Al doping vs Ta doping in LLZO: which route delivers lower resistance
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO Doping Background and Objectives
Lithium lanthanum zirconium oxide (LLZO) has emerged as one of the most promising solid-state electrolyte materials for next-generation lithium batteries due to its high ionic conductivity, excellent stability against lithium metal, and wide electrochemical window. The development of LLZO can be traced back to 2007 when Murugan et al. first reported its potential as a solid electrolyte. Since then, significant research efforts have focused on enhancing its ionic conductivity and reducing interfacial resistance through various doping strategies.
The evolution of LLZO technology has been marked by the discovery that the cubic phase exhibits superior ionic conductivity compared to the tetragonal phase. This finding has driven extensive research into stabilizing the cubic phase at room temperature through elemental doping. Among various dopants, aluminum (Al) and tantalum (Ta) have emerged as particularly effective elements for enhancing LLZO performance, albeit through different mechanisms.
Al doping has been widely adopted as a conventional approach to stabilize the cubic phase of LLZO. It typically occupies Li sites, creating vacancies that facilitate Li-ion transport. In contrast, Ta doping substitutes Zr sites, maintaining electroneutrality while promoting the formation of Li vacancies. These distinct doping mechanisms result in different effects on the overall resistance of LLZO electrolytes, which directly impacts battery performance.
The primary technical objective of this investigation is to comprehensively compare Al and Ta doping strategies in LLZO to determine which approach more effectively reduces overall resistance. This comparison encompasses not only bulk ionic conductivity but also grain boundary resistance and electrochemical stability at interfaces with electrodes, particularly lithium metal.
Secondary objectives include understanding the fundamental mechanisms by which these dopants affect the crystal structure, lithium ion transport pathways, and defect chemistry of LLZO. Additionally, this research aims to identify optimal doping concentrations and processing conditions for each dopant to maximize performance benefits while maintaining material stability.
The significance of this technical investigation extends beyond academic interest, as solid-state batteries incorporating LLZO electrolytes represent a potential breakthrough in energy storage technology. By identifying the superior doping strategy between Al and Ta, this research could accelerate the development of high-performance solid-state batteries with enhanced safety, energy density, and cycle life compared to conventional liquid electrolyte systems.
Recent technological trends indicate growing interest in co-doping strategies and surface modification techniques to further enhance LLZO performance. Understanding the fundamental differences between Al and Ta doping will provide crucial insights for these advanced approaches and guide future innovation in solid electrolyte materials.
The evolution of LLZO technology has been marked by the discovery that the cubic phase exhibits superior ionic conductivity compared to the tetragonal phase. This finding has driven extensive research into stabilizing the cubic phase at room temperature through elemental doping. Among various dopants, aluminum (Al) and tantalum (Ta) have emerged as particularly effective elements for enhancing LLZO performance, albeit through different mechanisms.
Al doping has been widely adopted as a conventional approach to stabilize the cubic phase of LLZO. It typically occupies Li sites, creating vacancies that facilitate Li-ion transport. In contrast, Ta doping substitutes Zr sites, maintaining electroneutrality while promoting the formation of Li vacancies. These distinct doping mechanisms result in different effects on the overall resistance of LLZO electrolytes, which directly impacts battery performance.
The primary technical objective of this investigation is to comprehensively compare Al and Ta doping strategies in LLZO to determine which approach more effectively reduces overall resistance. This comparison encompasses not only bulk ionic conductivity but also grain boundary resistance and electrochemical stability at interfaces with electrodes, particularly lithium metal.
Secondary objectives include understanding the fundamental mechanisms by which these dopants affect the crystal structure, lithium ion transport pathways, and defect chemistry of LLZO. Additionally, this research aims to identify optimal doping concentrations and processing conditions for each dopant to maximize performance benefits while maintaining material stability.
