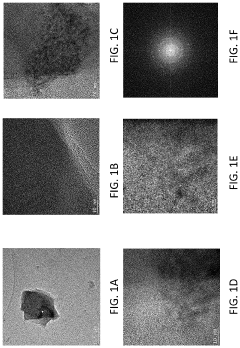
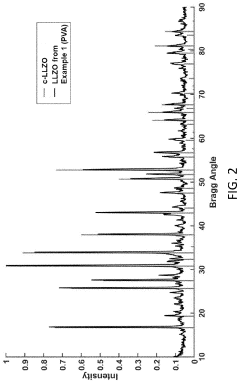
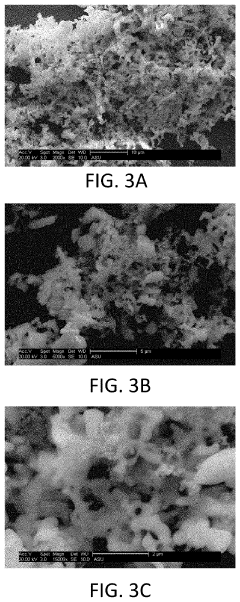
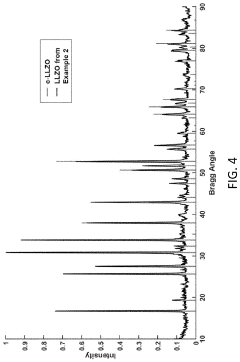
Cubic vs tetragonal LLZO: phase stability and ionic conductivity
AUG 25, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO Phase Stability Background and Research Objectives
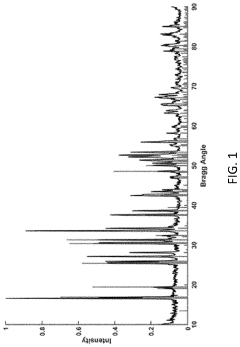
Lithium lanthanum zirconate (LLZO) has emerged as one of the most promising solid-state electrolyte materials for next-generation lithium-ion batteries due to its high ionic conductivity, excellent stability against lithium metal, and wide electrochemical window. The crystalline structure of LLZO exists primarily in two polymorphs: cubic and tetragonal phases, which exhibit significantly different lithium-ion conductivity properties.
The tetragonal phase of LLZO was first reported in 2007, demonstrating a conductivity of approximately 10^-6 S/cm at room temperature. Shortly thereafter, researchers discovered that doping LLZO with elements such as aluminum, gallium, or tantalum could stabilize the cubic phase, which exhibits ionic conductivity values two to three orders of magnitude higher (10^-3 to 10^-4 S/cm). This dramatic difference in conductivity has driven extensive research into understanding the phase stability mechanisms and optimizing the cubic phase for practical applications.
The phase transition between cubic and tetragonal LLZO occurs at approximately 630°C, with the high-temperature cubic phase being thermodynamically favored above this temperature. However, the cubic phase can be stabilized at room temperature through careful control of synthesis conditions and appropriate doping strategies. The stabilization mechanism involves the creation of lithium vacancies in the lattice structure, which facilitates the formation of the more conductive cubic phase.
Recent computational and experimental studies have revealed that the distribution and ordering of lithium ions and vacancies within the LLZO framework play crucial roles in determining phase stability. The tetragonal phase features ordered lithium arrangements, while the cubic phase exhibits disordered lithium distributions, creating more pathways for lithium-ion migration and thus enhancing ionic conductivity.
The primary research objectives in this field include developing reproducible synthesis methods for phase-pure cubic LLZO, understanding the fundamental mechanisms governing phase stability, and optimizing dopant concentrations to maximize ionic conductivity while maintaining mechanical and chemical stability. Additionally, researchers aim to elucidate the relationship between local structure, defect chemistry, and macroscopic transport properties to design improved solid electrolytes.
