Evaluation of Synthetic Biology in Pilot Tests for Optimization
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Objectives
Synthetic biology has evolved significantly since its conceptual inception in the early 2000s, transforming from theoretical frameworks to practical applications across multiple industries. The field emerged from the convergence of molecular biology, genetic engineering, and computational modeling, with the Human Genome Project serving as a critical catalyst by providing the foundational knowledge necessary for manipulating genetic material with precision. Early milestones included the development of BioBricks in 2003, standardized genetic components that could be assembled like building blocks, and the creation of the first synthetic bacterial genome by the J. Craig Venter Institute in 2010.
The evolution of synthetic biology has been characterized by increasing sophistication in genetic design tools, with CRISPR-Cas9 technology representing a revolutionary advancement that dramatically improved the precision and efficiency of genetic modifications. Concurrently, the development of high-throughput DNA synthesis and sequencing technologies has significantly reduced costs and accelerated research timelines, enabling more complex biological systems to be engineered.
Recent trends indicate a shift from proof-of-concept demonstrations to practical applications focused on optimization and scalability. The integration of artificial intelligence and machine learning algorithms has enhanced the predictive capabilities for genetic design, allowing researchers to navigate the vast design space more efficiently. Additionally, the emergence of cell-free systems has provided a simplified platform for testing and optimizing synthetic biological components without the complexities of whole-cell environments.
The primary objectives of synthetic biology in pilot testing contexts are multifaceted. First, researchers aim to validate the functionality and stability of engineered biological systems under controlled conditions that simulate real-world environments. Second, optimization efforts focus on enhancing performance metrics such as yield, specificity, and robustness through iterative design-build-test cycles. Third, scalability assessments determine whether laboratory-scale successes can be translated to industrial production volumes without compromising efficiency or quality.
Furthermore, synthetic biology pilot tests increasingly incorporate sustainability objectives, seeking to develop processes that minimize resource consumption and environmental impact. Safety and containment strategies are also paramount, with rigorous protocols being developed to prevent unintended consequences of engineered organisms. The field is progressively moving toward predictive design methodologies that can anticipate system behavior before physical implementation, reducing development cycles and costs.
Looking forward, the trajectory of synthetic biology is oriented toward creating increasingly complex, multi-functional biological systems capable of responding dynamically to environmental cues. The ultimate goal is to establish a robust engineering framework that enables the reliable design of biological systems with the same precision and predictability achieved in traditional engineering disciplines, facilitating transformative applications across medicine, agriculture, materials science, and environmental remediation.
The evolution of synthetic biology has been characterized by increasing sophistication in genetic design tools, with CRISPR-Cas9 technology representing a revolutionary advancement that dramatically improved the precision and efficiency of genetic modifications. Concurrently, the development of high-throughput DNA synthesis and sequencing technologies has significantly reduced costs and accelerated research timelines, enabling more complex biological systems to be engineered.
Recent trends indicate a shift from proof-of-concept demonstrations to practical applications focused on optimization and scalability. The integration of artificial intelligence and machine learning algorithms has enhanced the predictive capabilities for genetic design, allowing researchers to navigate the vast design space more efficiently. Additionally, the emergence of cell-free systems has provided a simplified platform for testing and optimizing synthetic biological components without the complexities of whole-cell environments.
The primary objectives of synthetic biology in pilot testing contexts are multifaceted. First, researchers aim to validate the functionality and stability of engineered biological systems under controlled conditions that simulate real-world environments. Second, optimization efforts focus on enhancing performance metrics such as yield, specificity, and robustness through iterative design-build-test cycles. Third, scalability assessments determine whether laboratory-scale successes can be translated to industrial production volumes without compromising efficiency or quality.
Furthermore, synthetic biology pilot tests increasingly incorporate sustainability objectives, seeking to develop processes that minimize resource consumption and environmental impact. Safety and containment strategies are also paramount, with rigorous protocols being developed to prevent unintended consequences of engineered organisms. The field is progressively moving toward predictive design methodologies that can anticipate system behavior before physical implementation, reducing development cycles and costs.
Looking forward, the trajectory of synthetic biology is oriented toward creating increasingly complex, multi-functional biological systems capable of responding dynamically to environmental cues. The ultimate goal is to establish a robust engineering framework that enables the reliable design of biological systems with the same precision and predictability achieved in traditional engineering disciplines, facilitating transformative applications across medicine, agriculture, materials science, and environmental remediation.