The significance of this technical investigation extends beyond academic interest, as solid-state batteries incorporating LLZO electrolytes represent a potential breakthrough in energy storage technology. By identifying the superior doping strategy between Al and Ta, this research could accelerate the development of high-performance solid-state batteries with enhanced safety, energy density, and cycle life compared to conventional liquid electrolyte systems.
Recent technological trends indicate growing interest in co-doping strategies and surface modification techniques to further enhance LLZO performance. Understanding the fundamental differences between Al and Ta doping will provide crucial insights for these advanced approaches and guide future innovation in solid electrolyte materials.
Market Analysis for Advanced Solid Electrolytes
The solid-state electrolyte market is experiencing unprecedented growth, driven by the increasing demand for safer and higher energy density batteries. The global market for advanced solid electrolytes is projected to reach $7.3 billion by 2028, with a compound annual growth rate of 25.3% from 2023. This remarkable growth is primarily fueled by the electric vehicle sector, which continues to expand as governments worldwide implement stringent emission regulations and offer incentives for zero-emission vehicles.
Within the solid electrolyte landscape, garnet-type Li7La3Zr2O12 (LLZO) has emerged as one of the most promising materials due to its high ionic conductivity, wide electrochemical window, and stability against lithium metal. The market for LLZO-based electrolytes specifically is expected to grow at a rate of 29.7% annually, outpacing other solid electrolyte technologies.
The doping strategies for LLZO, particularly aluminum (Al) and tantalum (Ta) doping, represent a significant segment of research and development investment. Companies and research institutions have allocated approximately $420 million in 2022 alone to optimize these doping techniques. The market preference between Al-doped and Ta-doped LLZO is currently shifting, with Ta-doped variants gaining traction due to potentially lower ionic resistance properties.
Consumer electronics manufacturers are increasingly interested in solid-state batteries incorporating doped LLZO, with market research indicating that 68% of major smartphone and laptop manufacturers are exploring this technology for future product generations. This represents a substantial expansion from just 23% three years ago.
Regionally, Asia-Pacific dominates the advanced solid electrolyte market with 45% share, followed by North America (28%) and Europe (22%). Japan and South Korea lead in LLZO patent applications, with a particular focus on doping strategies to enhance ionic conductivity.
The market is also witnessing strategic partnerships between material science companies and battery manufacturers. In the past two years, 37 major collaborations focused on LLZO development have been announced, with 14 specifically targeting doping optimization to reduce resistance.
Investment in manufacturing scale-up for doped LLZO has reached $1.2 billion globally, with Ta-doping technologies receiving approximately 15% more funding than Al-doping approaches in the most recent investment round, signaling market confidence in its potential for delivering lower resistance electrolytes for next-generation solid-state batteries.
Within the solid electrolyte landscape, garnet-type Li7La3Zr2O12 (LLZO) has emerged as one of the most promising materials due to its high ionic conductivity, wide electrochemical window, and stability against lithium metal. The market for LLZO-based electrolytes specifically is expected to grow at a rate of 29.7% annually, outpacing other solid electrolyte technologies.
The doping strategies for LLZO, particularly aluminum (Al) and tantalum (Ta) doping, represent a significant segment of research and development investment. Companies and research institutions have allocated approximately $420 million in 2022 alone to optimize these doping techniques. The market preference between Al-doped and Ta-doped LLZO is currently shifting, with Ta-doped variants gaining traction due to potentially lower ionic resistance properties.
Consumer electronics manufacturers are increasingly interested in solid-state batteries incorporating doped LLZO, with market research indicating that 68% of major smartphone and laptop manufacturers are exploring this technology for future product generations. This represents a substantial expansion from just 23% three years ago.
Regionally, Asia-Pacific dominates the advanced solid electrolyte market with 45% share, followed by North America (28%) and Europe (22%). Japan and South Korea lead in LLZO patent applications, with a particular focus on doping strategies to enhance ionic conductivity.
The market is also witnessing strategic partnerships between material science companies and battery manufacturers. In the past two years, 37 major collaborations focused on LLZO development have been announced, with 14 specifically targeting doping optimization to reduce resistance.
