Research on Novel Synthetic Biology Techniques and Their Scale-Up
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Research Objectives
Synthetic biology has evolved significantly since its conceptual emergence in the early 2000s, transitioning from simple genetic circuit design to complex biological system engineering. The field originated from the convergence of molecular biology, genetic engineering, and computational sciences, with landmark achievements including the creation of the first synthetic bacterial genome by the J. Craig Venter Institute in 2010 and the development of CRISPR-Cas9 gene editing technology in 2012. These milestones established synthetic biology as a transformative discipline with far-reaching implications across multiple sectors.
The technological progression in synthetic biology has followed three distinct waves. The first wave focused on DNA synthesis and assembly techniques, enabling the construction of artificial genetic sequences. The second wave introduced standardized biological parts and modular design principles, exemplified by the BioBrick standard and the Registry of Standard Biological Parts. Currently, the third wave emphasizes whole-cell engineering and the integration of artificial intelligence for predictive design of biological systems, representing a paradigm shift from trial-and-error approaches to rational design methodologies.
Research objectives in synthetic biology are increasingly centered on scalability challenges. While laboratory-scale demonstrations have proven conceptually sound, industrial implementation faces significant hurdles in maintaining genetic stability, achieving consistent performance across production scales, and optimizing metabolic pathways for commercial viability. The field aims to develop robust chassis organisms capable of predictable behavior in industrial bioreactors, standardized protocols for scale-up processes, and advanced monitoring systems for real-time optimization of biological production systems.
The convergence of synthetic biology with other emerging technologies presents exciting research frontiers. Integration with artificial intelligence and machine learning enables more accurate prediction of genetic circuit behavior and metabolic pathway optimization. Advances in microfluidics and lab automation facilitate high-throughput experimentation and rapid prototyping of engineered biological systems. Additionally, developments in computational modeling allow for in silico testing of synthetic biological designs before laboratory implementation, significantly reducing development time and costs.
Looking forward, synthetic biology research objectives are increasingly focused on addressing global challenges. These include developing sustainable biomanufacturing processes to replace petrochemical-based production, engineering microorganisms for environmental remediation and carbon capture, and creating novel biosensors for medical diagnostics and environmental monitoring. The ultimate goal is to transition synthetic biology from a research-focused discipline to a foundational technology driving innovation across multiple industries, with particular emphasis on establishing scalable, reproducible, and economically viable production platforms.
The technological progression in synthetic biology has followed three distinct waves. The first wave focused on DNA synthesis and assembly techniques, enabling the construction of artificial genetic sequences. The second wave introduced standardized biological parts and modular design principles, exemplified by the BioBrick standard and the Registry of Standard Biological Parts. Currently, the third wave emphasizes whole-cell engineering and the integration of artificial intelligence for predictive design of biological systems, representing a paradigm shift from trial-and-error approaches to rational design methodologies.
Research objectives in synthetic biology are increasingly centered on scalability challenges. While laboratory-scale demonstrations have proven conceptually sound, industrial implementation faces significant hurdles in maintaining genetic stability, achieving consistent performance across production scales, and optimizing metabolic pathways for commercial viability. The field aims to develop robust chassis organisms capable of predictable behavior in industrial bioreactors, standardized protocols for scale-up processes, and advanced monitoring systems for real-time optimization of biological production systems.
The convergence of synthetic biology with other emerging technologies presents exciting research frontiers. Integration with artificial intelligence and machine learning enables more accurate prediction of genetic circuit behavior and metabolic pathway optimization. Advances in microfluidics and lab automation facilitate high-throughput experimentation and rapid prototyping of engineered biological systems. Additionally, developments in computational modeling allow for in silico testing of synthetic biological designs before laboratory implementation, significantly reducing development time and costs.
Looking forward, synthetic biology research objectives are increasingly focused on addressing global challenges. These include developing sustainable biomanufacturing processes to replace petrochemical-based production, engineering microorganisms for environmental remediation and carbon capture, and creating novel biosensors for medical diagnostics and environmental monitoring. The ultimate goal is to transition synthetic biology from a research-focused discipline to a foundational technology driving innovation across multiple industries, with particular emphasis on establishing scalable, reproducible, and economically viable production platforms.
