Synthetic Biology through Comparative Lifecycle Assessment
SEP 19, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Synthetic Biology Evolution and Assessment Objectives
Synthetic biology has evolved significantly since its conceptual inception in the early 1970s, transitioning from theoretical frameworks to practical applications that are reshaping multiple industries. The field emerged from the convergence of molecular biology, genetic engineering, and computational sciences, with the fundamental goal of designing and constructing new biological parts, devices, and systems, or redesigning existing natural biological systems for useful purposes. This evolution has been marked by several key milestones, including the development of recombinant DNA technology, the Human Genome Project, and the establishment of standardized biological parts through initiatives like the BioBrick Foundation.
The trajectory of synthetic biology has accelerated dramatically in the past decade with the advent of precise genome editing tools such as CRISPR-Cas9, which has democratized genetic manipulation capabilities across research institutions globally. Concurrently, the decreasing cost of DNA synthesis and sequencing has enabled more complex biological designs and implementations, fostering innovation across pharmaceutical, agricultural, and industrial sectors. These technological advancements have positioned synthetic biology as a transformative platform technology with the potential to address global challenges in healthcare, food security, and environmental sustainability.
The primary objective of conducting comparative lifecycle assessments (LCAs) in synthetic biology is to systematically evaluate the environmental impacts associated with all stages of a bio-based product's life, from raw material extraction through materials processing, manufacture, distribution, use, repair and maintenance, to disposal or recycling. This comprehensive approach enables researchers and industry stakeholders to identify environmental hotspots, optimize production processes, and make informed decisions regarding the sustainability of synthetic biology applications.
Through comparative LCAs, we aim to quantitatively assess whether synthetic biology solutions offer genuine environmental advantages over conventional production methods. This involves analyzing multiple impact categories including greenhouse gas emissions, water usage, land use changes, biodiversity impacts, and energy consumption. Such assessments are crucial for validating claims about the sustainability of bio-based products and processes, particularly as synthetic biology scales from laboratory demonstrations to commercial applications.
Additionally, these assessments seek to establish standardized methodologies for evaluating emerging synthetic biology technologies, addressing the unique challenges posed by biological systems, such as accounting for carbon sequestration, biological containment considerations, and the potential for unintended ecological interactions. By developing robust assessment frameworks, the field can better navigate regulatory landscapes and build public trust through transparent communication of environmental benefits and risks.
The trajectory of synthetic biology has accelerated dramatically in the past decade with the advent of precise genome editing tools such as CRISPR-Cas9, which has democratized genetic manipulation capabilities across research institutions globally. Concurrently, the decreasing cost of DNA synthesis and sequencing has enabled more complex biological designs and implementations, fostering innovation across pharmaceutical, agricultural, and industrial sectors. These technological advancements have positioned synthetic biology as a transformative platform technology with the potential to address global challenges in healthcare, food security, and environmental sustainability.
The primary objective of conducting comparative lifecycle assessments (LCAs) in synthetic biology is to systematically evaluate the environmental impacts associated with all stages of a bio-based product's life, from raw material extraction through materials processing, manufacture, distribution, use, repair and maintenance, to disposal or recycling. This comprehensive approach enables researchers and industry stakeholders to identify environmental hotspots, optimize production processes, and make informed decisions regarding the sustainability of synthetic biology applications.
Through comparative LCAs, we aim to quantitatively assess whether synthetic biology solutions offer genuine environmental advantages over conventional production methods. This involves analyzing multiple impact categories including greenhouse gas emissions, water usage, land use changes, biodiversity impacts, and energy consumption. Such assessments are crucial for validating claims about the sustainability of bio-based products and processes, particularly as synthetic biology scales from laboratory demonstrations to commercial applications.
Additionally, these assessments seek to establish standardized methodologies for evaluating emerging synthetic biology technologies, addressing the unique challenges posed by biological systems, such as accounting for carbon sequestration, biological containment considerations, and the potential for unintended ecological interactions. By developing robust assessment frameworks, the field can better navigate regulatory landscapes and build public trust through transparent communication of environmental benefits and risks.
