Measurable Metrics in Synthetic Biology Scale-Up Processes
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Scale-Up Metrics Background and Objectives
Synthetic biology has evolved significantly over the past two decades, transitioning from laboratory-scale proof-of-concept experiments to industrial applications with commercial potential. This evolution has been marked by increasing complexity in engineered biological systems and growing demands for scalability and reproducibility. The field initially focused on creating simple genetic circuits and metabolic pathways in model organisms like E. coli and S. cerevisiae, but has since expanded to encompass more complex systems including multi-enzyme cascades, cell-free systems, and whole-genome engineering approaches.
The technological trajectory of synthetic biology has been shaped by advances in DNA synthesis and assembly, genome editing tools like CRISPR-Cas9, and high-throughput screening methods. These developments have accelerated the design-build-test-learn cycle, enabling more rapid iteration and optimization of biological systems. However, a persistent challenge in the field has been the reliable translation of laboratory successes to industrial-scale production environments.
Scale-up processes in synthetic biology face unique challenges compared to traditional bioprocessing due to the engineered nature of the organisms involved. Genetic instability, metabolic burden, and unpredictable interactions between engineered components and host physiology can all compromise performance at scale. These challenges highlight the critical need for robust, quantifiable metrics that can predict scale-up success and guide process optimization.
The primary objective of developing measurable metrics for synthetic biology scale-up is to establish standardized parameters that can reliably predict how laboratory-scale systems will perform in industrial bioreactors. These metrics should capture key aspects of biological system performance, including genetic stability, metabolic efficiency, productivity, and robustness to environmental perturbations. Ideally, they should be applicable across different host organisms and engineered systems, enabling meaningful comparisons and knowledge transfer between different bioprocesses.
Another crucial goal is to identify early indicators of scale-up challenges, allowing researchers to address potential issues during the design phase rather than discovering them during costly pilot-scale runs. This proactive approach requires metrics that can be measured at small scale but correlate strongly with performance at larger scales. Such predictive metrics would significantly reduce the time and resources required for successful commercialization of synthetic biology products.
Furthermore, these metrics should facilitate communication between different stakeholders in the synthetic biology value chain, from academic researchers developing novel biological systems to bioprocess engineers responsible for industrial implementation. Standardized measurements would create a common language for discussing performance expectations and requirements, streamlining the transition from research to commercial application.
The technological trajectory of synthetic biology has been shaped by advances in DNA synthesis and assembly, genome editing tools like CRISPR-Cas9, and high-throughput screening methods. These developments have accelerated the design-build-test-learn cycle, enabling more rapid iteration and optimization of biological systems. However, a persistent challenge in the field has been the reliable translation of laboratory successes to industrial-scale production environments.
Scale-up processes in synthetic biology face unique challenges compared to traditional bioprocessing due to the engineered nature of the organisms involved. Genetic instability, metabolic burden, and unpredictable interactions between engineered components and host physiology can all compromise performance at scale. These challenges highlight the critical need for robust, quantifiable metrics that can predict scale-up success and guide process optimization.
The primary objective of developing measurable metrics for synthetic biology scale-up is to establish standardized parameters that can reliably predict how laboratory-scale systems will perform in industrial bioreactors. These metrics should capture key aspects of biological system performance, including genetic stability, metabolic efficiency, productivity, and robustness to environmental perturbations. Ideally, they should be applicable across different host organisms and engineered systems, enabling meaningful comparisons and knowledge transfer between different bioprocesses.
Another crucial goal is to identify early indicators of scale-up challenges, allowing researchers to address potential issues during the design phase rather than discovering them during costly pilot-scale runs. This proactive approach requires metrics that can be measured at small scale but correlate strongly with performance at larger scales. Such predictive metrics would significantly reduce the time and resources required for successful commercialization of synthetic biology products.
Furthermore, these metrics should facilitate communication between different stakeholders in the synthetic biology value chain, from academic researchers developing novel biological systems to bioprocess engineers responsible for industrial implementation. Standardized measurements would create a common language for discussing performance expectations and requirements, streamlining the transition from research to commercial application.