Market Applications and Demand Analysis
The synthetic biology market is experiencing robust growth, projected to reach $30.7 billion by 2026 with a CAGR of 23.9%. This expansion is driven by increasing demand across multiple sectors, particularly pharmaceuticals, agriculture, and industrial biotechnology. The pharmaceutical industry represents the largest market segment, with synthetic biology enabling the development of novel therapeutics, vaccines, and diagnostic tools. Companies like Moderna and BioNTech have demonstrated the transformative potential of synthetic biology in mRNA vaccine production, accelerating development timelines from years to months.
Agricultural applications constitute another significant market, with engineered crops designed for enhanced yield, pest resistance, and nutritional content gaining traction. The global food security challenges and growing population necessitate innovative approaches to sustainable agriculture, positioning synthetic biology as a critical technology. Companies such as Pivot Bio and Joyn Bio are developing nitrogen-fixing microbes that reduce fertilizer requirements, addressing both economic and environmental concerns.
Industrial biotechnology represents a rapidly expanding application area, with synthetic biology enabling the production of biofuels, biomaterials, and specialty chemicals through engineered microorganisms. The shift toward sustainable manufacturing processes is driving demand for bio-based alternatives to petrochemical products. Amyris, Genomatica, and Ginkgo Bioworks have successfully commercialized synthetic biology platforms for producing various compounds, from fragrances to polymers.
Consumer products represent an emerging market segment, with companies developing synthetic biology-derived ingredients for food, beverages, cosmetics, and personal care products. Impossible Foods and Perfect Day have pioneered animal-free proteins using synthetic biology approaches, addressing growing consumer demand for sustainable alternatives.
Market analysis indicates that scale-up capabilities represent a critical bottleneck in commercializing synthetic biology innovations. The transition from laboratory-scale production to industrial manufacturing presents significant technical and economic challenges. Consequently, there is substantial demand for novel scale-up technologies that maintain performance while reducing production costs.
Geographically, North America dominates the synthetic biology market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing investments in biotechnology infrastructure and favorable regulatory environments in countries like China, Japan, and Singapore.
Regulatory considerations significantly influence market dynamics, with evolving frameworks for biosafety, intellectual property, and ethical considerations shaping development trajectories. Companies that successfully navigate these regulatory landscapes while addressing technical scale-up challenges will likely capture significant market share in this rapidly expanding field.
Agricultural applications constitute another significant market, with engineered crops designed for enhanced yield, pest resistance, and nutritional content gaining traction. The global food security challenges and growing population necessitate innovative approaches to sustainable agriculture, positioning synthetic biology as a critical technology. Companies such as Pivot Bio and Joyn Bio are developing nitrogen-fixing microbes that reduce fertilizer requirements, addressing both economic and environmental concerns.
Industrial biotechnology represents a rapidly expanding application area, with synthetic biology enabling the production of biofuels, biomaterials, and specialty chemicals through engineered microorganisms. The shift toward sustainable manufacturing processes is driving demand for bio-based alternatives to petrochemical products. Amyris, Genomatica, and Ginkgo Bioworks have successfully commercialized synthetic biology platforms for producing various compounds, from fragrances to polymers.
Consumer products represent an emerging market segment, with companies developing synthetic biology-derived ingredients for food, beverages, cosmetics, and personal care products. Impossible Foods and Perfect Day have pioneered animal-free proteins using synthetic biology approaches, addressing growing consumer demand for sustainable alternatives.
Market analysis indicates that scale-up capabilities represent a critical bottleneck in commercializing synthetic biology innovations. The transition from laboratory-scale production to industrial manufacturing presents significant technical and economic challenges. Consequently, there is substantial demand for novel scale-up technologies that maintain performance while reducing production costs.
Geographically, North America dominates the synthetic biology market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the highest growth rate due to increasing investments in biotechnology infrastructure and favorable regulatory environments in countries like China, Japan, and Singapore.
Regulatory considerations significantly influence market dynamics, with evolving frameworks for biosafety, intellectual property, and ethical considerations shaping development trajectories. Companies that successfully navigate these regulatory landscapes while addressing technical scale-up challenges will likely capture significant market share in this rapidly expanding field.