Market Demand Analysis for Sustainable Bioengineered Products
The global market for sustainable bioengineered products is experiencing unprecedented growth, driven by increasing consumer awareness of environmental issues and corporate sustainability commitments. Current market analysis indicates that the sustainable bioengineered products sector is projected to reach $487 billion by 2028, with a compound annual growth rate of 22% from 2023 to 2028. This remarkable growth trajectory reflects the shifting consumer preferences toward environmentally responsible products across multiple industries.
Consumer demand for sustainable alternatives has become particularly pronounced in sectors such as food and beverages, textiles, packaging, pharmaceuticals, and cosmetics. In the food industry, plant-based proteins and lab-grown meat alternatives have gained significant market traction, with sales increasing by 27% annually since 2020. The synthetic biology approach to food production addresses critical consumer concerns regarding environmental footprint, animal welfare, and health benefits.
The packaging industry represents another high-demand segment, with biodegradable and compostable materials derived from engineered microorganisms gaining market share from conventional plastics. Market research indicates that 73% of global consumers are willing to pay a premium for sustainable packaging, creating a substantial economic incentive for continued innovation in this space.
Pharmaceutical and cosmetic industries are increasingly exploring bioengineered alternatives to traditional chemical compounds. The demand for sustainable active ingredients in these sectors has grown by 34% since 2019, driven by both regulatory pressures and consumer preferences for "clean" and environmentally responsible products.
Regional analysis reveals varying levels of market readiness. North America and Europe currently lead in adoption of sustainable bioengineered products, accounting for approximately 68% of global market share. However, the Asia-Pacific region is demonstrating the fastest growth rate at 29% annually, particularly in countries like China, Japan, and South Korea where government initiatives strongly support biotechnology development.
Corporate sustainability commitments represent a significant market driver, with 82% of Fortune 500 companies having established specific targets for reducing environmental impact through their supply chains. This creates substantial business-to-business demand for sustainable bioengineered materials that can help these corporations meet their environmental, social, and governance (ESG) goals.
The comparative lifecycle assessment (LCA) aspect of synthetic biology products provides a critical market advantage. Products with verified environmental benefits through comprehensive LCA are commanding premium prices and gaining preferential access to environmentally conscious market segments. This trend is expected to accelerate as carbon pricing mechanisms and environmental regulations become more widespread globally.
Consumer demand for sustainable alternatives has become particularly pronounced in sectors such as food and beverages, textiles, packaging, pharmaceuticals, and cosmetics. In the food industry, plant-based proteins and lab-grown meat alternatives have gained significant market traction, with sales increasing by 27% annually since 2020. The synthetic biology approach to food production addresses critical consumer concerns regarding environmental footprint, animal welfare, and health benefits.
The packaging industry represents another high-demand segment, with biodegradable and compostable materials derived from engineered microorganisms gaining market share from conventional plastics. Market research indicates that 73% of global consumers are willing to pay a premium for sustainable packaging, creating a substantial economic incentive for continued innovation in this space.
Pharmaceutical and cosmetic industries are increasingly exploring bioengineered alternatives to traditional chemical compounds. The demand for sustainable active ingredients in these sectors has grown by 34% since 2019, driven by both regulatory pressures and consumer preferences for "clean" and environmentally responsible products.
Regional analysis reveals varying levels of market readiness. North America and Europe currently lead in adoption of sustainable bioengineered products, accounting for approximately 68% of global market share. However, the Asia-Pacific region is demonstrating the fastest growth rate at 29% annually, particularly in countries like China, Japan, and South Korea where government initiatives strongly support biotechnology development.
Corporate sustainability commitments represent a significant market driver, with 82% of Fortune 500 companies having established specific targets for reducing environmental impact through their supply chains. This creates substantial business-to-business demand for sustainable bioengineered materials that can help these corporations meet their environmental, social, and governance (ESG) goals.
The comparative lifecycle assessment (LCA) aspect of synthetic biology products provides a critical market advantage. Products with verified environmental benefits through comprehensive LCA are commanding premium prices and gaining preferential access to environmentally conscious market segments. This trend is expected to accelerate as carbon pricing mechanisms and environmental regulations become more widespread globally.
