Synthetic Biology's Role in Pilot-Based Assessments for Industrial Use
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Assessment Objectives
Synthetic biology has evolved significantly over the past two decades, transitioning from a nascent field focused on basic genetic circuit design to a sophisticated discipline with far-reaching industrial applications. The evolution began with the development of fundamental tools for DNA synthesis and assembly in the early 2000s, followed by the establishment of standardized biological parts and modular design principles. This progression has enabled increasingly complex biological systems to be engineered with greater precision and predictability.
The field's trajectory has been characterized by several key technological advancements, including CRISPR-Cas9 gene editing, which revolutionized the ability to make precise genetic modifications; high-throughput DNA synthesis and assembly methods that accelerated the design-build-test cycle; and computational tools that enhanced the ability to predict biological system behavior. These developments have collectively transformed synthetic biology from an academic curiosity into a powerful industrial technology platform.
Current trends indicate a shift toward more sophisticated applications, including the development of cell-free systems that eliminate the complexities of working with living organisms, the integration of artificial intelligence for biological design optimization, and the expansion into novel chassis organisms beyond traditional model systems like E. coli and yeast. The industrial application landscape has similarly expanded from initial focus areas in biofuels and pharmaceuticals to encompass materials science, agriculture, food technology, and environmental remediation.
The primary objective of pilot-based assessments for industrial synthetic biology applications is to bridge the gap between laboratory-scale proof-of-concept and full commercial implementation. These assessments aim to validate scalability by demonstrating that engineered biological systems can maintain performance characteristics when production volumes increase by several orders of magnitude. They seek to optimize process parameters including fermentation conditions, feedstock utilization efficiency, and downstream processing requirements.
Additionally, these assessments evaluate economic viability by analyzing production costs, capital expenditure requirements, and potential return on investment compared to conventional manufacturing processes. They also address regulatory compliance by generating safety data and environmental impact assessments necessary for approval by relevant authorities. The technical objectives include identifying and resolving scale-up challenges such as genetic stability, metabolic burden, and contamination risks that may not be apparent in laboratory settings.
The ultimate goal is to establish synthetic biology as a reliable, cost-effective, and sustainable manufacturing platform across diverse industries, capable of addressing global challenges in resource utilization, environmental impact, and production efficiency. This requires not only technical validation but also the development of standardized assessment frameworks that can accurately predict industrial performance from pilot-scale data.
The field's trajectory has been characterized by several key technological advancements, including CRISPR-Cas9 gene editing, which revolutionized the ability to make precise genetic modifications; high-throughput DNA synthesis and assembly methods that accelerated the design-build-test cycle; and computational tools that enhanced the ability to predict biological system behavior. These developments have collectively transformed synthetic biology from an academic curiosity into a powerful industrial technology platform.
Current trends indicate a shift toward more sophisticated applications, including the development of cell-free systems that eliminate the complexities of working with living organisms, the integration of artificial intelligence for biological design optimization, and the expansion into novel chassis organisms beyond traditional model systems like E. coli and yeast. The industrial application landscape has similarly expanded from initial focus areas in biofuels and pharmaceuticals to encompass materials science, agriculture, food technology, and environmental remediation.
The primary objective of pilot-based assessments for industrial synthetic biology applications is to bridge the gap between laboratory-scale proof-of-concept and full commercial implementation. These assessments aim to validate scalability by demonstrating that engineered biological systems can maintain performance characteristics when production volumes increase by several orders of magnitude. They seek to optimize process parameters including fermentation conditions, feedstock utilization efficiency, and downstream processing requirements.
Additionally, these assessments evaluate economic viability by analyzing production costs, capital expenditure requirements, and potential return on investment compared to conventional manufacturing processes. They also address regulatory compliance by generating safety data and environmental impact assessments necessary for approval by relevant authorities. The technical objectives include identifying and resolving scale-up challenges such as genetic stability, metabolic burden, and contamination risks that may not be apparent in laboratory settings.
The ultimate goal is to establish synthetic biology as a reliable, cost-effective, and sustainable manufacturing platform across diverse industries, capable of addressing global challenges in resource utilization, environmental impact, and production efficiency. This requires not only technical validation but also the development of standardized assessment frameworks that can accurately predict industrial performance from pilot-scale data.