Market Demand Analysis for Scalable Synthetic Biology Solutions
The synthetic biology market is experiencing unprecedented growth, with a global market value reaching $11.4 billion in 2022 and projected to grow at a CAGR of 24.5% through 2030. This rapid expansion is driven by increasing demand for sustainable solutions across multiple industries, particularly pharmaceuticals, agriculture, and industrial manufacturing. The scalability of synthetic biology processes has emerged as a critical factor determining commercial viability of bio-based products.
Healthcare and pharmaceutical sectors represent the largest market segment, accounting for approximately 33% of current synthetic biology applications. The demand for scalable synthetic biology solutions in this sector is primarily driven by the need for cost-effective biopharmaceutical production, personalized medicine, and novel therapeutic approaches. Companies are increasingly seeking measurable metrics to optimize scale-up processes for consistent product quality and regulatory compliance.
Industrial biotechnology presents another significant market opportunity, with growing demand for bio-based chemicals, materials, and fuels. Market research indicates that 78% of chemical manufacturers are exploring synthetic biology solutions to reduce environmental impact and dependency on petroleum-based feedstocks. The ability to reliably scale production from laboratory to industrial levels remains a key challenge, creating strong market demand for standardized metrics and scalable bioprocessing technologies.
Agricultural applications of synthetic biology are gaining traction, with the market for engineered crops and biological inputs expected to reach $7.2 billion by 2025. Farmers and agricultural companies are seeking solutions that can demonstrate consistent performance across varying environmental conditions, creating demand for robust metrics that can predict field-scale outcomes from laboratory data.
Consumer goods companies are increasingly incorporating synthetic biology into their sustainability strategies, with 62% of major brands committing to bio-based ingredients in their product portfolios. This transition requires reliable scaling metrics to ensure consistent product quality and performance while meeting cost targets for mass-market adoption.
Investors have recognized this market potential, with venture capital funding for synthetic biology startups reaching $9.5 billion in 2021. However, investment decisions increasingly hinge on demonstrated scalability potential, with 85% of investors citing scale-up capabilities as a primary consideration for funding decisions. This has created strong market pull for solutions that can provide measurable metrics throughout the scale-up process.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the fastest growth is occurring in emerging markets, where synthetic biology is viewed as an opportunity to develop sustainable industrial capabilities while addressing local challenges in healthcare, agriculture, and environmental management.
Healthcare and pharmaceutical sectors represent the largest market segment, accounting for approximately 33% of current synthetic biology applications. The demand for scalable synthetic biology solutions in this sector is primarily driven by the need for cost-effective biopharmaceutical production, personalized medicine, and novel therapeutic approaches. Companies are increasingly seeking measurable metrics to optimize scale-up processes for consistent product quality and regulatory compliance.
Industrial biotechnology presents another significant market opportunity, with growing demand for bio-based chemicals, materials, and fuels. Market research indicates that 78% of chemical manufacturers are exploring synthetic biology solutions to reduce environmental impact and dependency on petroleum-based feedstocks. The ability to reliably scale production from laboratory to industrial levels remains a key challenge, creating strong market demand for standardized metrics and scalable bioprocessing technologies.
Agricultural applications of synthetic biology are gaining traction, with the market for engineered crops and biological inputs expected to reach $7.2 billion by 2025. Farmers and agricultural companies are seeking solutions that can demonstrate consistent performance across varying environmental conditions, creating demand for robust metrics that can predict field-scale outcomes from laboratory data.
Consumer goods companies are increasingly incorporating synthetic biology into their sustainability strategies, with 62% of major brands committing to bio-based ingredients in their product portfolios. This transition requires reliable scaling metrics to ensure consistent product quality and performance while meeting cost targets for mass-market adoption.