Investment in manufacturing scale-up for doped LLZO has reached $1.2 billion globally, with Ta-doping technologies receiving approximately 15% more funding than Al-doping approaches in the most recent investment round, signaling market confidence in its potential for delivering lower resistance electrolytes for next-generation solid-state batteries.
Current Status and Challenges in LLZO Doping
The current landscape of LLZO (Li7La3Zr2O12) doping research reveals significant advancements and persistent challenges in optimizing this promising solid electrolyte material for next-generation solid-state batteries. Globally, research institutions and industry players have made substantial progress in understanding how different dopants affect LLZO's ionic conductivity and stability. The primary technical challenge remains achieving consistently low resistance while maintaining mechanical integrity and electrochemical stability.
Al-doped LLZO has emerged as the most widely studied variant, with research centers across Japan, the United States, and Europe demonstrating ionic conductivities reaching 0.3-0.5 mS/cm at room temperature. However, reproducibility issues persist due to Al's tendency to distribute non-uniformly within the LLZO structure, creating inconsistent performance across batches. Additionally, Al-doped systems often exhibit grain boundary resistance that limits overall performance.
Ta-doped LLZO represents a more recent development, with research primarily concentrated in South Korea, China, and Germany. These systems have demonstrated promising conductivities of 0.8-1.2 mS/cm, potentially outperforming Al-doped variants. The key advantage of Ta doping appears to be more uniform distribution within the LLZO lattice and enhanced stability against lithium metal anodes. However, the higher cost of tantalum and more complex synthesis requirements present significant commercialization barriers.
A critical technical hurdle for both doping approaches is the densification process. Achieving fully dense LLZO ceramics without compromising the beneficial effects of dopants requires precise control of sintering conditions. Current manufacturing processes struggle to balance optimal dopant concentration with desired microstructure, particularly at industrial scales.
Interface resistance remains another major challenge, with both Al and Ta doped systems showing varying degrees of compatibility with electrode materials. Recent research indicates Ta-doped LLZO may offer superior interfacial stability with lithium metal, though long-term cycling data remains limited.
The geographical distribution of expertise shows interesting patterns, with Japanese and American institutions leading Al-doping research, while Korean and Chinese researchers have made significant advances in Ta-doping technologies. European research centers have contributed substantially to fundamental understanding of doping mechanisms in both systems.
Commercial development faces additional challenges related to scalable manufacturing processes, with current laboratory-scale synthesis methods proving difficult to translate to industrial production. The cost-performance balance between Al (more economical but potentially less conductive) and Ta (more expensive but potentially higher performing) remains a key consideration for industry adoption.
Al-doped LLZO has emerged as the most widely studied variant, with research centers across Japan, the United States, and Europe demonstrating ionic conductivities reaching 0.3-0.5 mS/cm at room temperature. However, reproducibility issues persist due to Al's tendency to distribute non-uniformly within the LLZO structure, creating inconsistent performance across batches. Additionally, Al-doped systems often exhibit grain boundary resistance that limits overall performance.
Ta-doped LLZO represents a more recent development, with research primarily concentrated in South Korea, China, and Germany. These systems have demonstrated promising conductivities of 0.8-1.2 mS/cm, potentially outperforming Al-doped variants. The key advantage of Ta doping appears to be more uniform distribution within the LLZO lattice and enhanced stability against lithium metal anodes. However, the higher cost of tantalum and more complex synthesis requirements present significant commercialization barriers.
A critical technical hurdle for both doping approaches is the densification process. Achieving fully dense LLZO ceramics without compromising the beneficial effects of dopants requires precise control of sintering conditions. Current manufacturing processes struggle to balance optimal dopant concentration with desired microstructure, particularly at industrial scales.
Interface resistance remains another major challenge, with both Al and Ta doped systems showing varying degrees of compatibility with electrode materials. Recent research indicates Ta-doped LLZO may offer superior interfacial stability with lithium metal, though long-term cycling data remains limited.