Market Applications and Demand Analysis
Synthetic biology market applications have expanded significantly in recent years, driven by advancements in genetic engineering techniques and growing demand for sustainable solutions across multiple industries. The global synthetic biology market was valued at $9.5 billion in 2021 and is projected to reach $30.7 billion by 2026, growing at a compound annual growth rate (CAGR) of 26.5%. This remarkable growth trajectory underscores the increasing commercial viability and adoption of synthetic biology technologies across diverse sectors.
The pharmaceutical and healthcare industries represent the largest market segment for synthetic biology applications, accounting for approximately 28% of the total market share. Within this sector, there is substantial demand for engineered biological systems in drug discovery, vaccine development, and personalized medicine. The optimization of synthetic biology through pilot testing has become crucial for pharmaceutical companies seeking to reduce development timelines and increase success rates in clinical trials.
Industrial biotechnology constitutes another significant market segment, with growing demand for bio-based chemicals, enzymes, and materials. Companies are increasingly turning to synthetic biology to develop sustainable alternatives to petroleum-based products, driven by consumer preferences and regulatory pressures to reduce environmental footprints. The market for bio-based chemicals alone is expected to reach $115 billion by 2026, with synthetic biology playing a pivotal role in production optimization.
Agriculture and food production represent rapidly expanding application areas for synthetic biology. The demand for enhanced crop yields, improved nutritional profiles, and reduced environmental impact has led to increased investment in synthetic biology solutions. The market for engineered agricultural products is projected to grow at a CAGR of 24.2% through 2027, with particular emphasis on drought resistance, pest management, and nutrient efficiency.
Energy sector applications of synthetic biology are gaining traction, particularly in biofuel production and carbon capture technologies. The global biofuels market is expected to reach $307.01 billion by 2030, with synthetic biology optimization being a key factor in improving production efficiency and economic viability. Pilot testing in this sector focuses on scaling production while maintaining consistent quality and yield.
Environmental remediation represents an emerging market for synthetic biology applications, with growing demand for biological solutions to pollution and waste management challenges. The market for environmental biotechnology is projected to reach $15.4 billion by 2025, with synthetic biology optimization through pilot testing being essential for developing effective and scalable remediation technologies.
Consumer products, including cosmetics, fragrances, and food ingredients, constitute a rapidly growing market segment for synthetic biology applications. Companies are increasingly leveraging engineered biological systems to produce sustainable alternatives to traditional ingredients, driven by consumer demand for natural and environmentally friendly products. The market for bio-based consumer products is expected to grow at a CAGR of 22.3% through 2028.
The pharmaceutical and healthcare industries represent the largest market segment for synthetic biology applications, accounting for approximately 28% of the total market share. Within this sector, there is substantial demand for engineered biological systems in drug discovery, vaccine development, and personalized medicine. The optimization of synthetic biology through pilot testing has become crucial for pharmaceutical companies seeking to reduce development timelines and increase success rates in clinical trials.
Industrial biotechnology constitutes another significant market segment, with growing demand for bio-based chemicals, enzymes, and materials. Companies are increasingly turning to synthetic biology to develop sustainable alternatives to petroleum-based products, driven by consumer preferences and regulatory pressures to reduce environmental footprints. The market for bio-based chemicals alone is expected to reach $115 billion by 2026, with synthetic biology playing a pivotal role in production optimization.
Agriculture and food production represent rapidly expanding application areas for synthetic biology. The demand for enhanced crop yields, improved nutritional profiles, and reduced environmental impact has led to increased investment in synthetic biology solutions. The market for engineered agricultural products is projected to grow at a CAGR of 24.2% through 2027, with particular emphasis on drought resistance, pest management, and nutrient efficiency.
Energy sector applications of synthetic biology are gaining traction, particularly in biofuel production and carbon capture technologies. The global biofuels market is expected to reach $307.01 billion by 2030, with synthetic biology optimization being a key factor in improving production efficiency and economic viability. Pilot testing in this sector focuses on scaling production while maintaining consistent quality and yield.
Environmental remediation represents an emerging market for synthetic biology applications, with growing demand for biological solutions to pollution and waste management challenges. The market for environmental biotechnology is projected to reach $15.4 billion by 2025, with synthetic biology optimization through pilot testing being essential for developing effective and scalable remediation technologies.