Another critical research direction involves investigating the impact of processing conditions, such as sintering temperature, atmosphere, and cooling rates, on the resultant LLZO phase and microstructure. These factors significantly influence grain boundary properties, which can act as bottlenecks for overall ionic conductivity in polycrystalline samples.
Understanding and controlling the phase stability of LLZO represents a fundamental challenge that must be overcome to enable the commercial deployment of solid-state batteries with enhanced safety and energy density compared to conventional liquid electrolyte systems.
The tetragonal phase of LLZO was first reported in 2007, demonstrating a conductivity of approximately 10^-6 S/cm at room temperature. Shortly thereafter, researchers discovered that doping LLZO with elements such as aluminum, gallium, or tantalum could stabilize the cubic phase, which exhibits ionic conductivity values two to three orders of magnitude higher (10^-3 to 10^-4 S/cm). This dramatic difference in conductivity has driven extensive research into understanding the phase stability mechanisms and optimizing the cubic phase for practical applications.
The phase transition between cubic and tetragonal LLZO occurs at approximately 630°C, with the high-temperature cubic phase being thermodynamically favored above this temperature. However, the cubic phase can be stabilized at room temperature through careful control of synthesis conditions and appropriate doping strategies. The stabilization mechanism involves the creation of lithium vacancies in the lattice structure, which facilitates the formation of the more conductive cubic phase.
Recent computational and experimental studies have revealed that the distribution and ordering of lithium ions and vacancies within the LLZO framework play crucial roles in determining phase stability. The tetragonal phase features ordered lithium arrangements, while the cubic phase exhibits disordered lithium distributions, creating more pathways for lithium-ion migration and thus enhancing ionic conductivity.
The primary research objectives in this field include developing reproducible synthesis methods for phase-pure cubic LLZO, understanding the fundamental mechanisms governing phase stability, and optimizing dopant concentrations to maximize ionic conductivity while maintaining mechanical and chemical stability. Additionally, researchers aim to elucidate the relationship between local structure, defect chemistry, and macroscopic transport properties to design improved solid electrolytes.
Another critical research direction involves investigating the impact of processing conditions, such as sintering temperature, atmosphere, and cooling rates, on the resultant LLZO phase and microstructure. These factors significantly influence grain boundary properties, which can act as bottlenecks for overall ionic conductivity in polycrystalline samples.
Understanding and controlling the phase stability of LLZO represents a fundamental challenge that must be overcome to enable the commercial deployment of solid-state batteries with enhanced safety and energy density compared to conventional liquid electrolyte systems.
Market Analysis for LLZO-Based Solid Electrolytes
The global market for LLZO-based solid electrolytes is experiencing significant growth, driven primarily by the increasing demand for safer and higher-energy-density batteries. The market size for solid-state batteries, where LLZO serves as a critical component, is projected to reach $8.7 billion by 2027, with a compound annual growth rate of 34.2% from 2022 to 2027.
The automotive sector represents the largest market segment for LLZO-based electrolytes, accounting for approximately 45% of the total market share. Major automotive manufacturers are investing heavily in solid-state battery technology to overcome the limitations of conventional lithium-ion batteries, particularly regarding safety concerns and energy density requirements for electric vehicles.
Consumer electronics constitutes the second-largest market segment, representing about 30% of the market. The demand for longer-lasting, faster-charging, and safer batteries in smartphones, laptops, and wearable devices is driving the adoption of solid-state battery technology in this sector.
Market analysis indicates that the cubic phase LLZO garners significantly more commercial interest than the tetragonal phase due to its superior ionic conductivity. Companies developing LLZO-based products are predominantly focusing on stabilizing the cubic phase at room temperature, with several patents filed for doping strategies that enhance phase stability.
Regional analysis shows that Asia-Pacific dominates the LLZO market with 52% market share, led by Japan and South Korea where major battery manufacturers have established solid-state battery production facilities. North America follows with 28% market share, while Europe accounts for 17%.