Industrial Market Demand for Synthetic Biology Solutions
The synthetic biology market is experiencing unprecedented growth, with projections indicating a global market value reaching $30.7 billion by 2026, growing at a CAGR of 23.9%. This surge reflects the increasing industrial demand for sustainable, bio-based solutions across multiple sectors. The industrial landscape is actively seeking alternatives to traditional chemical processes, driven by environmental regulations, consumer preferences for sustainable products, and the need for resource efficiency.
Manufacturing industries, particularly in chemicals and materials, demonstrate the strongest demand for synthetic biology applications. Companies are investing heavily in bio-based production methods that reduce carbon footprints while maintaining or improving product performance. The pharmaceutical sector represents another significant market, with approximately 60% of new small-molecule drugs now utilizing enzymatic processes developed through synthetic biology approaches.
Energy companies are increasingly exploring synthetic biology for biofuel production, carbon capture technologies, and waste valorization. This sector's demand is expected to grow at 28.5% annually through 2030, outpacing the overall market as renewable energy mandates intensify globally.
Consumer goods manufacturers have shown remarkable interest in synthetic biology solutions, particularly for sustainable packaging materials and bio-based ingredients. Market research indicates that 73% of global consumers are willing to pay premium prices for products with environmentally friendly credentials, driving brands to seek synthetic biology innovations.
Agricultural applications represent a rapidly expanding market segment, with demand for bio-based fertilizers, crop protection products, and soil health solutions growing at 25.7% annually. This growth is fueled by increasing restrictions on conventional agrochemicals and the push toward regenerative agricultural practices.
Regional analysis reveals that North America currently leads industrial demand for synthetic biology solutions, accounting for 42% of the global market. However, Asia-Pacific regions are experiencing the fastest growth rate at 29.3% annually, driven by China's substantial investments in bioeconomy infrastructure and Japan's focus on high-value specialty chemicals.
The market demonstrates particular interest in scalable pilot technologies that can bridge the gap between laboratory success and industrial implementation. Industry surveys indicate that 67% of potential industrial adopters cite scalability concerns as their primary hesitation in implementing synthetic biology solutions, highlighting the critical importance of effective pilot-scale demonstrations and assessments.
Manufacturing industries, particularly in chemicals and materials, demonstrate the strongest demand for synthetic biology applications. Companies are investing heavily in bio-based production methods that reduce carbon footprints while maintaining or improving product performance. The pharmaceutical sector represents another significant market, with approximately 60% of new small-molecule drugs now utilizing enzymatic processes developed through synthetic biology approaches.
Energy companies are increasingly exploring synthetic biology for biofuel production, carbon capture technologies, and waste valorization. This sector's demand is expected to grow at 28.5% annually through 2030, outpacing the overall market as renewable energy mandates intensify globally.
Consumer goods manufacturers have shown remarkable interest in synthetic biology solutions, particularly for sustainable packaging materials and bio-based ingredients. Market research indicates that 73% of global consumers are willing to pay premium prices for products with environmentally friendly credentials, driving brands to seek synthetic biology innovations.
Agricultural applications represent a rapidly expanding market segment, with demand for bio-based fertilizers, crop protection products, and soil health solutions growing at 25.7% annually. This growth is fueled by increasing restrictions on conventional agrochemicals and the push toward regenerative agricultural practices.
Regional analysis reveals that North America currently leads industrial demand for synthetic biology solutions, accounting for 42% of the global market. However, Asia-Pacific regions are experiencing the fastest growth rate at 29.3% annually, driven by China's substantial investments in bioeconomy infrastructure and Japan's focus on high-value specialty chemicals.
The market demonstrates particular interest in scalable pilot technologies that can bridge the gap between laboratory success and industrial implementation. Industry surveys indicate that 67% of potential industrial adopters cite scalability concerns as their primary hesitation in implementing synthetic biology solutions, highlighting the critical importance of effective pilot-scale demonstrations and assessments.