Consumer products, including cosmetics, fragrances, and food ingredients, constitute a rapidly growing market segment for synthetic biology applications. Companies are increasingly leveraging engineered biological systems to produce sustainable alternatives to traditional ingredients, driven by consumer demand for natural and environmentally friendly products. The market for bio-based consumer products is expected to grow at a CAGR of 22.3% through 2028.
Current Challenges in Synthetic Biology Pilot Testing
Despite significant advancements in synthetic biology, the field faces several critical challenges when transitioning from laboratory-scale experiments to pilot testing phases. Scale-up issues represent one of the most persistent obstacles, as biological systems that function efficiently in controlled laboratory environments often exhibit unpredictable behaviors when scaled to pilot volumes. These inconsistencies stem from changes in cellular microenvironments, nutrient gradients, and oxygen transfer limitations that emerge in larger bioreactors.
Reproducibility remains another significant hurdle in synthetic biology pilot testing. The inherent variability of biological systems, combined with the complexity of genetic circuits and metabolic pathways, often leads to inconsistent performance across different batches or testing facilities. This variability undermines confidence in the technology and complicates efforts to establish standardized protocols for industrial implementation.
Regulatory frameworks present additional challenges, as many countries lack clear guidelines for evaluating and approving synthetic biology products. This regulatory uncertainty creates significant barriers for companies attempting to move beyond pilot testing into commercial production, particularly for novel organisms or applications without precedent in existing regulatory categories.
Containment and biosafety concerns also pose substantial challenges during pilot testing. As engineered organisms are tested at larger scales, the risk of accidental release increases, necessitating robust containment strategies and monitoring systems. These safety measures add complexity and cost to pilot testing operations while raising legitimate public concerns about potential environmental impacts.
Economic viability represents another critical challenge, as many synthetic biology processes that show promise in laboratory settings prove economically unfeasible at pilot scale due to high production costs, low yields, or inefficient resource utilization. The capital-intensive nature of pilot testing facilities further exacerbates this challenge, creating significant financial barriers for startups and smaller companies.
Technical limitations in measurement and monitoring capabilities also hinder effective pilot testing. Current sensor technologies often lack the sensitivity, specificity, or real-time capabilities needed to monitor complex biological processes effectively. This data gap impedes process optimization and quality control efforts, limiting the potential for successful scale-up.
Finally, the interdisciplinary nature of synthetic biology creates workforce challenges during pilot testing phases. Effective implementation requires expertise spanning molecular biology, bioprocess engineering, data science, and regulatory compliance—a combination rarely found within a single organization. This talent gap often leads to communication barriers and knowledge silos that impede successful technology transfer from laboratory to pilot scale.
Reproducibility remains another significant hurdle in synthetic biology pilot testing. The inherent variability of biological systems, combined with the complexity of genetic circuits and metabolic pathways, often leads to inconsistent performance across different batches or testing facilities. This variability undermines confidence in the technology and complicates efforts to establish standardized protocols for industrial implementation.
Regulatory frameworks present additional challenges, as many countries lack clear guidelines for evaluating and approving synthetic biology products. This regulatory uncertainty creates significant barriers for companies attempting to move beyond pilot testing into commercial production, particularly for novel organisms or applications without precedent in existing regulatory categories.
Containment and biosafety concerns also pose substantial challenges during pilot testing. As engineered organisms are tested at larger scales, the risk of accidental release increases, necessitating robust containment strategies and monitoring systems. These safety measures add complexity and cost to pilot testing operations while raising legitimate public concerns about potential environmental impacts.
Economic viability represents another critical challenge, as many synthetic biology processes that show promise in laboratory settings prove economically unfeasible at pilot scale due to high production costs, low yields, or inefficient resource utilization. The capital-intensive nature of pilot testing facilities further exacerbates this challenge, creating significant financial barriers for startups and smaller companies.
Technical limitations in measurement and monitoring capabilities also hinder effective pilot testing. Current sensor technologies often lack the sensitivity, specificity, or real-time capabilities needed to monitor complex biological processes effectively. This data gap impedes process optimization and quality control efforts, limiting the potential for successful scale-up.