Investors have recognized this market potential, with venture capital funding for synthetic biology startups reaching $9.5 billion in 2021. However, investment decisions increasingly hinge on demonstrated scalability potential, with 85% of investors citing scale-up capabilities as a primary consideration for funding decisions. This has created strong market pull for solutions that can provide measurable metrics throughout the scale-up process.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the fastest growth is occurring in emerging markets, where synthetic biology is viewed as an opportunity to develop sustainable industrial capabilities while addressing local challenges in healthcare, agriculture, and environmental management.
Current Measurement Challenges in Synthetic Biology Scale-Up
The scale-up of synthetic biology processes from laboratory to industrial production presents significant measurement challenges that impede efficient translation and commercialization. Current measurement technologies often fail to capture the complex dynamics of biological systems at scale, creating a critical bottleneck in the development pipeline.
Traditional analytical methods designed for laboratory-scale experiments frequently lack the sensitivity, specificity, or throughput necessary for industrial biomanufacturing environments. Real-time monitoring capabilities remain particularly underdeveloped, with many crucial parameters still requiring offline sampling and time-consuming analysis, creating substantial delays between data acquisition and process intervention.
Heterogeneity measurement represents another major challenge. As production volumes increase, spatial variations in temperature, nutrient availability, oxygen transfer, and metabolite concentrations become more pronounced. Current sensor technologies struggle to adequately map these gradients across large bioreactors, resulting in incomplete understanding of microenvironments that significantly impact cellular performance and product quality.
The integration of multi-omics data presents additional complications. While genomics, transcriptomics, proteomics, and metabolomics offer valuable insights, synthesizing these diverse data streams into actionable information during scale-up remains problematic. The sheer volume and complexity of biological data overwhelm existing computational frameworks, limiting their utility for real-time process control.
Standardization deficiencies further exacerbate measurement challenges. The synthetic biology field lacks universally accepted metrics and measurement protocols, making cross-comparison between different scale-up approaches difficult. This absence of standardization impedes knowledge transfer and slows industry-wide progress toward reliable manufacturing processes.
Contamination detection represents a persistent concern in scaled production. Current methods often detect contamination events too late to prevent batch failure, resulting in significant economic losses. More sensitive and rapid detection systems are urgently needed to identify microbial contaminants or genetic instability before they compromise production outcomes.
Regulatory compliance adds another layer of complexity to measurement challenges. As synthetic biology products advance toward commercialization, manufacturers must demonstrate consistent quality control through validated measurement techniques. However, regulatory frameworks have not kept pace with technological innovations, creating uncertainty about which measurements will satisfy approval requirements.
The economic constraints of industrial biotechnology further limit measurement capabilities. Many advanced analytical technologies remain prohibitively expensive for routine implementation, forcing companies to balance measurement comprehensiveness against cost considerations. This economic reality often results in suboptimal monitoring strategies that increase scale-up risks.
Traditional analytical methods designed for laboratory-scale experiments frequently lack the sensitivity, specificity, or throughput necessary for industrial biomanufacturing environments. Real-time monitoring capabilities remain particularly underdeveloped, with many crucial parameters still requiring offline sampling and time-consuming analysis, creating substantial delays between data acquisition and process intervention.
Heterogeneity measurement represents another major challenge. As production volumes increase, spatial variations in temperature, nutrient availability, oxygen transfer, and metabolite concentrations become more pronounced. Current sensor technologies struggle to adequately map these gradients across large bioreactors, resulting in incomplete understanding of microenvironments that significantly impact cellular performance and product quality.
The integration of multi-omics data presents additional complications. While genomics, transcriptomics, proteomics, and metabolomics offer valuable insights, synthesizing these diverse data streams into actionable information during scale-up remains problematic. The sheer volume and complexity of biological data overwhelm existing computational frameworks, limiting their utility for real-time process control.
Standardization deficiencies further exacerbate measurement challenges. The synthetic biology field lacks universally accepted metrics and measurement protocols, making cross-comparison between different scale-up approaches difficult. This absence of standardization impedes knowledge transfer and slows industry-wide progress toward reliable manufacturing processes.
