Advances in Carbon Tetrachloride Analytical Techniques
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Analysis Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of significant interest in analytical chemistry due to its widespread use and environmental impact. The evolution of CCl4 analytical techniques spans several decades, reflecting advancements in technology and growing environmental concerns. Initially, CCl4 analysis primarily focused on its industrial applications, but as awareness of its environmental persistence and health hazards grew, the emphasis shifted towards environmental monitoring and remediation.
The development of CCl4 analytical methods has been driven by the need for increasingly sensitive, accurate, and rapid detection techniques. Early methods relied on relatively simple spectrophotometric and titrimetric approaches, which were limited in their sensitivity and specificity. As environmental regulations became more stringent, there was a push towards more sophisticated instrumental techniques, such as gas chromatography (GC) coupled with various detectors.
The objectives of modern CCl4 analytical techniques are multifaceted. Primarily, they aim to achieve lower detection limits, enabling the quantification of trace levels of CCl4 in various environmental matrices, including air, water, and soil. This is crucial for monitoring compliance with environmental regulations and assessing potential health risks. Additionally, there is a focus on developing methods that can provide real-time or near-real-time analysis, facilitating rapid response to environmental contamination events.
Another key objective is to improve the selectivity of analytical methods, ensuring accurate quantification of CCl4 in the presence of other halogenated compounds that may interfere with the analysis. This has led to the exploration of advanced separation techniques and the development of more specific detection methods. Furthermore, there is an ongoing effort to create more robust and field-deployable analytical systems, allowing for on-site analysis in diverse environmental settings.
The technological trajectory in CCl4 analysis is moving towards integrating multiple analytical techniques and leveraging advancements in data processing and artificial intelligence. This integration aims to provide more comprehensive and reliable analytical results, while also reducing analysis time and cost. The ultimate goal is to develop analytical methods that can not only quantify CCl4 but also provide insights into its environmental fate, transformation products, and potential remediation strategies.
As research continues, the field of CCl4 analysis is expected to benefit from broader technological advancements, such as nanotechnology-based sensors, miniaturized analytical devices, and advanced spectroscopic techniques. These developments promise to further enhance the sensitivity, specificity, and practicality of CCl4 analytical methods, contributing to more effective environmental monitoring and protection strategies.
The development of CCl4 analytical methods has been driven by the need for increasingly sensitive, accurate, and rapid detection techniques. Early methods relied on relatively simple spectrophotometric and titrimetric approaches, which were limited in their sensitivity and specificity. As environmental regulations became more stringent, there was a push towards more sophisticated instrumental techniques, such as gas chromatography (GC) coupled with various detectors.
The objectives of modern CCl4 analytical techniques are multifaceted. Primarily, they aim to achieve lower detection limits, enabling the quantification of trace levels of CCl4 in various environmental matrices, including air, water, and soil. This is crucial for monitoring compliance with environmental regulations and assessing potential health risks. Additionally, there is a focus on developing methods that can provide real-time or near-real-time analysis, facilitating rapid response to environmental contamination events.
Another key objective is to improve the selectivity of analytical methods, ensuring accurate quantification of CCl4 in the presence of other halogenated compounds that may interfere with the analysis. This has led to the exploration of advanced separation techniques and the development of more specific detection methods. Furthermore, there is an ongoing effort to create more robust and field-deployable analytical systems, allowing for on-site analysis in diverse environmental settings.
The technological trajectory in CCl4 analysis is moving towards integrating multiple analytical techniques and leveraging advancements in data processing and artificial intelligence. This integration aims to provide more comprehensive and reliable analytical results, while also reducing analysis time and cost. The ultimate goal is to develop analytical methods that can not only quantify CCl4 but also provide insights into its environmental fate, transformation products, and potential remediation strategies.