The market faces several challenges, including high production costs and scalability issues. The current cost of LLZO-based electrolytes is approximately 5-7 times higher than conventional liquid electrolytes, primarily due to complex synthesis processes and the need for high-purity materials.
Supply chain analysis reveals potential bottlenecks in the availability of lithium and zirconium, with concerns about sustainable sourcing. Several mining companies are expanding their operations to meet the anticipated demand growth for these materials.
Market forecasts suggest that as manufacturing processes mature and economies of scale are achieved, the cost of LLZO-based electrolytes will decrease by approximately 40% over the next five years, potentially accelerating market adoption. The development of more efficient methods to stabilize cubic LLZO at room temperature without expensive dopants could serve as a significant market catalyst.
The automotive sector represents the largest market segment for LLZO-based electrolytes, accounting for approximately 45% of the total market share. Major automotive manufacturers are investing heavily in solid-state battery technology to overcome the limitations of conventional lithium-ion batteries, particularly regarding safety concerns and energy density requirements for electric vehicles.
Consumer electronics constitutes the second-largest market segment, representing about 30% of the market. The demand for longer-lasting, faster-charging, and safer batteries in smartphones, laptops, and wearable devices is driving the adoption of solid-state battery technology in this sector.
Market analysis indicates that the cubic phase LLZO garners significantly more commercial interest than the tetragonal phase due to its superior ionic conductivity. Companies developing LLZO-based products are predominantly focusing on stabilizing the cubic phase at room temperature, with several patents filed for doping strategies that enhance phase stability.
Regional analysis shows that Asia-Pacific dominates the LLZO market with 52% market share, led by Japan and South Korea where major battery manufacturers have established solid-state battery production facilities. North America follows with 28% market share, while Europe accounts for 17%.
The market faces several challenges, including high production costs and scalability issues. The current cost of LLZO-based electrolytes is approximately 5-7 times higher than conventional liquid electrolytes, primarily due to complex synthesis processes and the need for high-purity materials.
Supply chain analysis reveals potential bottlenecks in the availability of lithium and zirconium, with concerns about sustainable sourcing. Several mining companies are expanding their operations to meet the anticipated demand growth for these materials.
Market forecasts suggest that as manufacturing processes mature and economies of scale are achieved, the cost of LLZO-based electrolytes will decrease by approximately 40% over the next five years, potentially accelerating market adoption. The development of more efficient methods to stabilize cubic LLZO at room temperature without expensive dopants could serve as a significant market catalyst.
Current Challenges in Cubic-Tetragonal LLZO Development
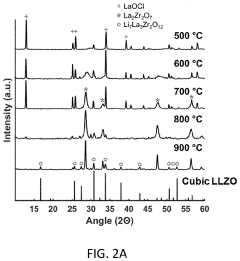
Despite significant advancements in LLZO solid electrolyte research, several critical challenges persist in the development and practical application of cubic and tetragonal LLZO phases. The phase stability between cubic and tetragonal LLZO remains a fundamental issue, as the high-conductivity cubic phase is metastable at room temperature without dopants. Researchers continue to struggle with precise control over phase transformation mechanisms, particularly during synthesis and subsequent processing steps.
Temperature sensitivity presents another significant challenge, as the cubic-to-tetragonal phase transition can occur during thermal cycling in battery operation. This transition is accompanied by a substantial volume change (approximately 2%), which can lead to mechanical stress, interfacial delamination, and ultimately performance degradation in solid-state batteries.
The ionic conductivity gap between the two phases (cubic: 10^-4 to 10^-3 S/cm; tetragonal: 10^-6 S/cm) creates a technological barrier that must be overcome for practical applications. Even minor tetragonal phase formation in predominantly cubic LLZO can create high-resistance pathways that significantly impair overall ionic transport properties.
Dopant optimization remains complex, with researchers still seeking the ideal combination and concentration of dopants (Al, Ga, Ta, Nb) to stabilize the cubic phase without compromising other properties. The distribution homogeneity of these dopants throughout the LLZO structure presents manufacturing challenges at scale, often resulting in localized phase variations and conductivity inconsistencies.