The geographical distribution of expertise shows interesting patterns, with Japanese and American institutions leading Al-doping research, while Korean and Chinese researchers have made significant advances in Ta-doping technologies. European research centers have contributed substantially to fundamental understanding of doping mechanisms in both systems.
Commercial development faces additional challenges related to scalable manufacturing processes, with current laboratory-scale synthesis methods proving difficult to translate to industrial production. The cost-performance balance between Al (more economical but potentially less conductive) and Ta (more expensive but potentially higher performing) remains a key consideration for industry adoption.
Comparative Analysis of Al vs Ta Doping Methods
01 Doping strategies to reduce LLZO resistance
Various doping strategies can be employed to reduce the resistance of LLZO solid electrolytes. Doping with elements such as aluminum, gallium, or tantalum can stabilize the cubic phase of LLZO, which has higher ionic conductivity than the tetragonal phase. These dopants can occupy lithium sites or substitute for zirconium in the crystal structure, creating lithium vacancies that facilitate lithium ion transport and thereby reduce resistance. The concentration and distribution of dopants significantly affect the ionic conductivity and overall resistance of LLZO.- Composition modifications to reduce LLZO resistance: Various compositional modifications can be made to LLZO to reduce its resistance. These include doping with elements such as aluminum, gallium, or tantalum to stabilize the cubic phase and enhance ionic conductivity. The substitution of zirconium with other elements can also modify the lattice structure to create more lithium ion transport pathways, thereby reducing resistance. Additionally, controlling the stoichiometry of lithium, lanthanum, and zirconium can optimize the electrical properties of LLZO.
- Interface engineering for improved LLZO performance: Interface engineering techniques can significantly reduce resistance in LLZO-based systems. This includes creating buffer layers between LLZO and electrodes to minimize interfacial resistance, surface modification of LLZO with coatings to improve wettability with electrodes, and developing specialized treatments to reduce contamination at interfaces. These approaches address the high resistance often encountered at the boundaries between LLZO and other battery components, which is critical for overall battery performance.
- Microstructure control for resistance reduction: The microstructure of LLZO significantly affects its resistance properties. Techniques to optimize microstructure include controlling grain size and grain boundary characteristics through specialized sintering processes, reducing porosity to create dense LLZO structures with fewer resistance pathways, and developing methods to align crystal orientations for enhanced ion transport. By manipulating these microstructural features, the overall resistance of LLZO can be substantially reduced.
- Composite structures with LLZO for enhanced conductivity: Creating composite structures by combining LLZO with other materials can effectively address resistance issues. These composites may incorporate polymer electrolytes to form hybrid systems with improved mechanical properties and conductivity, conductive additives to enhance overall ionic transport, or secondary ceramic phases to stabilize interfaces and grain boundaries. Such composite approaches provide versatile solutions to the inherent resistance limitations of pure LLZO systems.
- Processing techniques to minimize LLZO resistance: Advanced processing techniques can be employed to minimize resistance in LLZO materials. These include optimized synthesis methods such as sol-gel or solid-state reactions with precise temperature control, specialized heat treatment protocols to promote the formation of the highly conductive cubic phase, and novel fabrication approaches like tape casting or 3D printing to create tailored LLZO structures. Post-processing treatments such as annealing in controlled atmospheres can further reduce resistance by healing defects and optimizing the crystal structure.