Finally, the interdisciplinary nature of synthetic biology creates workforce challenges during pilot testing phases. Effective implementation requires expertise spanning molecular biology, bioprocess engineering, data science, and regulatory compliance—a combination rarely found within a single organization. This talent gap often leads to communication barriers and knowledge silos that impede successful technology transfer from laboratory to pilot scale.
Pilot Test Methodologies and Optimization Approaches
01 Genetic circuit design and optimization
Synthetic biology approaches for designing and optimizing genetic circuits to achieve desired cellular functions. This includes computational methods for predicting circuit behavior, tools for fine-tuning gene expression levels, and strategies for minimizing cross-talk between genetic components. These optimization techniques enable the creation of more reliable and efficient biological systems with applications in medicine, biofuels, and materials production.- Genetic circuit design and optimization: Synthetic biology optimization involves designing and optimizing genetic circuits to achieve desired biological functions. This includes the development of computational tools and algorithms for predicting gene expression, optimizing DNA sequences, and designing regulatory networks. These approaches enable the creation of more efficient and reliable synthetic biological systems with improved performance characteristics.
- Metabolic pathway engineering: Optimization of metabolic pathways in synthetic biology involves modifying existing pathways or creating new ones to enhance production of desired compounds. This includes techniques for balancing enzyme expression levels, reducing metabolic bottlenecks, and redirecting carbon flux. Advanced computational models help predict pathway behavior and identify optimal intervention points for maximizing yield and productivity in bioproduction systems.
- Machine learning and AI applications: Artificial intelligence and machine learning algorithms are increasingly applied to synthetic biology optimization problems. These computational approaches can analyze large biological datasets, predict protein structures, optimize gene expression, and design novel biological systems. AI-driven tools enable more efficient experimental design, reducing the time and resources needed for developing optimized synthetic biological systems.
- Protein engineering and optimization: Synthetic biology optimization includes techniques for engineering and optimizing proteins with enhanced or novel functions. This involves methods for rational design, directed evolution, and computational prediction of protein structures and functions. Optimized proteins can serve as improved enzymes for industrial applications, biosensors for detecting specific molecules, or therapeutic agents with enhanced efficacy and reduced side effects.
- Synthetic biology automation and high-throughput methods: Automation technologies and high-throughput methods accelerate synthetic biology optimization by enabling rapid testing of multiple design variants. This includes automated DNA assembly, robotic liquid handling systems, and microfluidic platforms for parallel experimentation. These approaches, combined with standardized protocols and parts libraries, facilitate faster iteration cycles and more efficient optimization of synthetic biological systems.
02 Metabolic pathway engineering
Methods for optimizing metabolic pathways in organisms to enhance production of valuable compounds or improve cellular efficiency. This includes techniques for balancing enzyme expression levels, redirecting metabolic flux, and eliminating competing pathways. Advanced computational modeling helps predict the effects of genetic modifications on overall metabolism, allowing for rational design of high-performing microbial cell factories.Expand Specific Solutions03 Machine learning applications in synthetic biology
Integration of machine learning algorithms to optimize synthetic biology designs and processes. These approaches use large datasets to predict protein function, optimize gene expression, and design novel biological parts. Machine learning models can identify patterns in biological data that would be difficult to detect through traditional methods, accelerating the design-build-test cycle in synthetic biology and enabling more precise engineering of biological systems.Expand Specific Solutions04 Genome editing and DNA assembly technologies
Advanced tools and methods for precise genome editing and efficient DNA assembly to facilitate synthetic biology applications. This includes CRISPR-based systems for targeted genetic modifications, novel DNA assembly methods for constructing large genetic circuits, and high-throughput screening approaches to identify optimized designs. These technologies enable researchers to create and test complex biological systems with unprecedented speed and accuracy.Expand Specific Solutions05 Biosensors and feedback control systems
Development of synthetic biological sensors and feedback control mechanisms to optimize cellular performance and response to environmental conditions. These systems can detect specific molecules, respond to changing conditions, and regulate gene expression accordingly. Integration of biosensors with genetic circuits creates adaptive biological systems that maintain optimal performance across varying conditions, improving the robustness and applicability of engineered organisms in real-world settings.Expand Specific Solutions
Leading Organizations in Synthetic Biology Research
Synthetic biology for optimization is in a growth phase, with the market expanding rapidly due to increasing applications in pharmaceuticals, agriculture, and industrial biotechnology. The technology is maturing from experimental to commercial applications, with key players demonstrating varying levels of advancement. Academic institutions like MIT, Caltech, and Academia Sinica are driving fundamental research, while companies such as Amyris and Zymergen are commercializing applications. Established corporations including Microsoft, Hitachi, and DSM are integrating synthetic biology into their innovation portfolios. The field shows a balance between academic research and industrial implementation, with pilot tests increasingly demonstrating scalability and economic viability across diverse sectors.