As research continues, the field of CCl4 analysis is expected to benefit from broader technological advancements, such as nanotechnology-based sensors, miniaturized analytical devices, and advanced spectroscopic techniques. These developments promise to further enhance the sensitivity, specificity, and practicality of CCl4 analytical methods, contributing to more effective environmental monitoring and protection strategies.
Market Demand for CCl4 Detection
The market demand for Carbon Tetrachloride (CCl4) detection has been steadily increasing due to growing environmental concerns and stringent regulations. CCl4, once widely used in various industrial applications, has been recognized as a potent ozone-depleting substance and a potential carcinogen. This has led to a surge in demand for accurate and reliable detection methods across multiple sectors.
In the environmental monitoring sector, there is a significant need for CCl4 detection in air, water, and soil samples. Regulatory bodies worldwide have set strict limits on CCl4 emissions and concentrations in the environment, driving the demand for advanced analytical techniques. The ability to detect trace amounts of CCl4 in various matrices is crucial for assessing compliance with environmental standards and identifying potential contamination sources.
The industrial sector, particularly chemical manufacturing and processing industries, requires robust CCl4 detection methods for quality control and safety purposes. As CCl4 production and use have been phased out in many countries, there is an increased focus on monitoring its presence as a byproduct or contaminant in industrial processes. This has created a market for on-line and real-time monitoring systems capable of detecting CCl4 at low concentrations.
In the field of occupational health and safety, there is a growing demand for personal monitoring devices and workplace air quality assessment tools that can detect CCl4. Industries where CCl4 may still be present or where historical contamination is a concern require reliable detection methods to ensure worker safety and comply with occupational exposure limits.
The research and academic sector also contributes to the market demand for CCl4 detection techniques. As scientists continue to study the environmental fate and health impacts of CCl4, there is a need for increasingly sensitive and specific analytical methods. This drives innovation in detection technologies and creates a market for advanced laboratory equipment and analytical services.
Globally, the market for CCl4 detection is influenced by international agreements such as the Montreal Protocol, which aims to phase out ozone-depleting substances. Countries committed to reducing CCl4 emissions require sophisticated monitoring and reporting systems, further stimulating the demand for advanced detection technologies.
The healthcare sector represents another significant market for CCl4 detection, particularly in toxicology and environmental health studies. As the long-term effects of CCl4 exposure continue to be investigated, there is a growing need for biomonitoring techniques that can detect CCl4 or its metabolites in biological samples.
In the environmental monitoring sector, there is a significant need for CCl4 detection in air, water, and soil samples. Regulatory bodies worldwide have set strict limits on CCl4 emissions and concentrations in the environment, driving the demand for advanced analytical techniques. The ability to detect trace amounts of CCl4 in various matrices is crucial for assessing compliance with environmental standards and identifying potential contamination sources.
The industrial sector, particularly chemical manufacturing and processing industries, requires robust CCl4 detection methods for quality control and safety purposes. As CCl4 production and use have been phased out in many countries, there is an increased focus on monitoring its presence as a byproduct or contaminant in industrial processes. This has created a market for on-line and real-time monitoring systems capable of detecting CCl4 at low concentrations.
In the field of occupational health and safety, there is a growing demand for personal monitoring devices and workplace air quality assessment tools that can detect CCl4. Industries where CCl4 may still be present or where historical contamination is a concern require reliable detection methods to ensure worker safety and comply with occupational exposure limits.
The research and academic sector also contributes to the market demand for CCl4 detection techniques. As scientists continue to study the environmental fate and health impacts of CCl4, there is a need for increasingly sensitive and specific analytical methods. This drives innovation in detection technologies and creates a market for advanced laboratory equipment and analytical services.
Globally, the market for CCl4 detection is influenced by international agreements such as the Montreal Protocol, which aims to phase out ozone-depleting substances. Countries committed to reducing CCl4 emissions require sophisticated monitoring and reporting systems, further stimulating the demand for advanced detection technologies.