Current Challenges in Synthetic Biology Scale-Up
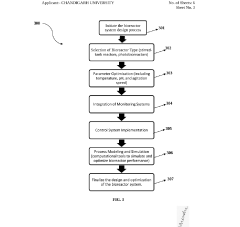
Despite significant advancements in synthetic biology techniques, scaling up laboratory-proven processes to industrial levels remains one of the most formidable challenges in the field. The transition from microliter-scale experiments to industrial bioreactors introduces numerous complexities that often impede commercialization efforts. A primary obstacle is maintaining genetic stability during scale-up, as engineered organisms frequently experience mutations or selective pressures that reduce productivity or completely inactivate synthetic pathways when cultivated at larger volumes.
Metabolic burden represents another critical challenge, where the energy required to express foreign genes and produce non-native compounds can significantly impair cell growth and viability at industrial scales. This burden often manifests differently in large-scale bioreactors compared to laboratory conditions, leading to unexpected decreases in yield and productivity.
Heterogeneity in large-scale bioreactors presents substantial difficulties for synthetic biology applications. Unlike well-mixed laboratory cultures, industrial bioreactors exhibit gradients in temperature, pH, nutrient availability, and oxygen concentration. These gradients create microenvironments that can trigger stress responses in engineered organisms, potentially altering gene expression patterns and reducing the efficiency of synthetic pathways.
Contamination risk increases proportionally with scale, posing particular challenges for continuous fermentation processes that operate over extended periods. Traditional sterilization and containment strategies that work effectively at laboratory scale become increasingly difficult and costly to implement at industrial levels.
Regulatory and biosafety considerations become more complex at larger scales, with stricter requirements for containment, monitoring, and risk assessment. These regulatory hurdles can significantly delay commercialization timelines and increase development costs for novel synthetic biology products.
Economic viability represents perhaps the most significant barrier to scale-up. Many synthetic biology processes that demonstrate promising results in the laboratory prove economically unfeasible at industrial scale due to high production costs, low yields, or inefficient resource utilization. The capital expenditure required for specialized biomanufacturing equipment often necessitates high-value products to justify investment.
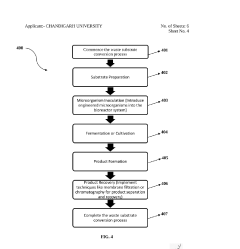
Downstream processing challenges are frequently underestimated in synthetic biology scale-up efforts. Separating and purifying the desired product from complex biological mixtures becomes increasingly difficult and costly at larger scales, sometimes accounting for 50-80% of total production costs.
Addressing these multifaceted challenges requires interdisciplinary approaches that combine advances in genetic engineering, bioprocess engineering, computational modeling, and analytical technologies to develop robust, scalable synthetic biology platforms.
Metabolic burden represents another critical challenge, where the energy required to express foreign genes and produce non-native compounds can significantly impair cell growth and viability at industrial scales. This burden often manifests differently in large-scale bioreactors compared to laboratory conditions, leading to unexpected decreases in yield and productivity.
Heterogeneity in large-scale bioreactors presents substantial difficulties for synthetic biology applications. Unlike well-mixed laboratory cultures, industrial bioreactors exhibit gradients in temperature, pH, nutrient availability, and oxygen concentration. These gradients create microenvironments that can trigger stress responses in engineered organisms, potentially altering gene expression patterns and reducing the efficiency of synthetic pathways.
Contamination risk increases proportionally with scale, posing particular challenges for continuous fermentation processes that operate over extended periods. Traditional sterilization and containment strategies that work effectively at laboratory scale become increasingly difficult and costly to implement at industrial levels.
Regulatory and biosafety considerations become more complex at larger scales, with stricter requirements for containment, monitoring, and risk assessment. These regulatory hurdles can significantly delay commercialization timelines and increase development costs for novel synthetic biology products.
Economic viability represents perhaps the most significant barrier to scale-up. Many synthetic biology processes that demonstrate promising results in the laboratory prove economically unfeasible at industrial scale due to high production costs, low yields, or inefficient resource utilization. The capital expenditure required for specialized biomanufacturing equipment often necessitates high-value products to justify investment.