Current Challenges in Pilot-Scale Synthetic Biology Implementation
Despite significant advancements in synthetic biology, scaling processes from laboratory to industrial pilot scale presents numerous technical and operational challenges. The transition from controlled laboratory environments to larger-scale operations introduces variables that often compromise reproducibility and efficiency. One primary challenge is maintaining genetic stability in engineered organisms during scale-up operations. As production volumes increase, selective pressures change, potentially leading to genetic mutations or loss of engineered functions that were stable at laboratory scale.
Process parameter optimization represents another significant hurdle. Parameters that work effectively in small-scale bioreactors—such as oxygen transfer rates, nutrient distribution, and temperature control—often behave differently at pilot scale due to altered mixing dynamics and heat transfer properties. This necessitates extensive re-optimization, consuming valuable time and resources before industrial implementation can proceed.
Contamination risks increase substantially at pilot scale, where maintaining strict sterility becomes more difficult across larger equipment and longer production runs. Even minor contamination can lead to batch failure or inconsistent product quality, creating significant economic losses and delaying commercialization timelines.
Regulatory compliance presents a complex challenge for pilot-scale implementation. Current regulatory frameworks were largely developed before the emergence of synthetic biology, creating uncertainty regarding approval pathways for novel organisms and processes. Companies must navigate evolving regulations while demonstrating safety and containment strategies appropriate for larger-scale operations.
Economic viability remains a persistent concern, as many synthetic biology processes that show promise in laboratories face prohibitive costs when scaled. Expensive feedstocks, low yields, and inefficient downstream processing can undermine the commercial potential of otherwise promising technologies. The capital investment required for pilot facilities often creates a "valley of death" where promising technologies stall before reaching commercial implementation.
Technical expertise limitations further complicate scale-up efforts. The interdisciplinary nature of synthetic biology requires teams with diverse expertise spanning molecular biology, bioprocess engineering, and industrial operations—a combination that remains scarce in the industry. This knowledge gap frequently leads to suboptimal design decisions during the transition to pilot scale.
Data integration and analysis capabilities are often insufficient to handle the increased complexity and volume of data generated at pilot scale. Without robust computational infrastructure and standardized metrics, organizations struggle to extract actionable insights from pilot operations, limiting their ability to optimize processes effectively for industrial implementation.
Process parameter optimization represents another significant hurdle. Parameters that work effectively in small-scale bioreactors—such as oxygen transfer rates, nutrient distribution, and temperature control—often behave differently at pilot scale due to altered mixing dynamics and heat transfer properties. This necessitates extensive re-optimization, consuming valuable time and resources before industrial implementation can proceed.
Contamination risks increase substantially at pilot scale, where maintaining strict sterility becomes more difficult across larger equipment and longer production runs. Even minor contamination can lead to batch failure or inconsistent product quality, creating significant economic losses and delaying commercialization timelines.
Regulatory compliance presents a complex challenge for pilot-scale implementation. Current regulatory frameworks were largely developed before the emergence of synthetic biology, creating uncertainty regarding approval pathways for novel organisms and processes. Companies must navigate evolving regulations while demonstrating safety and containment strategies appropriate for larger-scale operations.
Economic viability remains a persistent concern, as many synthetic biology processes that show promise in laboratories face prohibitive costs when scaled. Expensive feedstocks, low yields, and inefficient downstream processing can undermine the commercial potential of otherwise promising technologies. The capital investment required for pilot facilities often creates a "valley of death" where promising technologies stall before reaching commercial implementation.
Technical expertise limitations further complicate scale-up efforts. The interdisciplinary nature of synthetic biology requires teams with diverse expertise spanning molecular biology, bioprocess engineering, and industrial operations—a combination that remains scarce in the industry. This knowledge gap frequently leads to suboptimal design decisions during the transition to pilot scale.
Data integration and analysis capabilities are often insufficient to handle the increased complexity and volume of data generated at pilot scale. Without robust computational infrastructure and standardized metrics, organizations struggle to extract actionable insights from pilot operations, limiting their ability to optimize processes effectively for industrial implementation.