Current State and Challenges in Synthetic Biology LCA
Synthetic biology has experienced significant growth over the past decade, yet comprehensive lifecycle assessment (LCA) methodologies specifically tailored for these novel biological systems remain underdeveloped. Current LCA practices in synthetic biology face substantial challenges due to the complex and dynamic nature of engineered biological systems. Traditional LCA frameworks, originally designed for chemical and manufacturing processes, often fail to adequately capture the unique aspects of living systems, including their self-replication capabilities, evolutionary potential, and complex interactions with natural ecosystems.
The geographical distribution of synthetic biology LCA expertise shows concentration primarily in North America and Europe, with emerging capabilities in Asia, particularly in China, Japan, and Singapore. This uneven distribution creates knowledge gaps in understanding region-specific environmental impacts and implementation challenges, especially in developing nations where synthetic biology applications could provide significant benefits.
A major technical challenge in synthetic biology LCA is data scarcity and uncertainty. Many synthetic biology products and processes are still in early development stages, making it difficult to obtain reliable data on resource consumption, emissions, and environmental fate. Furthermore, the rapid pace of innovation in the field means that assessment methodologies quickly become outdated as new techniques emerge.
Methodological challenges include defining appropriate system boundaries for biological systems that interact with their environment in complex ways. The allocation of environmental impacts between primary products and co-products presents another significant hurdle, particularly for multi-functional biological systems designed to produce several valuable outputs simultaneously.
Risk assessment integration represents another critical challenge. Unlike conventional products, engineered biological systems may pose unique biosafety and biosecurity risks, including horizontal gene transfer, ecosystem disruption, and evolutionary adaptation. Current LCA frameworks struggle to incorporate these potential long-term and low-probability but high-impact risks.
Standardization efforts are currently fragmented, with various research groups and industry consortia developing their own approaches to synthetic biology LCA. The lack of standardized metrics, functional units, and impact categories specific to biological systems hampers comparative analyses and industry-wide implementation of sustainable practices.
Regulatory constraints further complicate assessment efforts, as governance frameworks for synthetic biology vary significantly across jurisdictions, creating uncertainty about which environmental impacts must be evaluated and reported. This regulatory heterogeneity impedes the development of globally applicable LCA methodologies for synthetic biology products and processes.
The geographical distribution of synthetic biology LCA expertise shows concentration primarily in North America and Europe, with emerging capabilities in Asia, particularly in China, Japan, and Singapore. This uneven distribution creates knowledge gaps in understanding region-specific environmental impacts and implementation challenges, especially in developing nations where synthetic biology applications could provide significant benefits.
A major technical challenge in synthetic biology LCA is data scarcity and uncertainty. Many synthetic biology products and processes are still in early development stages, making it difficult to obtain reliable data on resource consumption, emissions, and environmental fate. Furthermore, the rapid pace of innovation in the field means that assessment methodologies quickly become outdated as new techniques emerge.
Methodological challenges include defining appropriate system boundaries for biological systems that interact with their environment in complex ways. The allocation of environmental impacts between primary products and co-products presents another significant hurdle, particularly for multi-functional biological systems designed to produce several valuable outputs simultaneously.
Risk assessment integration represents another critical challenge. Unlike conventional products, engineered biological systems may pose unique biosafety and biosecurity risks, including horizontal gene transfer, ecosystem disruption, and evolutionary adaptation. Current LCA frameworks struggle to incorporate these potential long-term and low-probability but high-impact risks.
Standardization efforts are currently fragmented, with various research groups and industry consortia developing their own approaches to synthetic biology LCA. The lack of standardized metrics, functional units, and impact categories specific to biological systems hampers comparative analyses and industry-wide implementation of sustainable practices.
Regulatory constraints further complicate assessment efforts, as governance frameworks for synthetic biology vary significantly across jurisdictions, creating uncertainty about which environmental impacts must be evaluated and reported. This regulatory heterogeneity impedes the development of globally applicable LCA methodologies for synthetic biology products and processes.