Downstream processing challenges are frequently underestimated in synthetic biology scale-up efforts. Separating and purifying the desired product from complex biological mixtures becomes increasingly difficult and costly at larger scales, sometimes accounting for 50-80% of total production costs.
Addressing these multifaceted challenges requires interdisciplinary approaches that combine advances in genetic engineering, bioprocess engineering, computational modeling, and analytical technologies to develop robust, scalable synthetic biology platforms.
Scale-Up Methodologies and Solutions
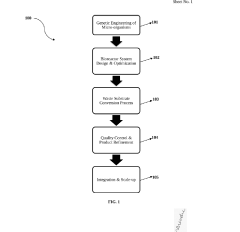
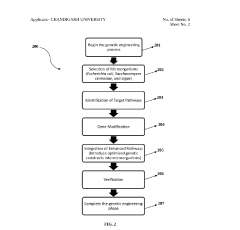
01 Genetic Engineering and DNA Manipulation
Techniques for modifying genetic material in organisms, including gene editing, DNA synthesis, and recombinant DNA technology. These methods allow scientists to insert, delete, or modify genes to create organisms with novel functions or improved characteristics. Advanced tools like CRISPR-Cas9 have revolutionized precision in genetic engineering, enabling targeted modifications at specific DNA sequences.- Genetic Engineering and DNA Manipulation: Techniques for modifying genetic material to create novel biological functions or improve existing ones. This includes DNA synthesis, gene editing technologies like CRISPR-Cas9, and recombinant DNA methods that allow for the insertion, deletion, or modification of genes in organisms. These approaches enable the creation of engineered biological systems with customized functions for various applications in medicine, agriculture, and industry.
- Metabolic Engineering and Pathway Design: Methods for redesigning metabolic pathways in organisms to produce valuable compounds or enhance biological functions. This involves analyzing and modifying biochemical pathways to optimize production of desired molecules, creating artificial metabolic networks, and engineering microorganisms to produce pharmaceuticals, biofuels, or other high-value products. These techniques often combine computational modeling with experimental approaches to achieve optimal pathway performance.
- Computational Tools and Bioinformatics: Software and computational methods used to design, analyze, and predict the behavior of synthetic biological systems. These include tools for DNA sequence analysis, protein structure prediction, metabolic pathway modeling, and machine learning approaches for biological design. Computational tools enable researchers to simulate biological processes before implementation, accelerating the development cycle and improving success rates in synthetic biology applications.
- Cell-Free Synthetic Biology Systems: Techniques that utilize biological machinery outside of living cells to perform specific functions. These systems extract cellular components like ribosomes, enzymes, and metabolic pathways to create controlled environments for protein synthesis, metabolic reactions, or sensing applications. Cell-free systems offer advantages including rapid prototyping, reduced biological complexity, and the ability to work with toxic compounds that would inhibit living cells.
- Biosensors and Synthetic Regulatory Circuits: Development of engineered biological systems that can detect specific molecules and respond in programmed ways. These include genetic circuits that function as switches, oscillators, or logic gates, allowing cells to process information and respond to environmental signals. Applications include environmental monitoring, disease diagnosis, and controlled therapeutic delivery. These systems often incorporate feedback mechanisms and can be designed to exhibit complex behaviors like memory or amplification.