Pilot-Scale Assessment Methodologies for Synthetic Biology
01 Genetic Engineering and DNA Manipulation
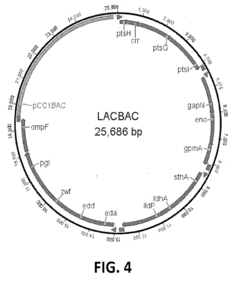
Synthetic biology approaches involving the engineering and manipulation of DNA to create novel biological systems or modify existing ones. This includes techniques for gene editing, DNA synthesis, and the construction of artificial genetic circuits that can perform specific functions. These technologies enable the design and creation of organisms with new capabilities for applications in medicine, agriculture, and industry.- Genetic Engineering and DNA Manipulation: Synthetic biology approaches involving the manipulation of genetic material to create novel biological systems or modify existing ones. This includes techniques for DNA synthesis, gene editing, and the creation of artificial genetic circuits that can perform specific functions. These technologies enable the design and construction of biological parts, devices, and systems that do not exist in nature.
- Biosensors and Detection Systems: Development of synthetic biology-based biosensors that can detect specific molecules, environmental conditions, or biological threats. These systems typically incorporate engineered biological components that produce measurable signals in response to target analytes. Applications include medical diagnostics, environmental monitoring, and security screening.
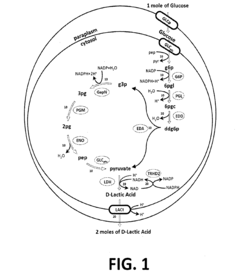
- Metabolic Engineering for Bioproduction: Application of synthetic biology principles to redesign metabolic pathways in microorganisms for the production of valuable compounds. This includes engineering cells to produce pharmaceuticals, biofuels, chemicals, and materials through optimized biosynthetic pathways. The approach often involves introducing new genes or modifying existing ones to create efficient cellular factories.
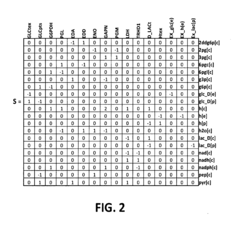
- Computational Tools and Artificial Intelligence in Synthetic Biology: Integration of computational methods, machine learning, and artificial intelligence to design and predict the behavior of synthetic biological systems. These tools help in modeling complex biological interactions, optimizing genetic circuits, and accelerating the design-build-test cycle in synthetic biology research and applications.
- Synthetic Biology Platforms and Standardization: Development of standardized platforms, protocols, and modular components for synthetic biology applications. This includes the creation of biological parts libraries, chassis organisms, and assembly methods that facilitate the engineering of complex biological systems. Standardization efforts aim to improve reproducibility, interoperability, and scalability in synthetic biology research and industrial applications.
02 Biosensors and Detection Systems
Development of synthetic biology-based biosensors and detection systems that can identify specific molecules, pathogens, or environmental conditions. These systems typically incorporate engineered biological components such as proteins or cells that produce detectable signals in response to target analytes. Applications include medical diagnostics, environmental monitoring, and food safety testing.Expand Specific Solutions03 Metabolic Engineering for Bioproduction
Application of synthetic biology principles to engineer metabolic pathways in microorganisms for the production of valuable compounds. This involves redesigning cellular metabolism to optimize the synthesis of target molecules such as pharmaceuticals, biofuels, chemicals, and materials. Techniques include pathway optimization, enzyme engineering, and the integration of novel biosynthetic routes into host organisms.Expand Specific Solutions04 Computational Tools and Artificial Intelligence in Synthetic Biology
Development and application of computational tools, algorithms, and artificial intelligence approaches to design, model, and analyze synthetic biological systems. These technologies enable the prediction of genetic circuit behavior, optimization of biological designs, and analysis of complex biological data. Machine learning methods are increasingly being used to accelerate the design-build-test cycle in synthetic biology research and applications.Expand Specific Solutions05 Cell-Free Synthetic Biology Systems
Development of cell-free systems that utilize biological machinery outside of living cells for various applications in synthetic biology. These systems extract and utilize cellular components such as enzymes, ribosomes, and metabolic pathways in controlled environments without the constraints of cell walls or growth requirements. Applications include rapid prototyping of genetic circuits, biosensing, biomanufacturing of proteins and chemicals, and educational tools.Expand Specific Solutions
Key Industry Players in Synthetic Biology Commercialization
Synthetic biology in industrial pilot assessments is currently in a growth phase, with the market expanding rapidly due to increasing applications in pharmaceuticals, chemicals, and biofuels. The global market is projected to reach significant scale as industries seek sustainable bio-manufacturing solutions. Technical maturity varies across applications, with companies like Amyris and Ginkgo Bioworks leading commercial-scale implementations through advanced fermentation platforms. Academic-industry partnerships are accelerating development, with institutions like MIT, Technical University of Denmark, and Chinese research institutes collaborating with BASF and DSM to bridge the gap between laboratory success and industrial viability. The field is characterized by increasing automation and standardization of pilot testing protocols to reduce scale-up failures.