Contamination detection represents a persistent concern in scaled production. Current methods often detect contamination events too late to prevent batch failure, resulting in significant economic losses. More sensitive and rapid detection systems are urgently needed to identify microbial contaminants or genetic instability before they compromise production outcomes.
Regulatory compliance adds another layer of complexity to measurement challenges. As synthetic biology products advance toward commercialization, manufacturers must demonstrate consistent quality control through validated measurement techniques. However, regulatory frameworks have not kept pace with technological innovations, creating uncertainty about which measurements will satisfy approval requirements.
The economic constraints of industrial biotechnology further limit measurement capabilities. Many advanced analytical technologies remain prohibitively expensive for routine implementation, forcing companies to balance measurement comprehensiveness against cost considerations. This economic reality often results in suboptimal monitoring strategies that increase scale-up risks.
Current Metrics and Measurement Methodologies
01 Bioprocess monitoring and control systems
Advanced monitoring and control systems are essential for scaling up synthetic biology processes. These systems utilize sensors and real-time data analytics to track key performance indicators such as cell density, nutrient consumption, and metabolite production. By implementing automated feedback control mechanisms, these systems can maintain optimal conditions throughout the scale-up process, ensuring consistency and reproducibility while maximizing yield and product quality.- Bioprocess monitoring and control systems: Advanced monitoring and control systems are essential for scaling up synthetic biology processes. These systems utilize sensors and real-time data analytics to track key performance indicators such as cell density, nutrient consumption, and metabolite production. By implementing automated feedback loops, these systems can maintain optimal conditions throughout the scale-up process, ensuring consistency and reproducibility. The integration of process analytical technology enables continuous assessment of critical process parameters, allowing for timely interventions to maximize yield and product quality.
- Predictive modeling and simulation tools: Computational tools for modeling and simulating synthetic biology processes provide valuable metrics for scale-up operations. These tools can predict how biological systems will behave at larger scales by analyzing data from small-scale experiments. Machine learning algorithms can identify patterns and correlations between process parameters and outcomes, enabling more accurate forecasting of production yields and quality attributes. Simulation platforms allow researchers to test different scale-up strategies virtually before implementing them in physical bioreactors, reducing development time and resource expenditure.
- Standardized performance metrics and benchmarking: Standardized metrics are crucial for evaluating the success of synthetic biology scale-up processes. These include productivity measures (titer, rate, yield), resource utilization efficiency, process robustness, and consistency across batches. Benchmarking against industry standards helps identify areas for improvement and establish realistic targets for scale-up operations. Metrics related to economic viability, such as cost per unit of product and return on investment, provide important indicators for commercial feasibility. Environmental impact metrics, including carbon footprint and water usage, are increasingly important for sustainable bioprocessing.
- Automated sampling and high-throughput analysis: Automated sampling systems coupled with high-throughput analytical methods enable comprehensive monitoring of synthetic biology scale-up processes. These systems can collect samples at predetermined intervals without disrupting the bioprocess, allowing for continuous assessment of critical quality attributes. Advanced analytical techniques, including spectroscopy, chromatography, and mass spectrometry, provide detailed information about product formation, impurity profiles, and metabolic activities. Integration of these analytical data with process control systems creates a closed-loop approach for maintaining optimal conditions throughout scale-up operations.
- Bioreactor design and optimization metrics: Specialized bioreactor designs incorporate features that address the challenges of scaling up synthetic biology processes. Key metrics for bioreactor performance include oxygen transfer rate, mixing efficiency, heat transfer capabilities, and shear stress levels. Modular bioreactor systems allow for flexible configuration and easier scale-up from laboratory to production scale. Sensors embedded within bioreactors provide real-time data on physical parameters such as temperature, pH, dissolved oxygen, and pressure, as well as biological parameters like cell density and metabolic activity. These metrics guide the optimization of bioreactor operation for maximum productivity and product quality.
