and engineered organisms to produce small molecules for industrial application
PatentInactiveUS20240084343A1
Innovation
- The use of engineered and natural microorganisms, such as Rhodococcus and Ralstonia strains, that can grow on carbon-containing gases like syngas to produce and secrete specific small molecules, involving genetic modifications to enhance enzyme activity and nutrient utilization, and subsequent extraction and processing techniques like chromatography and solvent extraction.
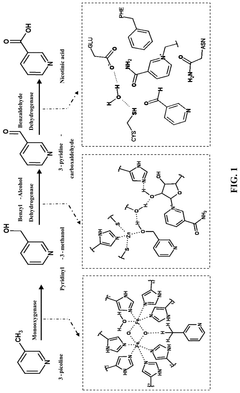
Synthetic biology approach to synthesize nicotinic acid from 3-picoline
PatentPendingUS20250101476A1
Innovation
- The development of a biosynthetic method using microbial biotransformation, involving the integration of monooxygenase, electron transfer component, benzyl alcohol dehydrogenase, and benzaldehyde dehydrogenase enzymes, optimized through gene isolation, enzyme engineering, and structural insights, to achieve a high conversion rate of 90% or higher from 3-picoline to nicotinic acid.
Regulatory Framework for Engineered Biological Systems
The regulatory landscape for engineered biological systems has evolved significantly in response to the rapid advancement of synthetic biology. Governments worldwide have established frameworks that balance innovation with safety concerns, creating a complex regulatory environment that varies by region. In the United States, oversight is shared among the FDA, EPA, and USDA, each regulating different aspects of engineered biological products based on their intended use rather than production method.
The European Union employs a more precautionary approach through its Directive on the Deliberate Release of GMOs, requiring extensive risk assessment before market approval. This contrasts with countries like Singapore and the UK that have implemented more streamlined regulatory pathways specifically designed to accelerate synthetic biology innovation while maintaining appropriate safeguards.
Risk assessment methodologies for engineered biological systems have become increasingly sophisticated, incorporating both quantitative and qualitative approaches. Modern frameworks evaluate containment strategies, horizontal gene transfer potential, and ecological impact through tiered assessment protocols. The development of standardized biosafety levels specifically for synthetic organisms represents a significant advancement in regulatory science.
International harmonization efforts have gained momentum through organizations like the OECD and WHO, which are working to establish common principles for risk assessment and management. These initiatives aim to reduce regulatory fragmentation while respecting sovereign regulatory authority, facilitating global research collaboration and commercial development in synthetic biology.
Emerging regulatory challenges include the governance of gene drives, cell-free systems, and distributed manufacturing models enabled by synthetic biology. Regulators are increasingly adopting adaptive licensing approaches that allow for staged market entry with enhanced post-market surveillance, recognizing the need for regulatory frameworks to evolve alongside technological capabilities.
Industry self-regulation has emerged as a complementary mechanism through initiatives like the International Gene Synthesis Consortium, which screens DNA synthesis orders against databases of pathogenic sequences. These voluntary standards often precede formal regulation and help establish industry best practices that inform subsequent regulatory development.
The integration of ethical considerations into regulatory frameworks represents another evolution, with many jurisdictions now requiring ethical impact assessments alongside traditional safety evaluations. This reflects growing recognition that synthetic biology applications raise not only technical safety questions but also profound societal and moral considerations that must be addressed through inclusive stakeholder engagement processes.
The European Union employs a more precautionary approach through its Directive on the Deliberate Release of GMOs, requiring extensive risk assessment before market approval. This contrasts with countries like Singapore and the UK that have implemented more streamlined regulatory pathways specifically designed to accelerate synthetic biology innovation while maintaining appropriate safeguards.
Risk assessment methodologies for engineered biological systems have become increasingly sophisticated, incorporating both quantitative and qualitative approaches. Modern frameworks evaluate containment strategies, horizontal gene transfer potential, and ecological impact through tiered assessment protocols. The development of standardized biosafety levels specifically for synthetic organisms represents a significant advancement in regulatory science.
International harmonization efforts have gained momentum through organizations like the OECD and WHO, which are working to establish common principles for risk assessment and management. These initiatives aim to reduce regulatory fragmentation while respecting sovereign regulatory authority, facilitating global research collaboration and commercial development in synthetic biology.
Emerging regulatory challenges include the governance of gene drives, cell-free systems, and distributed manufacturing models enabled by synthetic biology. Regulators are increasingly adopting adaptive licensing approaches that allow for staged market entry with enhanced post-market surveillance, recognizing the need for regulatory frameworks to evolve alongside technological capabilities.