Tianjin Institute of Industrial Biotechnology of CAS
Technical Solution: The Tianjin Institute of Industrial Biotechnology (TIB) has developed a comprehensive synthetic biology platform for industrial applications called "SynBio Foundry" that emphasizes systematic pilot-scale validation. Their approach integrates computational design, automated DNA assembly, and high-throughput screening with sophisticated scale-up methodologies. TIB has pioneered the development of chassis organisms specifically optimized for industrial conditions, including strains resistant to common manufacturing stressors. Their pilot assessment framework incorporates a multi-parameter optimization approach that simultaneously evaluates productivity, stability, and economic viability. A key innovation in their methodology is the "Scale-Down Model" system that recreates industrial-scale heterogeneities in laboratory settings to better predict large-scale performance[5]. TIB has established specialized pilot facilities for different bioprocess types, including both aerobic and anaerobic fermentation systems, solid-state fermentation, and enzymatic conversion processes. Their platform has been successfully applied to develop industrial processes for bio-based chemicals, enzymes, and biofuels, with particular strength in converting agricultural waste streams into value-added products. The institute has formed numerous industrial partnerships to accelerate technology transfer and commercialization.
Strengths: Strong integration of computational modeling with experimental validation; specialized expertise in developing robust industrial strains; extensive experience with diverse feedstocks including agricultural waste. Weaknesses: Potential challenges in international technology transfer; less commercial experience compared to established multinational corporations; may face limitations in accessing certain proprietary technologies.
Amyris, Inc.
Technical Solution: Amyris has developed a sophisticated synthetic biology platform specifically designed for industrial-scale implementation through their "No Compromise" approach. Their technology centers on precision fermentation using engineered yeast strains to convert plant-derived sugars into high-value molecules. For pilot-based assessments, Amyris employs a multi-stage evaluation process that begins with laboratory-scale testing, followed by 300L pilot fermentations, and culminating in commercial-scale production. Their proprietary Automated Strain Engineering (ASE) platform enables rapid prototyping and testing of thousands of strain variants to identify optimal performers. Amyris has pioneered advanced analytics and machine learning tools that correlate pilot-scale performance metrics with industrial outcomes, significantly reducing scale-up risks[3]. Their approach includes comprehensive techno-economic analysis at each development stage to ensure commercial viability. The company has successfully commercialized multiple products using this methodology, including specialty ingredients for cosmetics, flavors, fragrances, and pharmaceuticals, demonstrating reliable translation from pilot to industrial scale.
Strengths: Proven track record of successfully commercializing multiple synthetic biology products; sophisticated machine learning tools for predicting scale-up performance; extensive experience in optimizing fermentation economics. Weaknesses: Heavy focus on yeast platforms may limit application in certain product categories; reliance on agricultural feedstocks exposes operations to commodity price fluctuations; capital-intensive scale-up process requires significant investment.
Critical Patents and Innovations in Industrial Synthetic Biology
Expression of steady state metabolic pathways
PatentInactiveUS20130224804A1
Innovation
- Identifying and expressing all polypeptides of a steady state metabolic pathway within a host cell to decouple chemical production from biomass metabolism, allowing perpetual synthesis of desired products independent of biomass-related metabolites.
High-performance empirical analysis of metabolic pathways in microalgae using cell-free system
PatentInactiveUS20170130257A1
Innovation
- A high-performance empirical analysis method using a microalgae cell-free system, where a microalgae homogenate is prepared and treated with effector molecules or an effector molecule library, allowing for rapid biological effect measurement without complex genetic manipulation, such as gene delivery or knock-out, enabling high-throughput analysis of metabolic pathways.