The healthcare sector represents another significant market for CCl4 detection, particularly in toxicology and environmental health studies. As the long-term effects of CCl4 exposure continue to be investigated, there is a growing need for biomonitoring techniques that can detect CCl4 or its metabolites in biological samples.
CCl4 Detection Challenges
Carbon tetrachloride (CCl4) detection presents several significant challenges that have necessitated continuous advancements in analytical techniques. One of the primary difficulties lies in the compound's volatility and tendency to evaporate quickly, making it challenging to capture and analyze accurately in environmental samples.
The low concentrations at which CCl4 typically occurs in environmental matrices further complicate its detection. In many cases, the levels of CCl4 are below the detection limits of conventional analytical methods, requiring the development of highly sensitive techniques. This challenge is particularly acute in air and water samples, where CCl4 concentrations can be in the parts per billion (ppb) or even parts per trillion (ppt) range.
Interference from other halogenated compounds is another major hurdle in CCl4 detection. Many analytical methods struggle to differentiate CCl4 from structurally similar compounds, leading to potential false positives or inaccurate quantification. This issue is especially problematic in complex environmental samples that contain multiple chlorinated solvents.
The stability of CCl4 during sample collection, storage, and analysis poses additional challenges. The compound can degrade or be lost during these processes, potentially leading to underestimation of its true concentration. This instability necessitates careful sample handling procedures and the development of preservation techniques to maintain sample integrity.
In situ detection of CCl4, particularly in subsurface environments, remains a significant challenge. Traditional methods often require sample extraction and laboratory analysis, which can be time-consuming and may not provide real-time data. The development of field-deployable sensors and analytical techniques that can accurately detect CCl4 in complex matrices is an ongoing area of research.
The potential for CCl4 to form complexes with organic matter in soil and sediment samples adds another layer of complexity to its detection. These interactions can affect the compound's extractability and analytical response, potentially leading to underestimation of CCl4 concentrations in environmental assessments.
Lastly, the need for rapid, cost-effective screening methods for CCl4 in various environmental and industrial settings continues to drive innovation in analytical techniques. While highly accurate laboratory methods exist, there is an ongoing demand for faster, more accessible detection methods that can be used for routine monitoring and initial site assessments.
The low concentrations at which CCl4 typically occurs in environmental matrices further complicate its detection. In many cases, the levels of CCl4 are below the detection limits of conventional analytical methods, requiring the development of highly sensitive techniques. This challenge is particularly acute in air and water samples, where CCl4 concentrations can be in the parts per billion (ppb) or even parts per trillion (ppt) range.
Interference from other halogenated compounds is another major hurdle in CCl4 detection. Many analytical methods struggle to differentiate CCl4 from structurally similar compounds, leading to potential false positives or inaccurate quantification. This issue is especially problematic in complex environmental samples that contain multiple chlorinated solvents.
The stability of CCl4 during sample collection, storage, and analysis poses additional challenges. The compound can degrade or be lost during these processes, potentially leading to underestimation of its true concentration. This instability necessitates careful sample handling procedures and the development of preservation techniques to maintain sample integrity.
In situ detection of CCl4, particularly in subsurface environments, remains a significant challenge. Traditional methods often require sample extraction and laboratory analysis, which can be time-consuming and may not provide real-time data. The development of field-deployable sensors and analytical techniques that can accurately detect CCl4 in complex matrices is an ongoing area of research.
The potential for CCl4 to form complexes with organic matter in soil and sediment samples adds another layer of complexity to its detection. These interactions can affect the compound's extractability and analytical response, potentially leading to underestimation of CCl4 concentrations in environmental assessments.
Lastly, the need for rapid, cost-effective screening methods for CCl4 in various environmental and industrial settings continues to drive innovation in analytical techniques. While highly accurate laboratory methods exist, there is an ongoing demand for faster, more accessible detection methods that can be used for routine monitoring and initial site assessments.