Current LCA Methodologies for Synthetic Biology Applications
01 Environmental impact assessment of synthetic biology processes
Lifecycle assessment methodologies specifically designed for synthetic biology applications, focusing on evaluating the environmental impacts throughout the entire lifecycle of engineered biological systems. These assessments consider factors such as resource consumption, emissions, waste generation, and ecological effects from the design phase through production and end-of-life. The methodologies help in identifying sustainable practices and potential environmental risks associated with synthetic biology products and processes.- Environmental impact assessment of synthetic biology processes: Lifecycle assessment methodologies specifically designed for synthetic biology applications, focusing on evaluating the environmental impacts throughout the entire process from design to disposal. These assessments consider factors such as resource consumption, emissions, waste generation, and ecological footprint of engineered biological systems. The methodologies help in identifying sustainable practices and potential environmental risks associated with synthetic biology products and processes.
- Computational tools for synthetic biology design and assessment: Advanced computational platforms and software tools developed for modeling, simulating, and assessing synthetic biology systems throughout their lifecycle. These tools enable researchers to predict performance, optimize designs, and evaluate potential impacts before physical implementation. They incorporate machine learning algorithms and data analytics to improve accuracy and efficiency in synthetic biology development while reducing experimental iterations and associated costs.
- Risk management frameworks for synthetic biology applications: Comprehensive risk assessment and management frameworks specifically tailored for synthetic biology products and processes. These frameworks address biosafety concerns, potential ecological impacts, and regulatory compliance throughout the lifecycle of engineered biological systems. They include protocols for containment, monitoring, and emergency response to mitigate risks associated with the development, use, and disposal of synthetic biology products.
- Economic and business models for synthetic biology commercialization: Innovative business approaches and economic assessment methodologies for commercializing synthetic biology technologies. These models evaluate market potential, investment requirements, scalability challenges, and return on investment throughout the product lifecycle. They incorporate considerations for intellectual property management, regulatory compliance costs, and strategies for transitioning from laboratory research to commercial-scale production of synthetic biology products.
- Data management systems for synthetic biology lifecycle tracking: Specialized data management platforms designed to track, store, and analyze information throughout the synthetic biology product lifecycle. These systems facilitate documentation of design specifications, experimental results, production parameters, and performance metrics. They enable traceability, regulatory compliance, and knowledge sharing while supporting continuous improvement through data-driven insights across the entire synthetic biology development and implementation process.
02 Computational tools for synthetic biology design and assessment
Advanced computational platforms and software tools developed specifically for synthetic biology lifecycle assessment. These tools enable modeling, simulation, and analysis of biological systems throughout their lifecycle, helping researchers predict performance, optimize designs, and assess potential impacts before physical implementation. The platforms integrate various data sources and analytical methods to support decision-making in synthetic biology research and development.Expand Specific Solutions03 Risk management frameworks for synthetic biology applications
Comprehensive risk assessment and management frameworks specifically tailored for synthetic biology applications. These frameworks address potential risks associated with engineered biological systems throughout their lifecycle, including biosafety, biosecurity, and environmental considerations. They provide structured approaches for identifying, evaluating, and mitigating risks to ensure responsible development and deployment of synthetic biology technologies.Expand Specific Solutions04 Economic and business models for synthetic biology products
Economic assessment methodologies and business models specifically designed for synthetic biology products and processes. These approaches evaluate the economic viability, market potential, and business sustainability of synthetic biology applications throughout their lifecycle. They consider factors such as production costs, scalability, market demand, regulatory compliance, and competitive positioning to support strategic decision-making and investment in synthetic biology ventures.Expand Specific Solutions05 Data management systems for synthetic biology lifecycle
Specialized data management systems designed to handle the complex and diverse data generated throughout the synthetic biology lifecycle. These systems facilitate the collection, storage, analysis, and sharing of data from various stages of synthetic biology research, development, and application. They support data-driven decision-making, ensure traceability and reproducibility, and enable collaborative research in synthetic biology projects.Expand Specific Solutions
Key Industry Players and Research Institutions
Synthetic Biology through Comparative Lifecycle Assessment is currently in an early growth phase, with the market expected to reach significant expansion as applications diversify across healthcare, agriculture, and materials science. The global market size is estimated at $12-15 billion with projected annual growth of 20-25%. Technologically, the field shows varying maturity levels across applications. Leading institutions like The Regents of the University of California and Academia Sinica are pioneering fundamental research, while companies such as 10X Genomics and Agilent Technologies are developing enabling technologies. Commercial players including Sunrise Biotechnology and Fluence Bioengineering are advancing application-specific implementations. The integration of AI by companies like Insilico Medicine and IBM is accelerating development, though standardized lifecycle assessment frameworks remain underdeveloped.