Regulatory Framework for Synthetic Biology Industrial Deployment
The regulatory landscape for synthetic biology in industrial applications represents a complex and evolving framework that balances innovation with safety concerns. Current regulatory approaches vary significantly across jurisdictions, with the United States employing a coordinated framework involving the FDA, EPA, and USDA to oversee different aspects of synthetic biology products. The European Union, meanwhile, implements a more precautionary approach through its Directive on Contained Use of Genetically Modified Microorganisms and the Deliberate Release Directive.
Risk assessment protocols for synthetic biology industrial deployment typically follow a tiered approach, beginning with laboratory containment evaluations before progressing to pilot-scale assessments and eventually full industrial implementation. These protocols increasingly incorporate specific considerations for engineered biological systems, including horizontal gene transfer potential, ecological persistence, and interaction with native organisms in potential release environments.
International harmonization efforts have gained momentum through organizations such as the OECD Working Group on Harmonisation of Regulatory Oversight in Biotechnology and the Convention on Biological Diversity. These collaborative initiatives aim to establish consistent safety standards while facilitating responsible innovation across borders, though significant regulatory disparities remain between developed and developing nations.
Industry self-regulation has emerged as a complementary mechanism to formal government oversight, with consortia such as the International Gene Synthesis Consortium (IGSC) developing screening protocols for DNA synthesis orders and the BioBricks Foundation promoting responsible innovation through technical standards and open licensing frameworks.
Adaptive regulatory frameworks represent the frontier of synthetic biology governance, designed to evolve alongside technological advancements. These frameworks incorporate regular reassessment triggers based on predefined thresholds of scientific understanding, accumulated safety data, and emerging risk profiles. Pilot-based assessments serve as critical components within these adaptive frameworks, providing controlled environments for evaluating engineered organisms under near-industrial conditions while maintaining appropriate containment measures.
Stakeholder engagement has become increasingly recognized as essential to effective regulatory development, with structured consultation processes involving industry representatives, academic researchers, environmental organizations, and public interest groups. These multi-stakeholder dialogues help identify potential regulatory gaps while building broader societal acceptance of synthetic biology applications in industrial settings.
Risk assessment protocols for synthetic biology industrial deployment typically follow a tiered approach, beginning with laboratory containment evaluations before progressing to pilot-scale assessments and eventually full industrial implementation. These protocols increasingly incorporate specific considerations for engineered biological systems, including horizontal gene transfer potential, ecological persistence, and interaction with native organisms in potential release environments.
International harmonization efforts have gained momentum through organizations such as the OECD Working Group on Harmonisation of Regulatory Oversight in Biotechnology and the Convention on Biological Diversity. These collaborative initiatives aim to establish consistent safety standards while facilitating responsible innovation across borders, though significant regulatory disparities remain between developed and developing nations.
Industry self-regulation has emerged as a complementary mechanism to formal government oversight, with consortia such as the International Gene Synthesis Consortium (IGSC) developing screening protocols for DNA synthesis orders and the BioBricks Foundation promoting responsible innovation through technical standards and open licensing frameworks.
Adaptive regulatory frameworks represent the frontier of synthetic biology governance, designed to evolve alongside technological advancements. These frameworks incorporate regular reassessment triggers based on predefined thresholds of scientific understanding, accumulated safety data, and emerging risk profiles. Pilot-based assessments serve as critical components within these adaptive frameworks, providing controlled environments for evaluating engineered organisms under near-industrial conditions while maintaining appropriate containment measures.
Stakeholder engagement has become increasingly recognized as essential to effective regulatory development, with structured consultation processes involving industry representatives, academic researchers, environmental organizations, and public interest groups. These multi-stakeholder dialogues help identify potential regulatory gaps while building broader societal acceptance of synthetic biology applications in industrial settings.
Biosafety and Containment Strategies for Pilot Assessments
Biosafety and containment strategies represent critical components in the deployment of synthetic biology applications within industrial pilot assessments. The integration of engineered biological systems into production environments necessitates robust safety frameworks that address both environmental and human health concerns. Current biosafety protocols for synthetic biology pilots typically employ a multi-layered approach, combining physical, biological, and procedural containment measures to minimize risk.