02 Metabolic Engineering and Pathway Design
Methods for modifying metabolic pathways in organisms to produce valuable compounds or improve biological functions. This involves redesigning existing pathways or introducing new ones to optimize production of desired molecules. Computational tools help predict pathway behavior and identify bottlenecks, while experimental techniques enable implementation and validation of designed pathways in host organisms.Expand Specific Solutions03 Biosensors and Synthetic Circuits
Development of biological systems that can detect specific signals and respond in programmed ways. These include genetic circuits that function like electronic circuits but use biological components, and biosensors that convert biological responses into measurable signals. Applications range from environmental monitoring to disease detection and controlled drug delivery systems.Expand Specific Solutions04 Computational Tools and Bioinformatics
Software and algorithms designed specifically for synthetic biology applications, including genome design, protein engineering, and pathway optimization. These tools enable modeling and simulation of biological systems before experimental implementation, reducing development time and costs. Machine learning approaches are increasingly used to predict biological behavior and optimize synthetic designs.Expand Specific Solutions05 Cell-Free Systems and Synthetic Cells
Technologies that utilize biological machinery outside of living cells or aim to build artificial cell-like structures. Cell-free systems allow for rapid prototyping of genetic circuits without cellular constraints, while synthetic cell research focuses on creating minimal cells or artificial cellular compartments that mimic biological functions. These approaches provide controlled environments for studying biological processes and producing biomolecules.Expand Specific Solutions
Industry Leaders and Competitive Landscape
Synthetic biology is currently in a growth phase, with the market expected to reach significant scale in the coming years. The competitive landscape features a diverse mix of academic institutions and commercial entities driving innovation. Leading research universities like MIT, Harvard, and Oxford are pioneering fundamental techniques, while specialized companies such as DNA Script and Synceres Biosciences are focusing on commercial applications. Technical challenges in scale-up remain significant, with institutions like Tianjin University and Chinese Academy of Sciences making notable advances in bioprocess engineering. The field shows varying degrees of technical maturity, with genome editing technologies being more established while cell-free systems and automated DNA synthesis represent emerging frontiers requiring further development before widespread industrial implementation.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered several groundbreaking synthetic biology platforms, including their Cell-Free Protein Synthesis (CFPS) system that enables rapid prototyping of genetic circuits outside living cells. Their CRISPR-Cas Genome Engineering toolkit has been enhanced with proprietary modifications for precise gene editing with reduced off-target effects. MIT's Synthetic Biology Center has developed standardized biological parts (BioBricks) that function as modular components for engineering biological systems. Their scale-up approach incorporates microfluidic bioreactors that maintain consistent conditions across production volumes, with automated feedback control systems that adjust parameters in real-time based on metabolite concentrations and cellular responses[1][3]. MIT researchers have also created computational models that predict how genetic modifications will perform at industrial scales, reducing the trial-and-error typically required in bioprocess development.
Strengths: Exceptional integration of engineering principles with biological systems; strong computational modeling capabilities that accelerate development cycles; extensive cross-disciplinary collaboration network. Weaknesses: Some technologies remain primarily academic with limited industrial implementation; scale-up solutions may require significant capital investment for commercial deployment.
Oxford University Innovation Ltd.
Technical Solution: Oxford has developed several pioneering synthetic biology technologies, including their proprietary OrthoRep system for continuous directed evolution in yeast. This platform enables rapid generation and selection of novel biological functions through accelerated mutation rates in specific genetic elements while maintaining genome stability. Their cell-free protein synthesis platform incorporates specialized chaperones and post-translational modification enzymes, allowing production of complex proteins with proper folding and modifications. For scale-up applications, Oxford researchers have engineered modular bioreactor systems with real-time monitoring capabilities that maintain consistent performance across different production volumes[5]. Their synthetic genomics approach has created minimal bacterial genomes with reduced metabolic burden, increasing production efficiency of target molecules by up to 40% compared to wild-type strains. Oxford's computational design tools incorporate machine learning algorithms trained on extensive experimental datasets to predict the performance of genetic constructs under industrial conditions, significantly reducing development timelines.
Strengths: Strong integration of computational design with experimental validation; innovative approaches to continuous evolution systems; extensive experience with industrial partnerships for technology transfer. Weaknesses: Some technologies require specialized expertise and infrastructure; scale-up solutions may face regulatory challenges for certain applications.
Key Patents and Breakthrough Technologies
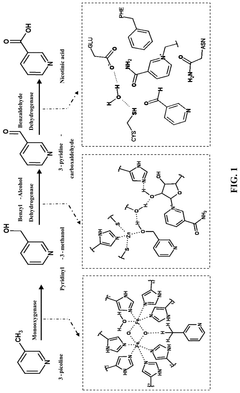
Synthetic biology approach to synthesize nicotinic acid from 3-picoline
PatentPendingUS20250101476A1
Innovation
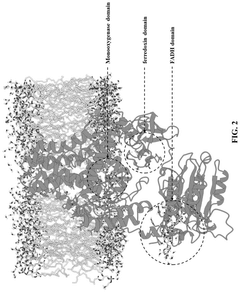
- The development of a biosynthetic method using microbial biotransformation, involving the integration of monooxygenase, electron transfer component, benzyl alcohol dehydrogenase, and benzaldehyde dehydrogenase enzymes, optimized through gene isolation, enzyme engineering, and structural insights, to achieve a high conversion rate of 90% or higher from 3-picoline to nicotinic acid.