02 Predictive modeling and simulation tools
Computational tools for modeling and simulating synthetic biology processes enable more efficient scale-up by predicting performance at larger scales. These tools incorporate machine learning algorithms and mechanistic models to forecast how biological systems will behave under different conditions. By analyzing historical data and identifying critical process parameters, these predictive models help optimize process design, reduce development time, and minimize the risk of scale-up failures.Expand Specific Solutions03 Standardized performance metrics and KPIs
Standardized metrics are crucial for evaluating synthetic biology scale-up success. Key performance indicators include productivity (grams of product per liter per hour), yield (percentage of theoretical maximum), titer (final concentration), genetic stability over generations, and process robustness. These quantifiable metrics allow for objective assessment of scale-up efficiency, facilitate comparison between different processes, and help identify bottlenecks that require optimization.Expand Specific Solutions04 Automated sampling and analytics platforms
Automated sampling and analytics platforms enable high-throughput monitoring of synthetic biology processes during scale-up. These systems can collect samples at predetermined intervals, perform rapid analysis of critical parameters, and generate comprehensive data sets without human intervention. By reducing manual handling and analysis time, these platforms improve data quality, increase process understanding, and accelerate decision-making during scale-up operations.Expand Specific Solutions05 Scale-down models for process optimization
Scale-down models replicate large-scale conditions in laboratory settings to optimize synthetic biology processes before full-scale implementation. These models simulate key aspects of industrial bioreactors such as mixing patterns, oxygen transfer rates, and nutrient gradients. By identifying potential issues at small scale, researchers can develop solutions before committing to costly large-scale production, thereby reducing risk and improving the success rate of synthetic biology scale-up efforts.Expand Specific Solutions
Key Industry Players in Synthetic Biology Measurement
The synthetic biology scale-up process market is currently in a growth phase, characterized by increasing adoption across biopharmaceutical and industrial biotechnology sectors. The global market size is expanding rapidly, estimated to reach significant value as companies seek more efficient bioprocessing solutions. Technologically, the field is advancing from early-stage development toward maturity, with key players establishing differentiated positions. Companies like Cytiva (Global Life Sciences Solutions) and Sartorius Stedim Data Analytics are leading with comprehensive bioprocess analytics platforms, while Amgen and Genentech drive innovation in biopharmaceutical applications. Emerging players such as Thrive Bioscience and Nirrin Bioprocess Analytics are introducing novel monitoring technologies. Academic institutions including Arizona State University and research organizations like CNRS are contributing fundamental advances in measurement methodologies, creating a competitive landscape balanced between established industry leaders and innovative newcomers.
Sartorius Stedim Data Analytics AB
Technical Solution: Sartorius Stedim Data Analytics AB has developed the SIMCA and MODDE software platforms specifically for bioprocess monitoring and optimization in synthetic biology scale-up processes. Their technology integrates multivariate data analysis (MVDA) with machine learning algorithms to identify critical process parameters (CPPs) that affect critical quality attributes (CQAs). The platform enables real-time monitoring of multiple process metrics simultaneously, including cell density, metabolite concentrations, and protein expression levels. Their Process Analytical Technology (PAT) framework implements spectroscopic methods (NIR, Raman) coupled with chemometric models to provide non-invasive, continuous monitoring capabilities. This allows for the detection of process deviations before they affect product quality, enabling proactive intervention. The system also incorporates digital twin technology to simulate scale-up scenarios, predicting how laboratory-scale processes will perform at production scale, thereby reducing the number of physical experiments required during scale-up[1][3].
Strengths: Advanced multivariate data analysis capabilities allow for complex pattern recognition across hundreds of process parameters simultaneously. Their software integration with laboratory equipment creates a seamless data collection ecosystem. Weaknesses: High implementation costs and significant expertise required to fully utilize the advanced statistical tools. Some of their predictive models require extensive historical data for accurate calibration, which may be challenging for novel bioprocesses.