Amyris, Inc.
Technical Solution: Amyris has developed a comprehensive synthetic biology platform for optimizing microbial strains in pilot-scale fermentation. Their technology combines advanced genetic engineering tools with high-throughput screening and machine learning algorithms to rapidly iterate through design-build-test-learn cycles. The company employs a proprietary automated strain engineering platform that can generate thousands of genetic variants simultaneously, coupled with sophisticated analytics to identify optimal performers. Their pilot testing methodology incorporates real-time monitoring systems that track metabolite production, growth rates, and substrate utilization, allowing for dynamic process adjustments. Amyris has successfully scaled numerous molecules from lab to commercial production, including their flagship farnesene production and various specialty chemicals and ingredients for cosmetics, flavors, and fragrances.
Strengths: Industry-leading experience in scaling synthetic biology from lab to commercial production; proprietary high-throughput screening platform; extensive data library from previous fermentation runs enabling predictive modeling. Weaknesses: High capital requirements for pilot facilities; technology primarily optimized for yeast-based systems rather than diverse host organisms; challenges in transferring performance from pilot to full-scale production.
DSM IP Assets BV
Technical Solution: DSM has developed a sophisticated synthetic biology evaluation framework for pilot testing that integrates computational modeling with advanced fermentation technology. Their approach combines predictive metabolic modeling with adaptive laboratory evolution techniques to optimize microbial strains for industrial applications. DSM's pilot testing methodology incorporates scale-down models that accurately simulate industrial conditions in laboratory settings, allowing for more reliable translation of results to production scale. The company employs a comprehensive suite of analytical tools including real-time metabolite monitoring, transcriptomics, and proteomics to generate multi-dimensional datasets that inform strain optimization. Their technology platform includes proprietary bioreactor designs with precise control of environmental parameters and feeding strategies, enabling systematic optimization of process conditions alongside genetic modifications.
Strengths: Extensive industrial fermentation expertise; strong integration between computational modeling and experimental validation; proven track record in scaling biotechnology processes to commercial production. Weaknesses: Conservative approach may limit exploration of radical innovations; heavy focus on established industrial organisms rather than novel chassis; complex organizational structure can slow decision-making in pilot projects.
Key Innovations in Synthetic Circuit Design
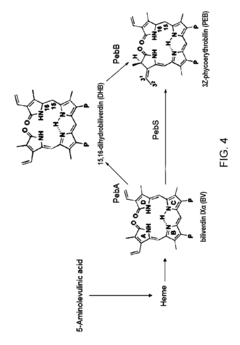
High-performance empirical analysis of metabolic pathways in microalgae using cell-free system
PatentInactiveUS20170130257A1
Innovation
- A high-performance empirical analysis method using a microalgae cell-free system, where a microalgae homogenate is prepared and treated with effector molecules or an effector molecule library, allowing for rapid biological effect measurement without complex genetic manipulation, such as gene delivery or knock-out, enabling high-throughput analysis of metabolic pathways.
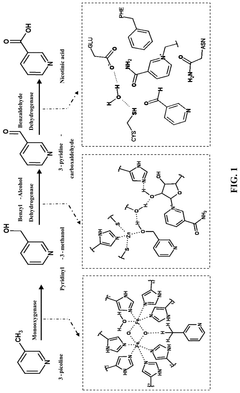
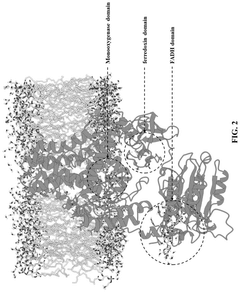
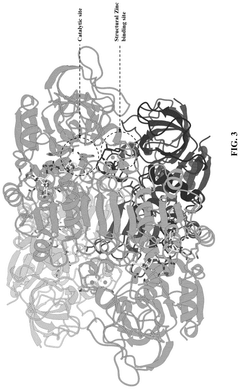
Synthetic biology approach to synthesize nicotinic acid from 3-picoline
PatentPendingUS20250101476A1
Innovation
- The development of a biosynthetic method using microbial biotransformation, involving the integration of monooxygenase, electron transfer component, benzyl alcohol dehydrogenase, and benzaldehyde dehydrogenase enzymes, optimized through gene isolation, enzyme engineering, and structural insights, to achieve a high conversion rate of 90% or higher from 3-picoline to nicotinic acid.