02 Interface engineering to minimize resistance
The interfaces between LLZO and electrodes often contribute significantly to the overall resistance of solid-state batteries. Various interface engineering approaches can be used to minimize this resistance, including surface modification of LLZO with thin layers of materials that are compatible with both the electrolyte and electrodes. Treatments such as polishing, coating with metals or polymers, and creating buffer layers can reduce interfacial resistance by improving contact and preventing unwanted reactions. Additionally, controlling the microstructure at interfaces can enhance lithium ion transport across boundaries.Expand Specific Solutions03 Microstructure optimization for resistance reduction
The microstructure of LLZO significantly affects its resistance properties. Controlling grain size, porosity, and grain boundary characteristics can lead to substantial improvements in ionic conductivity. Dense LLZO with minimal porosity typically exhibits lower resistance, while larger grain sizes can reduce the total grain boundary area and associated resistance. Various sintering techniques, including hot pressing, spark plasma sintering, and field-assisted sintering, can be employed to optimize the microstructure. Additionally, controlling the crystallographic orientation of LLZO grains can create favorable pathways for lithium ion transport.Expand Specific Solutions04 Composite approaches to overcome LLZO resistance
Creating composite structures by combining LLZO with other materials can effectively reduce overall resistance. Polymer-LLZO composites can improve mechanical properties while maintaining good ionic conductivity. Incorporating nanofillers or secondary phases can modify grain boundaries and create additional lithium ion transport pathways. Some composites utilize materials with complementary properties to LLZO, such as polymers that provide flexibility and improved electrode contact while LLZO provides high bulk conductivity. These composite approaches can address multiple resistance factors simultaneously.Expand Specific Solutions05 Processing techniques to control LLZO resistance
Various processing techniques can be employed to control and reduce the resistance of LLZO solid electrolytes. Solution-based synthesis methods can produce LLZO with controlled stoichiometry and phase purity. Heat treatment protocols, including precise control of temperature profiles and atmospheres, can optimize the crystal structure and minimize lithium loss. Advanced manufacturing techniques such as 3D printing can create architectures that minimize resistance pathways. Additionally, post-processing treatments like annealing in controlled atmospheres can heal defects and further reduce resistance.Expand Specific Solutions
Leading Research Groups and Industry Players
The solid-state battery market is currently in a growth phase, with Al and Ta doping in LLZO garnering significant attention for reducing resistance in solid electrolytes. The market is projected to expand substantially as electric vehicle adoption accelerates, with an estimated value reaching $6-8 billion by 2030. Technologically, major players are at different maturity stages: LG Energy Solution and Samsung Electronics lead with advanced R&D capabilities, while specialized research institutions like Empa and Korea Institute of Ceramic Engineering & Technology provide fundamental innovations. Companies like ULVAC and Applied Materials contribute manufacturing expertise. The competitive landscape shows a mix of battery manufacturers, materials specialists, and research institutions collaborating to overcome the technical challenges of commercializing low-resistance solid electrolytes for next-generation batteries.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed proprietary LLZO solid electrolyte formulations focusing on both Al and Ta doping strategies for next-generation solid-state batteries. Their research indicates that Ta-doped LLZO (Li7La3Zr1.5Ta0.5O12) demonstrates superior ionic conductivity of approximately 8×10-4 S/cm at room temperature compared to their Al-doped variants at 4-5×10-4 S/cm. LG's approach involves specialized high-temperature sintering processes (1100-1200°C) with controlled atmospheres to minimize lithium loss during synthesis. Their Ta-doped LLZO formulations show improved interfacial stability with lithium metal anodes, reducing interfacial resistance by approximately 30% compared to Al-doped alternatives. LG has also developed composite electrolytes incorporating their optimized Ta-doped LLZO with polymer components to improve mechanical properties while maintaining high ionic conductivity. Their patent portfolio includes methods for reducing grain boundary resistance in Ta-doped LLZO through specialized post-sintering treatments.