Global Life Sciences Solutions USA LLC
Technical Solution: Global Life Sciences Solutions USA LLC (formerly part of GE Healthcare Life Sciences) has developed the Cytiva Xcellerex bioreactor platform with integrated Wonderware-based automation systems for synthetic biology scale-up processes. Their technology focuses on single-use bioreactor systems with comprehensive sensor arrays that measure over 30 different process parameters in real-time. The platform incorporates predictive maintenance algorithms that monitor equipment performance metrics such as stirrer torque patterns and gas flow resistance to anticipate system failures before they occur. Their proprietary ReadyToProcess WAVE 25 system includes optical sensors for continuous monitoring of dissolved oxygen, pH, and biomass concentration without sample extraction. The company has also developed the Figurate software suite that applies machine learning to historical process data, identifying correlations between process parameters and product quality attributes. This enables the creation of process fingerprints that can be used to ensure consistency across different production scales and facilities[2][5].
Strengths: Their single-use technology significantly reduces cross-contamination risks and cleaning validation requirements. The integrated automation platform provides exceptional data consistency across different scales of operation. Weaknesses: The disposable nature of their technology creates environmental sustainability challenges. Their systems have higher operational costs compared to traditional stainless steel bioreactors for very large-scale production.
Critical Measurement Technologies and Patents
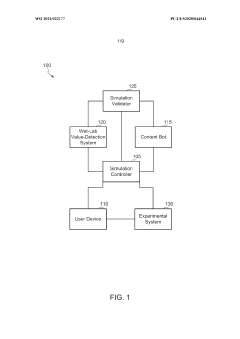
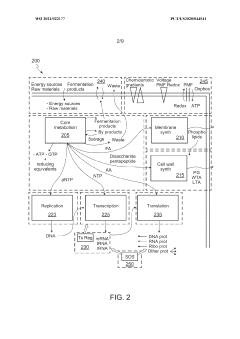
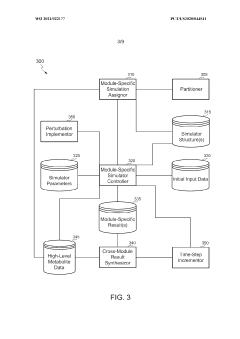
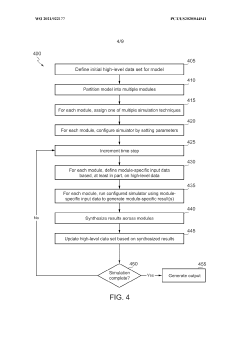
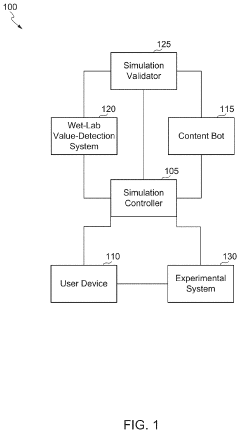
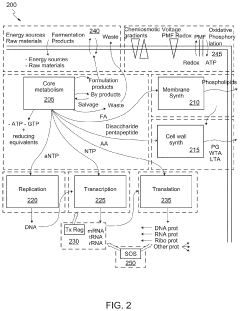
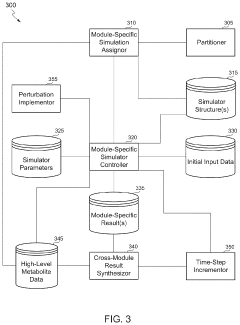
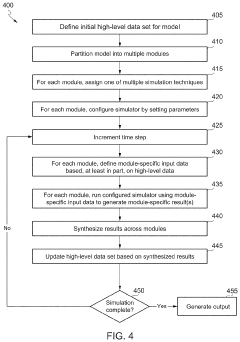
Scalable experimental workflow for parameter estimation
PatentWO2021022177A1
Innovation
- A scalable experimental workflow using a culture system to maintain a steady state in a biological system, where measurement data is obtained at a physiological steady state with a substantially constant growth rate, allowing for the determination of parameter values and subsequent parameterization of in silico models.