Industry self-regulation has emerged as a complementary mechanism through initiatives like the International Gene Synthesis Consortium, which screens DNA synthesis orders against databases of pathogenic sequences. These voluntary standards often precede formal regulation and help establish industry best practices that inform subsequent regulatory development.
The integration of ethical considerations into regulatory frameworks represents another evolution, with many jurisdictions now requiring ethical impact assessments alongside traditional safety evaluations. This reflects growing recognition that synthetic biology applications raise not only technical safety questions but also profound societal and moral considerations that must be addressed through inclusive stakeholder engagement processes.
Environmental Sustainability Impact Assessment
Synthetic biology's integration into industrial processes presents significant environmental implications that warrant comprehensive assessment. The environmental sustainability impact of this emerging field extends across multiple dimensions, from resource utilization to waste management and ecological footprints.
The application of synthetic biology in industrial microstructure development demonstrates promising potential for reducing environmental burdens compared to traditional manufacturing processes. Engineered microorganisms can produce chemicals, materials, and fuels with significantly lower carbon emissions than conventional petrochemical routes. For instance, microbial fermentation processes typically operate at ambient temperatures and pressures, requiring substantially less energy input than traditional chemical synthesis methods that often demand extreme conditions.
Water conservation represents another critical environmental benefit. Synthetic biology platforms generally consume less water per unit of production than conventional industrial processes. Quantitative analyses indicate that biomanufacturing approaches can reduce water usage by 30-50% in certain applications, particularly in sectors like textile production and specialty chemicals manufacturing.
The transition toward bio-based feedstocks enabled by synthetic biology contributes to circular economy principles. Rather than relying exclusively on fossil resources, engineered biological systems can utilize renewable carbon sources, including agricultural residues, forestry byproducts, and even captured carbon dioxide. This feedstock flexibility reduces pressure on virgin resource extraction while potentially valorizing waste streams.
However, potential environmental risks must be acknowledged alongside these benefits. The release of engineered organisms, whether intentional or accidental, raises concerns about ecological disruption and genetic transfer to wild populations. Robust containment strategies and thorough risk assessment protocols are essential to mitigate these concerns. The scientific community has developed multiple biocontainment approaches, including genetic safeguards and physical isolation systems, though their long-term effectiveness remains under evaluation.
Life cycle assessment (LCA) studies comparing synthetic biology-based production with conventional methods reveal complex sustainability profiles. While bio-based processes often demonstrate advantages in greenhouse gas emissions and fossil resource depletion, they may present trade-offs in land use, eutrophication potential, and water quality impacts depending on feedstock selection and process configuration.
Regulatory frameworks worldwide are evolving to address these environmental considerations, with particular attention to biosafety protocols, waste management requirements, and environmental monitoring standards. The development of internationally harmonized approaches to environmental risk assessment represents a crucial step toward responsible industry advancement.
The application of synthetic biology in industrial microstructure development demonstrates promising potential for reducing environmental burdens compared to traditional manufacturing processes. Engineered microorganisms can produce chemicals, materials, and fuels with significantly lower carbon emissions than conventional petrochemical routes. For instance, microbial fermentation processes typically operate at ambient temperatures and pressures, requiring substantially less energy input than traditional chemical synthesis methods that often demand extreme conditions.
Water conservation represents another critical environmental benefit. Synthetic biology platforms generally consume less water per unit of production than conventional industrial processes. Quantitative analyses indicate that biomanufacturing approaches can reduce water usage by 30-50% in certain applications, particularly in sectors like textile production and specialty chemicals manufacturing.
The transition toward bio-based feedstocks enabled by synthetic biology contributes to circular economy principles. Rather than relying exclusively on fossil resources, engineered biological systems can utilize renewable carbon sources, including agricultural residues, forestry byproducts, and even captured carbon dioxide. This feedstock flexibility reduces pressure on virgin resource extraction while potentially valorizing waste streams.
However, potential environmental risks must be acknowledged alongside these benefits. The release of engineered organisms, whether intentional or accidental, raises concerns about ecological disruption and genetic transfer to wild populations. Robust containment strategies and thorough risk assessment protocols are essential to mitigate these concerns. The scientific community has developed multiple biocontainment approaches, including genetic safeguards and physical isolation systems, though their long-term effectiveness remains under evaluation.
Life cycle assessment (LCA) studies comparing synthetic biology-based production with conventional methods reveal complex sustainability profiles. While bio-based processes often demonstrate advantages in greenhouse gas emissions and fossil resource depletion, they may present trade-offs in land use, eutrophication potential, and water quality impacts depending on feedstock selection and process configuration.
Regulatory frameworks worldwide are evolving to address these environmental considerations, with particular attention to biosafety protocols, waste management requirements, and environmental monitoring standards. The development of internationally harmonized approaches to environmental risk assessment represents a crucial step toward responsible industry advancement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!