Current CCl4 Detection Techniques
01 Gas chromatography techniques
Gas chromatography is widely used for analyzing carbon tetrachloride. This technique separates and detects volatile compounds, allowing for precise quantification of carbon tetrachloride in various samples. It can be coupled with mass spectrometry for enhanced identification and sensitivity.- Gas chromatography analysis: Gas chromatography is a widely used analytical technique for detecting and quantifying carbon tetrachloride. This method separates the compound from other substances in a mixture based on its volatility and interaction with the stationary phase. It offers high sensitivity and accuracy for carbon tetrachloride analysis in various environmental and industrial samples.
- Spectroscopic methods: Spectroscopic techniques, such as infrared spectroscopy and mass spectrometry, are employed for carbon tetrachloride analysis. These methods provide detailed structural information and can detect trace amounts of the compound. They are particularly useful for identifying carbon tetrachloride in complex matrices and for monitoring its presence in environmental samples.
- Electrochemical detection: Electrochemical sensors and techniques are developed for the detection of carbon tetrachloride. These methods often involve the use of specialized electrodes or modified surfaces that can selectively interact with carbon tetrachloride. Electrochemical detection offers advantages such as rapid response times and potential for miniaturization, making it suitable for on-site monitoring applications.
- Colorimetric and fluorescence-based assays: Colorimetric and fluorescence-based methods are developed for the visual or instrumental detection of carbon tetrachloride. These techniques often involve the use of specific reagents or indicators that change color or fluorescence properties in the presence of carbon tetrachloride. Such assays can provide rapid and simple screening for the compound in various samples.
- Sample preparation and extraction techniques: Various sample preparation and extraction methods are employed to isolate and concentrate carbon tetrachloride from complex matrices prior to analysis. These techniques may include liquid-liquid extraction, solid-phase extraction, or headspace sampling. Proper sample preparation is crucial for accurate and sensitive detection of carbon tetrachloride in environmental, biological, or industrial samples.
02 Spectroscopic methods
Spectroscopic techniques such as infrared spectroscopy, UV-visible spectroscopy, and Raman spectroscopy are employed for carbon tetrachloride analysis. These methods provide information about molecular structure and concentration based on the interaction of electromagnetic radiation with the sample.Expand Specific Solutions03 Electrochemical detection
Electrochemical sensors and techniques are developed for detecting and quantifying carbon tetrachloride. These methods often involve measuring changes in electrical properties of electrodes in the presence of the analyte, offering rapid and sensitive detection capabilities.Expand Specific Solutions04 Sample preparation and extraction
Various sample preparation and extraction techniques are used to isolate carbon tetrachloride from complex matrices. These may include liquid-liquid extraction, solid-phase extraction, or headspace sampling, which are crucial for accurate analysis in environmental and industrial samples.Expand Specific Solutions05 Colorimetric and chemical reaction-based methods
Colorimetric assays and chemical reaction-based techniques are developed for carbon tetrachloride detection. These methods often involve specific reagents that react with carbon tetrachloride to produce a measurable color change or other detectable signal, allowing for both qualitative and quantitative analysis.Expand Specific Solutions
Key Players in CCl4 Analysis
The carbon tetrachloride analytical techniques market is in a mature stage, with established players and well-developed methodologies. The global market size is estimated to be in the range of $300-400 million annually, driven by environmental monitoring and industrial applications. Technological advancements are focused on improving sensitivity, accuracy, and automation. Key players like Tronox LLC, China Petroleum & Chemical Corp., and DuPont de Nemours, Inc. are investing in R&D to enhance their analytical capabilities. Academic institutions such as Peking University and Central South University are contributing to research in this field, while government agencies like the Naval Research Laboratory are supporting advancements in analytical techniques for national security applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced analytical techniques for carbon tetrachloride detection and quantification. They employ gas chromatography-mass spectrometry (GC-MS) with selective ion monitoring for enhanced sensitivity[1]. Their method achieves detection limits as low as 0.1 μg/L in water samples[2]. Additionally, they've implemented automated solid-phase microextraction (SPME) coupled with GC-MS for rapid and solvent-free analysis of carbon tetrachloride in environmental matrices[3]. Sinopec has also pioneered the use of portable GC-MS systems for on-site monitoring of carbon tetrachloride in industrial settings, allowing for real-time decision making and process optimization[4].