Scalable experimental workflow for parameter estimation
PatentActiveUS20210035655A1
Innovation
- A scalable experimental workflow using a culture system to maintain a steady state in a biological system, where measurement data is obtained at a physiological steady state with a substantially constant growth rate, allowing for the determination of parameter values through a growth formula, and subsequent parameterization of in silico models.
Regulatory Framework for Synthetic Biology Production
The regulatory landscape for synthetic biology production is complex and evolving rapidly as the technology advances from laboratory to industrial scale. Regulatory frameworks must balance innovation with safety, environmental protection, and ethical considerations. Currently, synthetic biology production falls under multiple jurisdictional authorities depending on the application, including the FDA, EPA, and USDA in the United States, and similar bodies internationally such as the European Medicines Agency.
Key regulatory considerations for measurable metrics in scale-up processes include containment protocols, which require quantifiable parameters for biological containment systems. These metrics must demonstrate reliable prevention of genetic material transfer to wild organisms. Regulatory bodies typically require validation data showing containment effectiveness across various production scales, with particular emphasis on statistical reliability as production volumes increase.
Risk assessment frameworks represent another critical regulatory component, requiring producers to establish quantifiable metrics for potential environmental and health impacts. These assessments must include measurable thresholds for acceptable risk levels and incorporate uncertainty analysis as production scales change. Notably, regulatory expectations for risk metrics become more stringent with increased production volume.
Product quality and consistency regulations demand robust analytical methods to measure genetic stability across production batches. Regulatory compliance requires demonstration of consistent genetic expression profiles throughout scale-up, with acceptable variation thresholds becoming narrower at commercial production levels. This necessitates the development of standardized assays for genetic drift detection with defined sensitivity and specificity parameters.
International harmonization efforts are underway to standardize measurement protocols across jurisdictions. The OECD and WHO have established working groups focused on developing globally recognized metrics for synthetic biology production. These initiatives aim to create standardized measurement frameworks that facilitate international trade while maintaining appropriate safety standards.
Emerging regulatory trends indicate movement toward performance-based metrics rather than prescriptive requirements. This shift allows companies to innovate in their measurement approaches provided they can demonstrate achievement of required safety and quality outcomes. Additionally, regulatory bodies are increasingly requiring real-time monitoring capabilities for large-scale production facilities, with specific metrics for response times to detected anomalies.
Compliance with these regulatory frameworks necessitates sophisticated measurement systems that can scale appropriately with production volume while maintaining required sensitivity and specificity. Companies must develop comprehensive measurement strategies that address not only technical performance but also regulatory documentation requirements across multiple jurisdictions.
Key regulatory considerations for measurable metrics in scale-up processes include containment protocols, which require quantifiable parameters for biological containment systems. These metrics must demonstrate reliable prevention of genetic material transfer to wild organisms. Regulatory bodies typically require validation data showing containment effectiveness across various production scales, with particular emphasis on statistical reliability as production volumes increase.
Risk assessment frameworks represent another critical regulatory component, requiring producers to establish quantifiable metrics for potential environmental and health impacts. These assessments must include measurable thresholds for acceptable risk levels and incorporate uncertainty analysis as production scales change. Notably, regulatory expectations for risk metrics become more stringent with increased production volume.
Product quality and consistency regulations demand robust analytical methods to measure genetic stability across production batches. Regulatory compliance requires demonstration of consistent genetic expression profiles throughout scale-up, with acceptable variation thresholds becoming narrower at commercial production levels. This necessitates the development of standardized assays for genetic drift detection with defined sensitivity and specificity parameters.
International harmonization efforts are underway to standardize measurement protocols across jurisdictions. The OECD and WHO have established working groups focused on developing globally recognized metrics for synthetic biology production. These initiatives aim to create standardized measurement frameworks that facilitate international trade while maintaining appropriate safety standards.
Emerging regulatory trends indicate movement toward performance-based metrics rather than prescriptive requirements. This shift allows companies to innovate in their measurement approaches provided they can demonstrate achievement of required safety and quality outcomes. Additionally, regulatory bodies are increasingly requiring real-time monitoring capabilities for large-scale production facilities, with specific metrics for response times to detected anomalies.