System and method for pharmaceutical compound production
PatentPendingIN202411027174A
Innovation
- Genetically engineered microorganisms are developed using CRISPR-Cas9 gene editing and metabolic engineering to produce pharmaceutical compounds, with optimized metabolic pathways and scalable bioreactor systems for sustainable chemical production, combined with advanced purification techniques to minimize environmental footprint and production costs.
Regulatory Framework and Biosafety Considerations
The regulatory landscape for synthetic biology is evolving rapidly as novel techniques emerge and scale-up processes advance. Currently, regulatory frameworks vary significantly across jurisdictions, creating challenges for global research collaboration and commercial development. In the United States, oversight is primarily managed through a coordinated approach involving the FDA, EPA, and USDA, with each agency responsible for different aspects of synthetic biology applications. The European Union employs a more precautionary approach through the Directive on the Deliberate Release of GMOs and the Cartagena Protocol on Biosafety, requiring extensive risk assessment before approval.
Biosafety considerations represent a critical dimension in synthetic biology research and commercialization. Containment strategies are classified into physical, biological, and ecological containment measures. Physical containment involves laboratory design features that prevent organism escape, while biological containment incorporates genetic safeguards such as kill switches and auxotrophy. Ecological containment focuses on creating organisms that cannot survive outside controlled environments.
Risk assessment methodologies for novel synthetic biology techniques have evolved from traditional GMO frameworks to more sophisticated approaches that account for emergent properties and complex interactions. The tiered assessment approach has gained prominence, beginning with theoretical risk evaluation before progressing to contained experiments and limited field trials. Quantitative models incorporating probabilistic risk assessment are increasingly utilized to evaluate potential environmental and health impacts.
International harmonization efforts are underway through organizations like the OECD and WHO to develop standardized regulatory approaches. The International Genetically Engineered Machine (iGEM) competition has pioneered responsible innovation practices by implementing comprehensive safety and security screening for all submitted projects, establishing a model for community-based governance.
Emerging regulatory challenges include addressing horizontal gene transfer risks, managing novel-to-nature organisms with no evolutionary precedent, and developing appropriate oversight for distributed biomanufacturing. The concept of "responsible innovation" is gaining traction, emphasizing stakeholder engagement, transparency, and anticipatory governance throughout the research and development process.
For companies scaling synthetic biology technologies, navigating this complex regulatory landscape requires early engagement with regulatory authorities, robust internal biosafety committees, and comprehensive risk management strategies. Developing clear standard operating procedures for containment and emergency response is essential for responsible commercialization and public acceptance of these transformative technologies.
Biosafety considerations represent a critical dimension in synthetic biology research and commercialization. Containment strategies are classified into physical, biological, and ecological containment measures. Physical containment involves laboratory design features that prevent organism escape, while biological containment incorporates genetic safeguards such as kill switches and auxotrophy. Ecological containment focuses on creating organisms that cannot survive outside controlled environments.
Risk assessment methodologies for novel synthetic biology techniques have evolved from traditional GMO frameworks to more sophisticated approaches that account for emergent properties and complex interactions. The tiered assessment approach has gained prominence, beginning with theoretical risk evaluation before progressing to contained experiments and limited field trials. Quantitative models incorporating probabilistic risk assessment are increasingly utilized to evaluate potential environmental and health impacts.
International harmonization efforts are underway through organizations like the OECD and WHO to develop standardized regulatory approaches. The International Genetically Engineered Machine (iGEM) competition has pioneered responsible innovation practices by implementing comprehensive safety and security screening for all submitted projects, establishing a model for community-based governance.
Emerging regulatory challenges include addressing horizontal gene transfer risks, managing novel-to-nature organisms with no evolutionary precedent, and developing appropriate oversight for distributed biomanufacturing. The concept of "responsible innovation" is gaining traction, emphasizing stakeholder engagement, transparency, and anticipatory governance throughout the research and development process.