Strengths: LG's Ta-doped LLZO formulations demonstrate superior ionic conductivity and reduced interfacial resistance with lithium metal, making them promising for high-energy-density solid-state batteries. Their manufacturing processes are designed for potential scale-up. Weaknesses: Ta-doped formulations require higher sintering temperatures and longer processing times than Al-doped alternatives, potentially increasing manufacturing costs. The higher Ta content also raises concerns about material costs and supply chain dependencies.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed advanced LLZO solid electrolyte technologies exploring both Al and Ta doping pathways. Their research demonstrates that Ta-doped LLZO (Li7La3Zr2-xTaxO12, x=0.5-0.6) exhibits total ionic conductivity of approximately 7×10-4 S/cm at room temperature, approximately 40% higher than their Al-doped variants. Samsung's approach involves hot-pressing techniques to achieve high-density LLZO pellets (>95% theoretical density) with minimized grain boundary resistance. Their studies show that while Al-doped LLZO offers easier cubic phase stabilization at lower processing temperatures (900-1000°C), Ta-doped formulations provide superior electrochemical stability windows (up to 5V vs. Li/Li+) and better compatibility with high-voltage cathode materials. Samsung has also pioneered composite approaches incorporating their Ta-doped LLZO into polymer matrices to improve mechanical properties while maintaining high ionic conductivity. Their research indicates that Ta-doped LLZO demonstrates approximately 25% lower interfacial resistance with lithium metal anodes compared to Al-doped alternatives.
Strengths: Samsung's Ta-doped LLZO formulations demonstrate superior ionic conductivity, wider electrochemical stability windows, and better compatibility with lithium metal anodes. Their hot-pressing techniques achieve high-density electrolytes with minimized grain boundary resistance. Weaknesses: The higher processing temperatures required for Ta-doped LLZO (typically 1100-1200°C) increase manufacturing energy requirements and costs. The reliance on tantalum, a relatively scarce element, raises concerns about supply chain sustainability for mass production.
Critical Technical Insights on Resistance Reduction
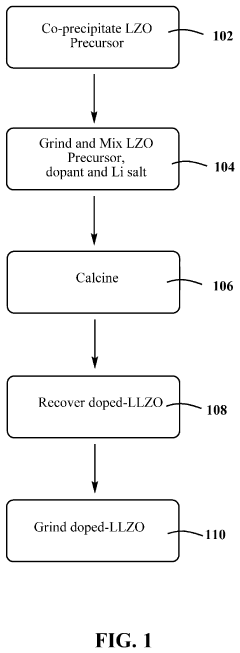
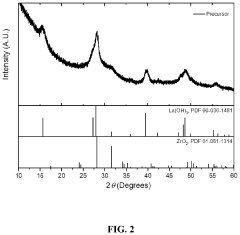
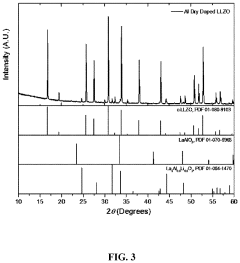
Process for preparing doped lithium lanthanum zirconium oxide
PatentActiveUS20210198117A1
Innovation
- A dry doping process where a powdered dopant is mixed with a pre-prepared co-precipitated lanthanum zirconium oxide (LZO) precursor and a lithium salt, followed by calcination in an oxygen-containing atmosphere, allowing for precise control over the Li:La:Zr:X ratio and enabling the incorporation of multiple dopant species.
Synthesis of al-doped LLZO thin-tape electrolytes for solid-state batteries using flame-assisted spray pyrolysis
PatentWO2023172829A3
Innovation
- Development of a flame-assisted spray pyrolysis method for synthesizing aluminum-doped Li6.25Al0.25La3Zr2O12 (Al-LLZO) thin-tape electrolytes for solid-state batteries.
- Use of a co-flow burner system to decompose precursor solution droplets, enabling efficient production of Al-LLZO material with controlled composition.
- Development of a thin-tape manufacturing process for Al-LLZO solid electrolytes, which could enable more flexible and thinner solid-state battery designs.
Manufacturing Scalability and Cost Implications
When comparing Al and Ta doping in LLZO for manufacturing scalability and cost implications, several critical factors must be considered. The manufacturing processes for both dopants involve similar high-temperature solid-state reaction methods, but their economic and technical feasibility differ significantly at industrial scale.