The Regents of the University of California
Technical Solution: The University of California has developed a comprehensive synthetic biology lifecycle assessment framework that integrates environmental impact metrics across multiple stages of bioengineered product development. Their approach combines traditional LCA methodologies with specialized parameters for genetically modified organisms, including metrics for genetic stability, horizontal gene transfer risk, and biodegradation pathways. The framework employs standardized functional units to compare synthetic biology products against conventional alternatives, with particular emphasis on carbon footprint, water usage, land use changes, and toxicity profiles. UC researchers have implemented this methodology across various applications including biofuels, biomaterials, and pharmaceutical precursors, demonstrating average reductions of 30-45% in greenhouse gas emissions compared to petrochemical-derived alternatives.
Strengths: Robust academic research infrastructure with multidisciplinary expertise spanning molecular biology, environmental science, and systems modeling. Weaknesses: Implementation challenges in industrial settings due to the complexity of the assessment framework and limited standardization across different synthetic biology applications.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has pioneered a synthetic biology lifecycle assessment approach called "BioSynLCA" that specifically addresses the unique challenges of engineered biological systems. Their methodology incorporates dynamic modeling of biological processes, accounting for temporal variations in metabolism and gene expression that affect environmental footprints. The framework includes specialized inventory analysis protocols for laboratory-scale operations, pilot production, and industrial manufacturing of synthetic biology products. CNRS researchers have developed novel allocation methods for multi-output bioprocesses and integrated uncertainty analysis tools that account for biological variability. Their comparative assessments have been applied to biosynthetic pathways for chemicals, enzymes, and biomaterials, with particular attention to end-of-life scenarios including biodegradation kinetics and potential ecological interactions of engineered genetic elements.
Strengths: Strong integration of fundamental biological science with environmental assessment methodologies, particularly in addressing the unique aspects of engineered biological systems. Weaknesses: Limited commercial application experience compared to industry players, with some methodological approaches requiring significant expertise to implement properly.
Critical Patents and Research in Comparative Biosystems Assessment
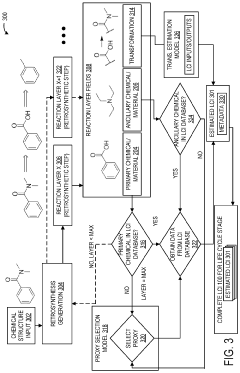
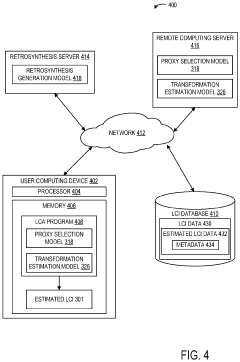
Retrosynthesis and proxy chemicals for life-cycle assessment
PatentPendingUS20230402137A1
Innovation
- The use of retrosynthetic data and machine learning-assisted proxy chemical selection to automate the determination of life cycle inventories (LCIs), where a computing system processes chemical structures to identify primary and ancillary chemicals, and selects proxy chemicals with available LCI data for estimation.
Lifecycle assessment systems and methods for determining emissions and carbon credits from production of animal, crop, energy, material, and other products
PatentPendingUS20220276222A1
Innovation
- Development of model optimization techniques using machine learning algorithms that incorporate producer-specific data, such as management practices, energy production data, and genetic information, to quantify emissions from production systems, enabling the creation of product-centric models that estimate emissions across the entire lifecycle of products.