Interface stability issues are particularly problematic, as the cubic-tetragonal phase boundary can serve as a site for lithium dendrite nucleation and growth. This significantly increases the risk of internal short circuits in solid-state batteries incorporating LLZO electrolytes.
Manufacturing scalability presents substantial hurdles, as current laboratory-scale synthesis methods that successfully produce phase-pure cubic LLZO often prove difficult to scale up while maintaining phase purity and performance. The high sintering temperatures required (typically >1100°C) complicate mass production and increase manufacturing costs.
Long-term cycling stability remains inadequately understood, with limited data available on how repeated lithium transport affects the phase stability of LLZO over thousands of cycles. Early evidence suggests gradual phase transformation may occur during extended cycling, potentially limiting battery lifespan.
Environmental factors such as moisture sensitivity further complicate development efforts, as LLZO can react with atmospheric moisture to form lithium hydroxide and carbonate surface layers that impede ionic conductivity and promote undesired phase transformations.
Temperature sensitivity presents another significant challenge, as the cubic-to-tetragonal phase transition can occur during thermal cycling in battery operation. This transition is accompanied by a substantial volume change (approximately 2%), which can lead to mechanical stress, interfacial delamination, and ultimately performance degradation in solid-state batteries.
The ionic conductivity gap between the two phases (cubic: 10^-4 to 10^-3 S/cm; tetragonal: 10^-6 S/cm) creates a technological barrier that must be overcome for practical applications. Even minor tetragonal phase formation in predominantly cubic LLZO can create high-resistance pathways that significantly impair overall ionic transport properties.
Dopant optimization remains complex, with researchers still seeking the ideal combination and concentration of dopants (Al, Ga, Ta, Nb) to stabilize the cubic phase without compromising other properties. The distribution homogeneity of these dopants throughout the LLZO structure presents manufacturing challenges at scale, often resulting in localized phase variations and conductivity inconsistencies.
Interface stability issues are particularly problematic, as the cubic-tetragonal phase boundary can serve as a site for lithium dendrite nucleation and growth. This significantly increases the risk of internal short circuits in solid-state batteries incorporating LLZO electrolytes.
Manufacturing scalability presents substantial hurdles, as current laboratory-scale synthesis methods that successfully produce phase-pure cubic LLZO often prove difficult to scale up while maintaining phase purity and performance. The high sintering temperatures required (typically >1100°C) complicate mass production and increase manufacturing costs.
Long-term cycling stability remains inadequately understood, with limited data available on how repeated lithium transport affects the phase stability of LLZO over thousands of cycles. Early evidence suggests gradual phase transformation may occur during extended cycling, potentially limiting battery lifespan.
Environmental factors such as moisture sensitivity further complicate development efforts, as LLZO can react with atmospheric moisture to form lithium hydroxide and carbonate surface layers that impede ionic conductivity and promote undesired phase transformations.
Comparative Analysis of Cubic vs Tetragonal LLZO Properties
01 Doping strategies for enhancing LLZO ionic conductivity
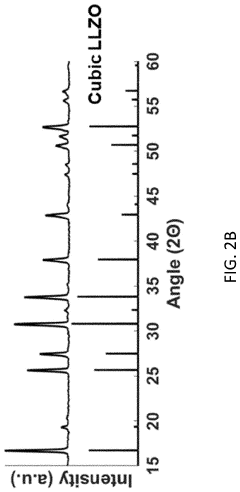
Various dopants can be incorporated into LLZO to stabilize the cubic phase and enhance ionic conductivity. Elements such as Al, Ga, Ta, and Nb are commonly used to substitute for Li or Zr sites, creating lithium vacancies and stabilizing the high-conductivity cubic structure. These dopants can significantly increase room temperature ionic conductivity from 10^-6 S/cm (tetragonal phase) to 10^-3 S/cm (cubic phase), making LLZO more suitable for solid-state battery applications.- Doping strategies for LLZO phase stability: Various doping strategies can be employed to stabilize the cubic phase of LLZO, which has higher ionic conductivity than other phases. Dopants such as aluminum, gallium, and tantalum can substitute for lithium or zirconium sites in the LLZO structure, effectively stabilizing the cubic phase at room temperature. These dopants can suppress phase transitions and reduce the formation of grain boundaries, leading to improved ionic conductivity and electrochemical performance.