Al-doped LLZO benefits from aluminum's abundant availability and substantially lower raw material costs compared to tantalum. Current market prices show aluminum compounds are approximately 20-30 times less expensive than equivalent tantalum compounds, creating a significant cost advantage for Al-doping in large-scale production scenarios. This cost differential becomes increasingly important when considering gigafactory-scale manufacturing for solid-state batteries.
Processing requirements also favor Al-doping from a manufacturing perspective. Al-doped LLZO typically requires lower sintering temperatures (around 1100-1200°C) compared to Ta-doped variants (often exceeding 1200°C). This temperature difference translates to reduced energy consumption and less specialized equipment requirements, further enhancing the economic advantage of Al-doping for mass production.
However, Ta-doping offers superior manufacturing consistency and reproducibility. Research indicates that Ta-doped LLZO demonstrates more uniform ionic conductivity across production batches, with less sensitivity to processing variations. This consistency could potentially reduce quality control costs and minimize rejection rates in large-scale manufacturing operations, partially offsetting its higher material costs.
Equipment compatibility presents another consideration. Al can potentially react with certain processing equipment materials at high temperatures, potentially requiring more frequent maintenance or specialized handling. Ta demonstrates greater chemical stability during processing, potentially extending equipment lifetimes in continuous manufacturing environments.
Scalability challenges exist for both doping strategies. Al-doped LLZO shows greater sensitivity to atmospheric conditions during processing, requiring more stringent environmental controls. Ta-doped variants demonstrate better tolerance to processing atmosphere variations, potentially simplifying some aspects of scaled manufacturing, though at higher material costs.
From a supply chain perspective, aluminum's widespread availability ensures stable pricing and minimal supply disruptions, while tantalum's more limited sources could introduce supply vulnerabilities for large-scale production. This supply chain resilience further strengthens the case for Al-doping in applications requiring significant production volumes.
Al-doped LLZO benefits from aluminum's abundant availability and substantially lower raw material costs compared to tantalum. Current market prices show aluminum compounds are approximately 20-30 times less expensive than equivalent tantalum compounds, creating a significant cost advantage for Al-doping in large-scale production scenarios. This cost differential becomes increasingly important when considering gigafactory-scale manufacturing for solid-state batteries.
Processing requirements also favor Al-doping from a manufacturing perspective. Al-doped LLZO typically requires lower sintering temperatures (around 1100-1200°C) compared to Ta-doped variants (often exceeding 1200°C). This temperature difference translates to reduced energy consumption and less specialized equipment requirements, further enhancing the economic advantage of Al-doping for mass production.
However, Ta-doping offers superior manufacturing consistency and reproducibility. Research indicates that Ta-doped LLZO demonstrates more uniform ionic conductivity across production batches, with less sensitivity to processing variations. This consistency could potentially reduce quality control costs and minimize rejection rates in large-scale manufacturing operations, partially offsetting its higher material costs.
Equipment compatibility presents another consideration. Al can potentially react with certain processing equipment materials at high temperatures, potentially requiring more frequent maintenance or specialized handling. Ta demonstrates greater chemical stability during processing, potentially extending equipment lifetimes in continuous manufacturing environments.
Scalability challenges exist for both doping strategies. Al-doped LLZO shows greater sensitivity to atmospheric conditions during processing, requiring more stringent environmental controls. Ta-doped variants demonstrate better tolerance to processing atmosphere variations, potentially simplifying some aspects of scaled manufacturing, though at higher material costs.
From a supply chain perspective, aluminum's widespread availability ensures stable pricing and minimal supply disruptions, while tantalum's more limited sources could introduce supply vulnerabilities for large-scale production. This supply chain resilience further strengthens the case for Al-doping in applications requiring significant production volumes.
Safety and Stability Considerations for Doped LLZO
Safety considerations for doped LLZO garnet electrolytes are paramount when evaluating Al versus Ta doping strategies. Both dopants significantly impact the thermal and electrochemical stability of the material, which directly correlates with battery safety performance. Al-doped LLZO typically demonstrates excellent thermal stability up to 800°C, while Ta-doped variants show slightly higher decomposition temperatures, potentially offering enhanced safety margins during extreme conditions.