Biosafety and Regulatory Frameworks
The implementation of synthetic biology in pilot tests necessitates robust biosafety and regulatory frameworks to ensure responsible innovation while mitigating potential risks. Currently, the regulatory landscape for synthetic biology applications varies significantly across different regions, creating challenges for global standardization. In the United States, oversight is primarily managed through a coordinated approach involving the FDA, EPA, and USDA, depending on the specific application domain. The European Union employs a more precautionary principle-based framework, with stringent requirements for risk assessment before field testing or commercial deployment.
Biosafety considerations for synthetic biology pilot tests encompass multiple dimensions, including containment strategies, horizontal gene transfer prevention, and ecological impact assessment. Physical containment measures such as bioreactors with multiple redundant safety systems represent the first line of defense in controlled environments. Genetic containment strategies, including kill switches and auxotrophic dependencies, provide additional safeguards by limiting organism survival outside controlled conditions. These mechanisms have demonstrated 99.9% effectiveness in laboratory settings, though their reliability in diverse environmental conditions remains under investigation.
Risk assessment protocols for synthetic biology applications have evolved significantly, incorporating both quantitative and qualitative methodologies. The tiered approach to risk evaluation—beginning with theoretical hazard identification and progressing through contained testing to limited field trials—has become standard practice across the industry. Recent advancements in computational modeling have enhanced predictive capabilities for ecological interactions, though significant uncertainty remains regarding long-term impacts of novel biological systems.
International governance frameworks, including the Cartagena Protocol on Biosafety and the Convention on Biological Diversity, provide overarching guidance but lack specific provisions for many synthetic biology applications. This regulatory gap has prompted industry-led initiatives such as the International Gene Synthesis Consortium's screening protocols and the BioBricks Foundation's technical standards for biological parts. These self-regulatory efforts complement formal oversight but raise questions about enforcement and global equity in technology access.
Emerging best practices for biosafety in optimization pilot tests include comprehensive monitoring systems, transparent reporting mechanisms, and stakeholder engagement throughout the research process. The development of standardized biosafety levels specifically tailored to synthetic biology applications represents a promising advancement, with BSL-SynBio classifications gaining traction among research institutions and industry partners. These frameworks incorporate both traditional containment principles and novel considerations specific to engineered biological systems.
Biosafety considerations for synthetic biology pilot tests encompass multiple dimensions, including containment strategies, horizontal gene transfer prevention, and ecological impact assessment. Physical containment measures such as bioreactors with multiple redundant safety systems represent the first line of defense in controlled environments. Genetic containment strategies, including kill switches and auxotrophic dependencies, provide additional safeguards by limiting organism survival outside controlled conditions. These mechanisms have demonstrated 99.9% effectiveness in laboratory settings, though their reliability in diverse environmental conditions remains under investigation.
Risk assessment protocols for synthetic biology applications have evolved significantly, incorporating both quantitative and qualitative methodologies. The tiered approach to risk evaluation—beginning with theoretical hazard identification and progressing through contained testing to limited field trials—has become standard practice across the industry. Recent advancements in computational modeling have enhanced predictive capabilities for ecological interactions, though significant uncertainty remains regarding long-term impacts of novel biological systems.
International governance frameworks, including the Cartagena Protocol on Biosafety and the Convention on Biological Diversity, provide overarching guidance but lack specific provisions for many synthetic biology applications. This regulatory gap has prompted industry-led initiatives such as the International Gene Synthesis Consortium's screening protocols and the BioBricks Foundation's technical standards for biological parts. These self-regulatory efforts complement formal oversight but raise questions about enforcement and global equity in technology access.