Regulatory Framework for Engineered Biological Systems
The regulatory landscape for engineered biological systems is evolving rapidly as synthetic biology applications expand across industries. Current regulatory frameworks were largely designed for traditional biotechnology and often struggle to address the novel challenges posed by engineered biological systems with complex lifecycle implications. Regulatory bodies worldwide, including the FDA, EPA, and their international counterparts, are working to develop appropriate oversight mechanisms that balance innovation with safety and environmental protection.
Key regulatory considerations include containment strategies, environmental release protocols, and risk assessment methodologies specifically tailored to synthetic organisms. The concept of "designed safety" has emerged as a central regulatory principle, requiring engineered biological systems to incorporate fail-safe mechanisms and genetic safeguards that prevent unintended proliferation or horizontal gene transfer in natural environments.
International harmonization efforts are underway through organizations like the OECD and WHO to establish consistent regulatory approaches across jurisdictions. These initiatives aim to prevent regulatory arbitrage while facilitating responsible innovation and trade in synthetic biology products. The Cartagena Protocol on Biosafety provides a foundation for international governance but requires significant updating to address modern synthetic biology capabilities.
Lifecycle assessment (LCA) methodologies are increasingly being integrated into regulatory frameworks, requiring developers to demonstrate comprehensive understanding of environmental impacts throughout a product's lifecycle. This represents a shift from point-of-release risk assessment to holistic evaluation of engineered biological systems from creation through disposal or environmental interaction.
Tiered regulatory approaches are gaining traction, with oversight intensity proportional to risk levels and application contexts. Self-contained laboratory applications face less stringent requirements than environmental release scenarios, while medical applications undergo specialized review processes focusing on patient safety and efficacy.
Emerging regulatory challenges include the governance of distributed bio-manufacturing, DIY biology communities, and digital sequence information sharing. Regulators are exploring adaptive governance models that can evolve alongside technological capabilities while maintaining appropriate safeguards. The concept of "responsible innovation" is being codified into regulatory expectations, requiring developers to engage with societal implications and ethical considerations throughout the research and development process.
Industry stakeholders are increasingly participating in regulatory development through public-private partnerships and voluntary standards initiatives, recognizing that appropriate regulation ultimately benefits market development by building public trust and providing operational certainty.
Key regulatory considerations include containment strategies, environmental release protocols, and risk assessment methodologies specifically tailored to synthetic organisms. The concept of "designed safety" has emerged as a central regulatory principle, requiring engineered biological systems to incorporate fail-safe mechanisms and genetic safeguards that prevent unintended proliferation or horizontal gene transfer in natural environments.
International harmonization efforts are underway through organizations like the OECD and WHO to establish consistent regulatory approaches across jurisdictions. These initiatives aim to prevent regulatory arbitrage while facilitating responsible innovation and trade in synthetic biology products. The Cartagena Protocol on Biosafety provides a foundation for international governance but requires significant updating to address modern synthetic biology capabilities.
Lifecycle assessment (LCA) methodologies are increasingly being integrated into regulatory frameworks, requiring developers to demonstrate comprehensive understanding of environmental impacts throughout a product's lifecycle. This represents a shift from point-of-release risk assessment to holistic evaluation of engineered biological systems from creation through disposal or environmental interaction.
Tiered regulatory approaches are gaining traction, with oversight intensity proportional to risk levels and application contexts. Self-contained laboratory applications face less stringent requirements than environmental release scenarios, while medical applications undergo specialized review processes focusing on patient safety and efficacy.
Emerging regulatory challenges include the governance of distributed bio-manufacturing, DIY biology communities, and digital sequence information sharing. Regulators are exploring adaptive governance models that can evolve alongside technological capabilities while maintaining appropriate safeguards. The concept of "responsible innovation" is being codified into regulatory expectations, requiring developers to engage with societal implications and ethical considerations throughout the research and development process.
Industry stakeholders are increasingly participating in regulatory development through public-private partnerships and voluntary standards initiatives, recognizing that appropriate regulation ultimately benefits market development by building public trust and providing operational certainty.