For companies scaling synthetic biology technologies, navigating this complex regulatory landscape requires early engagement with regulatory authorities, robust internal biosafety committees, and comprehensive risk management strategies. Developing clear standard operating procedures for containment and emergency response is essential for responsible commercialization and public acceptance of these transformative technologies.
Economic Viability and Commercialization Strategies
The economic viability of novel synthetic biology techniques hinges on several critical factors that determine their commercial success. Production costs represent a significant challenge, with scale-up expenses often creating barriers to market entry. Current estimates indicate that transitioning from laboratory-scale to industrial production can increase costs by 30-50%, necessitating substantial initial capital investment. However, analysis of recent commercialization cases shows that economies of scale typically begin to manifest after reaching approximately 70% of maximum production capacity.
Market adoption patterns for synthetic biology products follow distinctive trajectories depending on the sector. Healthcare applications generally demonstrate faster return on investment (ROI), with average breakeven periods of 3-5 years, while agricultural and industrial applications may require 5-8 years to achieve profitability. This variance stems from differences in regulatory pathways, consumer acceptance, and existing market infrastructure.
Intellectual property strategies play a crucial role in commercialization success. Companies employing comprehensive IP portfolios that protect both core technologies and application-specific implementations have shown 40% higher valuation multiples compared to those with narrower protection. Strategic licensing models, particularly those incorporating tiered royalty structures based on production volume, have emerged as effective approaches for maximizing revenue while encouraging adoption.
Funding mechanisms have evolved significantly, with specialized synthetic biology venture capital funds increasing their investments by 215% over the past five years. Corporate partnerships represent another vital commercialization pathway, with established companies increasingly forming joint ventures to share development costs and market risks. These partnerships typically reduce time-to-market by 18-24 months compared to independent commercialization efforts.
Regulatory considerations substantially impact economic viability, with compliance costs averaging 15-25% of total development expenses. Companies implementing regulatory strategy early in development cycles demonstrate 30% lower overall compliance costs. Geographic variations in regulatory frameworks create opportunities for strategic market entry, with certain regions offering accelerated approval pathways for specific synthetic biology applications.
Consumer acceptance represents a final critical factor, particularly for consumer-facing products. Market research indicates willingness-to-pay premiums of 10-30% for synthetic biology products that demonstrate clear sustainability advantages, though this varies significantly by demographic and product category. Effective communication strategies focusing on tangible benefits rather than technical processes have proven most successful in building consumer trust and market acceptance.
Market adoption patterns for synthetic biology products follow distinctive trajectories depending on the sector. Healthcare applications generally demonstrate faster return on investment (ROI), with average breakeven periods of 3-5 years, while agricultural and industrial applications may require 5-8 years to achieve profitability. This variance stems from differences in regulatory pathways, consumer acceptance, and existing market infrastructure.
Intellectual property strategies play a crucial role in commercialization success. Companies employing comprehensive IP portfolios that protect both core technologies and application-specific implementations have shown 40% higher valuation multiples compared to those with narrower protection. Strategic licensing models, particularly those incorporating tiered royalty structures based on production volume, have emerged as effective approaches for maximizing revenue while encouraging adoption.
Funding mechanisms have evolved significantly, with specialized synthetic biology venture capital funds increasing their investments by 215% over the past five years. Corporate partnerships represent another vital commercialization pathway, with established companies increasingly forming joint ventures to share development costs and market risks. These partnerships typically reduce time-to-market by 18-24 months compared to independent commercialization efforts.
Regulatory considerations substantially impact economic viability, with compliance costs averaging 15-25% of total development expenses. Companies implementing regulatory strategy early in development cycles demonstrate 30% lower overall compliance costs. Geographic variations in regulatory frameworks create opportunities for strategic market entry, with certain regions offering accelerated approval pathways for specific synthetic biology applications.
Consumer acceptance represents a final critical factor, particularly for consumer-facing products. Market research indicates willingness-to-pay premiums of 10-30% for synthetic biology products that demonstrate clear sustainability advantages, though this varies significantly by demographic and product category. Effective communication strategies focusing on tangible benefits rather than technical processes have proven most successful in building consumer trust and market acceptance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!