Emerging best practices for biosafety in optimization pilot tests include comprehensive monitoring systems, transparent reporting mechanisms, and stakeholder engagement throughout the research process. The development of standardized biosafety levels specifically tailored to synthetic biology applications represents a promising advancement, with BSL-SynBio classifications gaining traction among research institutions and industry partners. These frameworks incorporate both traditional containment principles and novel considerations specific to engineered biological systems.
Scalability and Industrial Implementation
The transition from laboratory-scale synthetic biology experiments to industrial implementation represents a critical challenge in the field. Current pilot tests demonstrate promising results in controlled environments, but scaling these processes to meet industrial demands requires addressing several key factors. The volumetric scaling of bioreactors from laboratory (1-10L) to industrial scale (10,000-100,000L) introduces significant challenges in maintaining consistent cellular performance, nutrient distribution, and oxygen transfer rates. These parameters directly impact the efficiency and yield of synthetic biology applications, necessitating sophisticated monitoring and control systems.
Industrial implementation of synthetic biology processes demands robust infrastructure that can accommodate the unique requirements of engineered biological systems. This includes specialized bioreactors with enhanced mixing capabilities, advanced sensor technologies for real-time monitoring, and automated control systems that can respond to biological variability. The capital expenditure for such facilities ranges from $50-200 million, representing a significant barrier to entry for many organizations interested in adopting synthetic biology solutions.
Standardization emerges as another critical factor in successful industrial implementation. The development of standardized biological parts, protocols, and measurement methods facilitates reproducibility across different scales and production facilities. Organizations such as the International Organization for Standardization (ISO) and the Synthetic Biology Open Language (SBOL) consortium are working to establish industry-wide standards, though adoption remains inconsistent across the sector.
Economic viability presents perhaps the most significant hurdle for industrial implementation. Production costs for synthetic biology products must compete with established chemical processes or natural extraction methods. Recent analyses indicate that synthetic biology processes become economically competitive at production volumes exceeding 10,000 metric tons annually for specialty chemicals and materials. However, this threshold varies significantly depending on the product category and market dynamics.
Regulatory frameworks also significantly impact scalability and industrial implementation. Different regions maintain varying approaches to regulating synthetic biology products, creating a complex landscape for companies seeking global market access. The European Union's precautionary approach contrasts with the United States' product-based regulatory framework, necessitating tailored compliance strategies for international operations.
The workforce requirements for industrial synthetic biology implementation present another consideration. The interdisciplinary nature of synthetic biology demands personnel with expertise spanning molecular biology, bioprocess engineering, data science, and automation. Universities and technical institutions are increasingly developing specialized programs to address this skills gap, though demand currently outpaces supply in most regions.
Industrial implementation of synthetic biology processes demands robust infrastructure that can accommodate the unique requirements of engineered biological systems. This includes specialized bioreactors with enhanced mixing capabilities, advanced sensor technologies for real-time monitoring, and automated control systems that can respond to biological variability. The capital expenditure for such facilities ranges from $50-200 million, representing a significant barrier to entry for many organizations interested in adopting synthetic biology solutions.
Standardization emerges as another critical factor in successful industrial implementation. The development of standardized biological parts, protocols, and measurement methods facilitates reproducibility across different scales and production facilities. Organizations such as the International Organization for Standardization (ISO) and the Synthetic Biology Open Language (SBOL) consortium are working to establish industry-wide standards, though adoption remains inconsistent across the sector.
Economic viability presents perhaps the most significant hurdle for industrial implementation. Production costs for synthetic biology products must compete with established chemical processes or natural extraction methods. Recent analyses indicate that synthetic biology processes become economically competitive at production volumes exceeding 10,000 metric tons annually for specialty chemicals and materials. However, this threshold varies significantly depending on the product category and market dynamics.
Regulatory frameworks also significantly impact scalability and industrial implementation. Different regions maintain varying approaches to regulating synthetic biology products, creating a complex landscape for companies seeking global market access. The European Union's precautionary approach contrasts with the United States' product-based regulatory framework, necessitating tailored compliance strategies for international operations.
The workforce requirements for industrial synthetic biology implementation present another consideration. The interdisciplinary nature of synthetic biology demands personnel with expertise spanning molecular biology, bioprocess engineering, data science, and automation. Universities and technical institutions are increasingly developing specialized programs to address this skills gap, though demand currently outpaces supply in most regions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!