Physical containment strategies include the implementation of closed-system bioreactors, negative pressure environments, HEPA filtration systems, and dedicated waste treatment processes. These engineering controls create barriers that prevent the unintended release of genetically modified organisms (GMOs) or their genetic material into surrounding environments. Advanced facilities often incorporate real-time monitoring systems that detect breaches in containment barriers and automatically trigger isolation protocols.
Biological containment approaches have evolved significantly, featuring engineered safeguards such as kill switches, auxotrophic dependencies, and orthogonal genetic systems. These mechanisms ensure that modified organisms cannot survive outside controlled conditions or transfer genetic material to wild populations. Recent innovations include genetic firewalls that prevent horizontal gene transfer and conditional viability systems that require specific laboratory-provided compounds for survival.
Procedural containment encompasses comprehensive training programs, standardized operating procedures, and regular safety audits. Personnel working in synthetic biology pilot facilities must demonstrate competency in biosafety practices before gaining access to biological materials. Documentation requirements include detailed risk assessments for each organism and process, emergency response protocols, and continuous validation of containment efficacy.
Regulatory frameworks governing biosafety in industrial synthetic biology applications vary globally, creating challenges for international deployment. The Cartagena Protocol on Biosafety provides baseline guidance, but implementation differs significantly between jurisdictions. Leading industrial players have advocated for harmonized standards that balance innovation with appropriate precaution, particularly for pilot-scale operations that bridge laboratory and full industrial implementation.
Risk assessment methodologies for synthetic biology pilots have become increasingly sophisticated, incorporating quantitative models that account for organism characteristics, process parameters, and potential exposure pathways. These assessments inform the design of tailored containment strategies proportionate to identified risks, allowing for resource-efficient safety implementations without compromising protection standards.
Future developments in biosafety for synthetic biology pilots will likely focus on adaptive containment systems that respond dynamically to changing conditions, improved monitoring technologies for real-time detection of containment failures, and standardized safety frameworks specifically designed for industrial-scale applications of engineered biological systems.
Physical containment strategies include the implementation of closed-system bioreactors, negative pressure environments, HEPA filtration systems, and dedicated waste treatment processes. These engineering controls create barriers that prevent the unintended release of genetically modified organisms (GMOs) or their genetic material into surrounding environments. Advanced facilities often incorporate real-time monitoring systems that detect breaches in containment barriers and automatically trigger isolation protocols.
Biological containment approaches have evolved significantly, featuring engineered safeguards such as kill switches, auxotrophic dependencies, and orthogonal genetic systems. These mechanisms ensure that modified organisms cannot survive outside controlled conditions or transfer genetic material to wild populations. Recent innovations include genetic firewalls that prevent horizontal gene transfer and conditional viability systems that require specific laboratory-provided compounds for survival.
Procedural containment encompasses comprehensive training programs, standardized operating procedures, and regular safety audits. Personnel working in synthetic biology pilot facilities must demonstrate competency in biosafety practices before gaining access to biological materials. Documentation requirements include detailed risk assessments for each organism and process, emergency response protocols, and continuous validation of containment efficacy.
Regulatory frameworks governing biosafety in industrial synthetic biology applications vary globally, creating challenges for international deployment. The Cartagena Protocol on Biosafety provides baseline guidance, but implementation differs significantly between jurisdictions. Leading industrial players have advocated for harmonized standards that balance innovation with appropriate precaution, particularly for pilot-scale operations that bridge laboratory and full industrial implementation.
Risk assessment methodologies for synthetic biology pilots have become increasingly sophisticated, incorporating quantitative models that account for organism characteristics, process parameters, and potential exposure pathways. These assessments inform the design of tailored containment strategies proportionate to identified risks, allowing for resource-efficient safety implementations without compromising protection standards.
Future developments in biosafety for synthetic biology pilots will likely focus on adaptive containment systems that respond dynamically to changing conditions, improved monitoring technologies for real-time detection of containment failures, and standardized safety frameworks specifically designed for industrial-scale applications of engineered biological systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!