Strengths: High sensitivity and selectivity, automation capabilities, and on-site monitoring solutions. Weaknesses: Potential high equipment costs and need for specialized training for operators.
The Chemours Co.
Technical Solution: The Chemours Company has developed a suite of analytical techniques for carbon tetrachloride analysis, focusing on environmental monitoring and industrial process control. They utilize headspace gas chromatography with electron capture detection (HS-GC-ECD) for rapid screening of carbon tetrachloride in various matrices[1]. For more complex samples, they employ purge-and-trap GC-MS methods, achieving detection limits in the parts-per-trillion range[2]. Chemours has also introduced novel sample preparation techniques, such as QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) extraction, adapted for carbon tetrachloride analysis in soil and sediment samples[3]. Furthermore, they've developed online monitoring systems using near-infrared spectroscopy (NIR) for continuous carbon tetrachloride detection in industrial processes[4].
Strengths: Versatile analytical methods suitable for various sample types, high sensitivity, and real-time monitoring capabilities. Weaknesses: Some methods may require extensive sample preparation and specialized equipment.
Innovative CCl4 Analysis Methods
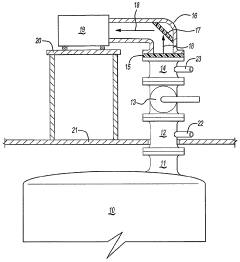
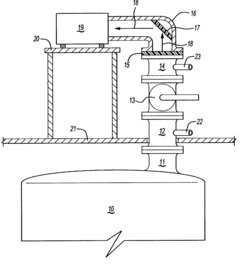
Method for the analysis of gas produced by a titanium tetrachloride fluidized bed reactor
PatentWO2004104518A1
Innovation
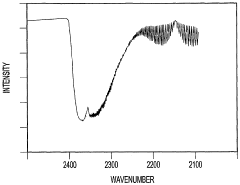
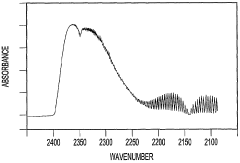
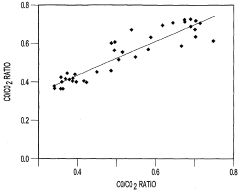
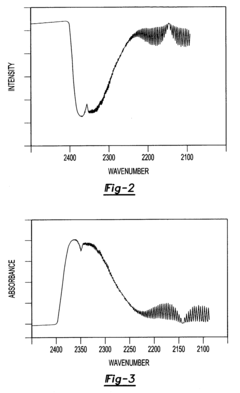
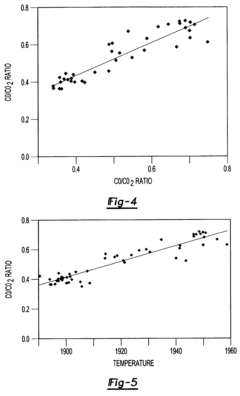
- A method that uses infrared radiation from the fluidized bed reactor to determine the ratio of infrared absorption intensity of components like carbon monoxide and carbon dioxide or carbonyl sulfide and sulfur dioxide without sampling, by directing infrared radiation through the gaseous products to an infrared spectrometer, analyzing specific wavenumbers for absorption intensity, and calculating concentration ratios using calibration factors.