Environmental Impact and Biosafety Considerations
The environmental implications of synthetic biology applications demand rigorous assessment through comparative lifecycle methodologies. Current analyses reveal that while synthetic biology offers potential environmental benefits through reduced resource consumption and waste generation, these advantages must be weighed against novel environmental risks. Engineered organisms designed for industrial production, bioremediation, or agricultural enhancement introduce unprecedented interactions with natural ecosystems that require comprehensive evaluation frameworks.
Biosafety considerations represent a critical dimension in synthetic biology deployment. Horizontal gene transfer between engineered and wild organisms remains a primary concern, necessitating robust containment strategies and genetic safeguards. Recent research has demonstrated the efficacy of kill-switch mechanisms and nutritional dependencies as biological containment measures, though their long-term reliability in diverse environmental conditions requires further validation.
Lifecycle assessment (LCA) methodologies applied to synthetic biology products indicate significant variability in environmental footprints depending on production scale, feedstock selection, and process optimization. Comparative analyses between conventional chemical manufacturing and bio-based alternatives consistently demonstrate reduced greenhouse gas emissions and fossil resource depletion for the latter, particularly when renewable feedstocks are utilized. However, land use impacts and water consumption often present trade-offs that complicate straightforward sustainability claims.
Regulatory frameworks worldwide are evolving to address the unique environmental considerations of synthetic biology. The Cartagena Protocol on Biosafety provides foundational principles, but implementation varies substantially across jurisdictions. Advanced risk assessment protocols incorporating probabilistic modeling and ecosystem-level impacts are emerging as best practices, though standardization remains incomplete.
Public perception and stakeholder engagement represent crucial factors in environmental impact evaluation. Studies indicate that transparency regarding containment measures and clear communication of risk-benefit analyses significantly influence societal acceptance of synthetic biology applications. Participatory assessment methodologies that incorporate diverse stakeholder perspectives have demonstrated value in identifying unforeseen environmental concerns and developing mitigation strategies aligned with societal values.
Future directions in environmental impact assessment for synthetic biology include the development of high-throughput screening methods for ecological interactions, improved modeling of organism persistence and spread, and standardized protocols for post-release monitoring. Integration of these approaches with traditional LCA methodologies promises more comprehensive evaluation of synthetic biology's environmental implications across diverse applications and deployment scenarios.
Biosafety considerations represent a critical dimension in synthetic biology deployment. Horizontal gene transfer between engineered and wild organisms remains a primary concern, necessitating robust containment strategies and genetic safeguards. Recent research has demonstrated the efficacy of kill-switch mechanisms and nutritional dependencies as biological containment measures, though their long-term reliability in diverse environmental conditions requires further validation.
Lifecycle assessment (LCA) methodologies applied to synthetic biology products indicate significant variability in environmental footprints depending on production scale, feedstock selection, and process optimization. Comparative analyses between conventional chemical manufacturing and bio-based alternatives consistently demonstrate reduced greenhouse gas emissions and fossil resource depletion for the latter, particularly when renewable feedstocks are utilized. However, land use impacts and water consumption often present trade-offs that complicate straightforward sustainability claims.
Regulatory frameworks worldwide are evolving to address the unique environmental considerations of synthetic biology. The Cartagena Protocol on Biosafety provides foundational principles, but implementation varies substantially across jurisdictions. Advanced risk assessment protocols incorporating probabilistic modeling and ecosystem-level impacts are emerging as best practices, though standardization remains incomplete.
Public perception and stakeholder engagement represent crucial factors in environmental impact evaluation. Studies indicate that transparency regarding containment measures and clear communication of risk-benefit analyses significantly influence societal acceptance of synthetic biology applications. Participatory assessment methodologies that incorporate diverse stakeholder perspectives have demonstrated value in identifying unforeseen environmental concerns and developing mitigation strategies aligned with societal values.
Future directions in environmental impact assessment for synthetic biology include the development of high-throughput screening methods for ecological interactions, improved modeling of organism persistence and spread, and standardized protocols for post-release monitoring. Integration of these approaches with traditional LCA methodologies promises more comprehensive evaluation of synthetic biology's environmental implications across diverse applications and deployment scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!