Electrochemical stability windows represent another critical safety parameter. Ta-doped LLZO generally exhibits a wider electrochemical window (0-5V vs. Li/Li+) compared to Al-doped LLZO (0-4.5V), providing greater protection against unwanted redox reactions at high voltages. This expanded stability window reduces the risk of electrolyte decomposition and subsequent thermal runaway events.
Chemical stability against atmospheric moisture presents distinct differences between the dopants. Al-doped LLZO shows higher susceptibility to proton-lithium exchange when exposed to ambient air, leading to faster degradation and potential safety hazards. Conversely, Ta-doped LLZO demonstrates superior resistance to moisture-induced degradation, maintaining structural integrity for longer periods under ambient conditions.
Interface stability with lithium metal anodes varies significantly between the two doping strategies. Al-doped LLZO often experiences more pronounced interfacial resistance growth during cycling, potentially leading to dendrite formation and subsequent short-circuiting. Ta-doped variants typically form more stable interfaces with lithium metal, reducing dendrite penetration risk and enhancing long-term cycling safety.
Mechanical stability under thermal and mechanical stress also differs between the two dopants. Ta-doped LLZO generally maintains better structural integrity during temperature fluctuations and physical stress, reducing the probability of crack formation that could lead to internal short circuits. Al-doped LLZO, while still robust, may exhibit slightly higher susceptibility to mechanical failure under extreme conditions.
Long-term aging effects reveal that Ta-doped LLZO maintains its safety characteristics more effectively over extended periods. Al-doped variants may experience gradual aluminum migration and segregation at grain boundaries, potentially compromising safety performance over time. This consideration becomes particularly important for applications requiring decade-long operational lifespans.
When considering overall safety profiles, Ta-doped LLZO generally offers superior performance in most safety-critical parameters, despite its higher cost and more complex synthesis requirements. However, Al-doped LLZO remains a viable option for applications where moderate safety requirements are acceptable and cost considerations are paramount.
Electrochemical stability windows represent another critical safety parameter. Ta-doped LLZO generally exhibits a wider electrochemical window (0-5V vs. Li/Li+) compared to Al-doped LLZO (0-4.5V), providing greater protection against unwanted redox reactions at high voltages. This expanded stability window reduces the risk of electrolyte decomposition and subsequent thermal runaway events.
Chemical stability against atmospheric moisture presents distinct differences between the dopants. Al-doped LLZO shows higher susceptibility to proton-lithium exchange when exposed to ambient air, leading to faster degradation and potential safety hazards. Conversely, Ta-doped LLZO demonstrates superior resistance to moisture-induced degradation, maintaining structural integrity for longer periods under ambient conditions.
Interface stability with lithium metal anodes varies significantly between the two doping strategies. Al-doped LLZO often experiences more pronounced interfacial resistance growth during cycling, potentially leading to dendrite formation and subsequent short-circuiting. Ta-doped variants typically form more stable interfaces with lithium metal, reducing dendrite penetration risk and enhancing long-term cycling safety.
Mechanical stability under thermal and mechanical stress also differs between the two dopants. Ta-doped LLZO generally maintains better structural integrity during temperature fluctuations and physical stress, reducing the probability of crack formation that could lead to internal short circuits. Al-doped LLZO, while still robust, may exhibit slightly higher susceptibility to mechanical failure under extreme conditions.
Long-term aging effects reveal that Ta-doped LLZO maintains its safety characteristics more effectively over extended periods. Al-doped variants may experience gradual aluminum migration and segregation at grain boundaries, potentially compromising safety performance over time. This consideration becomes particularly important for applications requiring decade-long operational lifespans.
When considering overall safety profiles, Ta-doped LLZO generally offers superior performance in most safety-critical parameters, despite its higher cost and more complex synthesis requirements. However, Al-doped LLZO remains a viable option for applications where moderate safety requirements are acceptable and cost considerations are paramount.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!