Method for the analysis of gas produced by a titanium tetrachloride fluidized bed reactor
PatentInactiveUS7183114B2
Innovation
- A method using infrared radiation from the hot fluidized bed to direct radiation through the reactor to an infrared spectrometer, determining absorption intensities at specific wavenumbers to calculate the ratio of components like carbon monoxide to carbon dioxide or carbonyl sulfide to sulfur dioxide without sampling, and using this ratio to control the reactor temperature by feedback mechanisms.
Environmental Impact of CCl4
Carbon tetrachloride (CCl4) has been recognized as a significant environmental pollutant due to its ozone-depleting properties and potential health hazards. The environmental impact of CCl4 is multifaceted, affecting various ecosystems and contributing to global atmospheric changes.
In the atmosphere, CCl4 acts as a potent ozone-depleting substance. When released, it rises to the stratosphere where it breaks down under ultraviolet radiation, releasing chlorine atoms that catalyze the destruction of ozone molecules. This process contributes to the depletion of the ozone layer, which is crucial for protecting life on Earth from harmful ultraviolet radiation. The long atmospheric lifetime of CCl4, estimated at 26 years, exacerbates its impact on stratospheric ozone.
CCl4 also contributes to global warming as a greenhouse gas. Although its global warming potential is lower than that of carbon dioxide on a per-molecule basis, its long atmospheric lifetime allows it to accumulate and persist in the atmosphere, amplifying its overall impact on climate change. The radiative forcing effect of CCl4 further adds to its role in altering global climate patterns.
In aquatic environments, CCl4 can contaminate both surface and groundwater sources. Its high volatility allows it to evaporate from surface waters, but it can also percolate through soil and rock formations to reach groundwater aquifers. Once in groundwater, CCl4 can persist for extended periods due to limited degradation processes in these environments. This persistence poses long-term risks to drinking water supplies and aquatic ecosystems.
Soil contamination by CCl4 is another significant environmental concern. When released into soil, CCl4 can volatilize into soil gas, potentially leading to vapor intrusion in buildings. It can also adsorb to soil particles or leach into groundwater, creating complex remediation challenges. The presence of CCl4 in soil can negatively impact soil microorganisms and plant life, disrupting local ecosystems and potentially entering the food chain.
The bioaccumulation potential of CCl4 in aquatic organisms is relatively low due to its high volatility. However, its presence in water bodies can still have acute and chronic effects on aquatic life, including fish, invertebrates, and algae. These effects can range from behavioral changes to reproductive impairment and, in severe cases, mortality.
Human exposure to CCl4 through environmental contamination is a significant health concern. Inhalation of CCl4 vapors or ingestion of contaminated water can lead to various health effects, including liver and kidney damage, central nervous system depression, and potential carcinogenicity. These health risks underscore the importance of monitoring and controlling CCl4 releases into the environment.
In the atmosphere, CCl4 acts as a potent ozone-depleting substance. When released, it rises to the stratosphere where it breaks down under ultraviolet radiation, releasing chlorine atoms that catalyze the destruction of ozone molecules. This process contributes to the depletion of the ozone layer, which is crucial for protecting life on Earth from harmful ultraviolet radiation. The long atmospheric lifetime of CCl4, estimated at 26 years, exacerbates its impact on stratospheric ozone.
CCl4 also contributes to global warming as a greenhouse gas. Although its global warming potential is lower than that of carbon dioxide on a per-molecule basis, its long atmospheric lifetime allows it to accumulate and persist in the atmosphere, amplifying its overall impact on climate change. The radiative forcing effect of CCl4 further adds to its role in altering global climate patterns.
In aquatic environments, CCl4 can contaminate both surface and groundwater sources. Its high volatility allows it to evaporate from surface waters, but it can also percolate through soil and rock formations to reach groundwater aquifers. Once in groundwater, CCl4 can persist for extended periods due to limited degradation processes in these environments. This persistence poses long-term risks to drinking water supplies and aquatic ecosystems.