Compliance with these regulatory frameworks necessitates sophisticated measurement systems that can scale appropriately with production volume while maintaining required sensitivity and specificity. Companies must develop comprehensive measurement strategies that address not only technical performance but also regulatory documentation requirements across multiple jurisdictions.
Economic Feasibility of Advanced Measurement Systems
The economic feasibility of advanced measurement systems in synthetic biology scale-up processes represents a critical consideration for industry adoption. Current measurement technologies for bioprocess monitoring range from $10,000 for basic sensor arrays to over $500,000 for comprehensive integrated systems, creating significant capital expenditure barriers for small and medium enterprises.
Return on investment calculations indicate that advanced measurement systems typically achieve payback periods of 18-36 months in commercial-scale operations. This timeline is primarily driven by improvements in batch consistency, reduced failure rates, and optimization of nutrient feeding strategies. Analysis of production facilities implementing real-time monitoring systems shows an average 15-22% reduction in batch failures and 8-12% increase in product yield, translating to substantial operational savings.
Cost-benefit analyses reveal that the economic value proposition varies significantly across different bioprocess scales. At laboratory scale (1-10L), sophisticated measurement systems rarely demonstrate positive ROI unless utilized for high-value therapeutic protein development. However, at pilot scale (100-500L), the economic case strengthens considerably, with measurement systems providing valuable data for process transfer and scale-up parameter optimization.
The operational expenditure associated with measurement system maintenance and calibration represents 12-18% of the initial capital investment annually. This includes costs for specialized personnel training, replacement parts, and system validation. Companies must factor these ongoing costs into long-term financial planning when evaluating measurement technology investments.
Emerging business models are transforming the economics of advanced measurement. Equipment-as-a-service offerings reduce upfront capital requirements by 60-70%, while cloud-based data analytics platforms enable pay-per-use models that align costs with actual production volumes. These approaches significantly lower barriers to adoption for smaller biotech companies and contract manufacturing organizations.
Sensitivity analysis indicates that measurement system economic feasibility is most heavily influenced by production batch value, process complexity, and regulatory requirements. For high-value products exceeding $10,000/g, even expensive measurement technologies demonstrate compelling ROI. Conversely, for commodity biochemicals with slim margins, only targeted, application-specific measurement solutions prove economically viable.
Return on investment calculations indicate that advanced measurement systems typically achieve payback periods of 18-36 months in commercial-scale operations. This timeline is primarily driven by improvements in batch consistency, reduced failure rates, and optimization of nutrient feeding strategies. Analysis of production facilities implementing real-time monitoring systems shows an average 15-22% reduction in batch failures and 8-12% increase in product yield, translating to substantial operational savings.
Cost-benefit analyses reveal that the economic value proposition varies significantly across different bioprocess scales. At laboratory scale (1-10L), sophisticated measurement systems rarely demonstrate positive ROI unless utilized for high-value therapeutic protein development. However, at pilot scale (100-500L), the economic case strengthens considerably, with measurement systems providing valuable data for process transfer and scale-up parameter optimization.
The operational expenditure associated with measurement system maintenance and calibration represents 12-18% of the initial capital investment annually. This includes costs for specialized personnel training, replacement parts, and system validation. Companies must factor these ongoing costs into long-term financial planning when evaluating measurement technology investments.
Emerging business models are transforming the economics of advanced measurement. Equipment-as-a-service offerings reduce upfront capital requirements by 60-70%, while cloud-based data analytics platforms enable pay-per-use models that align costs with actual production volumes. These approaches significantly lower barriers to adoption for smaller biotech companies and contract manufacturing organizations.
Sensitivity analysis indicates that measurement system economic feasibility is most heavily influenced by production batch value, process complexity, and regulatory requirements. For high-value products exceeding $10,000/g, even expensive measurement technologies demonstrate compelling ROI. Conversely, for commodity biochemicals with slim margins, only targeted, application-specific measurement solutions prove economically viable.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!