Soil contamination by CCl4 is another significant environmental concern. When released into soil, CCl4 can volatilize into soil gas, potentially leading to vapor intrusion in buildings. It can also adsorb to soil particles or leach into groundwater, creating complex remediation challenges. The presence of CCl4 in soil can negatively impact soil microorganisms and plant life, disrupting local ecosystems and potentially entering the food chain.
The bioaccumulation potential of CCl4 in aquatic organisms is relatively low due to its high volatility. However, its presence in water bodies can still have acute and chronic effects on aquatic life, including fish, invertebrates, and algae. These effects can range from behavioral changes to reproductive impairment and, in severe cases, mortality.
Human exposure to CCl4 through environmental contamination is a significant health concern. Inhalation of CCl4 vapors or ingestion of contaminated water can lead to various health effects, including liver and kidney damage, central nervous system depression, and potential carcinogenicity. These health risks underscore the importance of monitoring and controlling CCl4 releases into the environment.
CCl4 Regulations and Standards
Carbon tetrachloride (CCl4) has been subject to increasingly stringent regulations and standards worldwide due to its harmful effects on human health and the environment. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of CCl4 since its inception in 1987. Signatories to the protocol have committed to eliminating the use of CCl4 in various applications, with exceptions for essential uses and as feedstock for other chemicals.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on CCl4 under the Clean Air Act and the Toxic Substances Control Act. The EPA has classified CCl4 as a hazardous air pollutant and a toxic chemical, requiring facilities to report releases and implement control measures. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for workers, setting the threshold at 10 parts per million (ppm) for an 8-hour time-weighted average.
The European Union has also taken significant steps to regulate CCl4. The REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation has placed CCl4 on the list of substances of very high concern, subjecting it to authorization requirements. The EU has set a maximum contaminant level for CCl4 in drinking water at 3 μg/L, which is more stringent than the World Health Organization's guideline value of 4 μg/L.
In response to these regulations, analytical techniques for CCl4 detection and quantification have evolved to meet the demands of lower detection limits and higher accuracy. Standard methods for CCl4 analysis in various matrices have been developed by organizations such as the EPA, ASTM International, and ISO. These methods typically involve gas chromatography coupled with mass spectrometry (GC-MS) or electron capture detection (GC-ECD) for high sensitivity and specificity.
The push for more stringent regulations has also driven the development of real-time monitoring systems and portable analytical devices for CCl4 detection. These advancements aim to improve workplace safety and environmental monitoring capabilities. As global efforts to reduce CCl4 emissions continue, it is likely that regulations and standards will become even more stringent, necessitating further improvements in analytical techniques to ensure compliance and protect public health and the environment.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on CCl4 under the Clean Air Act and the Toxic Substances Control Act. The EPA has classified CCl4 as a hazardous air pollutant and a toxic chemical, requiring facilities to report releases and implement control measures. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for workers, setting the threshold at 10 parts per million (ppm) for an 8-hour time-weighted average.
The European Union has also taken significant steps to regulate CCl4. The REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation has placed CCl4 on the list of substances of very high concern, subjecting it to authorization requirements. The EU has set a maximum contaminant level for CCl4 in drinking water at 3 μg/L, which is more stringent than the World Health Organization's guideline value of 4 μg/L.
In response to these regulations, analytical techniques for CCl4 detection and quantification have evolved to meet the demands of lower detection limits and higher accuracy. Standard methods for CCl4 analysis in various matrices have been developed by organizations such as the EPA, ASTM International, and ISO. These methods typically involve gas chromatography coupled with mass spectrometry (GC-MS) or electron capture detection (GC-ECD) for high sensitivity and specificity.
The push for more stringent regulations has also driven the development of real-time monitoring systems and portable analytical devices for CCl4 detection. These advancements aim to improve workplace safety and environmental monitoring capabilities. As global efforts to reduce CCl4 emissions continue, it is likely that regulations and standards will become even more stringent, necessitating further improvements in analytical techniques to ensure compliance and protect public health and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!