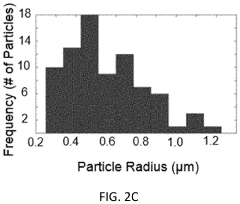
- Synthesis methods affecting LLZO phase composition: Different synthesis methods significantly impact the phase composition and stability of LLZO. Solid-state reactions, sol-gel processes, and hydrothermal methods each produce LLZO with varying phase purity and microstructures. Processing parameters such as sintering temperature, duration, and atmosphere play crucial roles in determining whether tetragonal or cubic phases form. Advanced synthesis techniques can reduce impurity phases and promote the formation of the desired cubic phase with enhanced ionic conductivity.
- Grain boundary engineering for improved ionic conductivity: The ionic conductivity of LLZO is significantly affected by grain boundaries, which often act as barriers to lithium ion transport. Engineering approaches to modify grain boundaries include controlling grain size, reducing porosity, and introducing specific additives that segregate at grain boundaries. These methods can decrease the resistance at grain boundaries and enhance the overall ionic conductivity of LLZO solid electrolytes, making them more suitable for high-performance solid-state batteries.
- Interface modification between LLZO and electrodes: The interface between LLZO solid electrolytes and electrodes plays a critical role in battery performance. Various interface modification strategies have been developed to improve contact and reduce interfacial resistance. These include surface coating of LLZO with materials like Al2O3 or LiPON, creating buffer layers, and developing composite interfaces. Such modifications can enhance the electrochemical stability of the interface, prevent side reactions, and maintain phase stability during battery operation.
- Advanced characterization techniques for LLZO phase analysis: Advanced characterization techniques are essential for understanding LLZO phase stability and ionic conductivity mechanisms. Methods such as synchrotron X-ray diffraction, neutron diffraction, and nuclear magnetic resonance spectroscopy provide detailed insights into crystal structure, phase transitions, and lithium ion transport pathways. In-situ and operando techniques allow for real-time monitoring of phase changes during battery operation, helping to identify degradation mechanisms and optimize electrolyte compositions for enhanced performance.
02 Synthesis methods affecting LLZO phase stability
The synthesis method significantly impacts LLZO phase stability and ionic conductivity. Techniques such as solid-state reaction, sol-gel processing, and hydrothermal synthesis yield different microstructures and phase compositions. High-temperature sintering (>1100°C) typically promotes cubic phase formation, while rapid cooling can preserve this high-conductivity phase. Advanced synthesis approaches like spark plasma sintering can reduce processing time while maintaining phase purity and achieving high relative density, which is crucial for optimal ionic conductivity.Expand Specific Solutions03 Grain boundary engineering for improved LLZO performance
Grain boundaries in LLZO significantly affect overall ionic conductivity. Engineering these interfaces through controlled sintering, grain size optimization, and surface treatments can reduce grain boundary resistance. Techniques such as hot pressing, addition of sintering aids, and creation of composite structures help minimize porosity and improve grain-to-grain contact. Reducing the Li+ transport resistance across grain boundaries is essential for achieving bulk-like conductivity in polycrystalline LLZO samples.Expand Specific Solutions04 Environmental stability and moisture sensitivity of LLZO
LLZO is sensitive to atmospheric conditions, particularly moisture and CO2, which can lead to Li+ exchange with H+ and formation of Li2CO3 on the surface. These reactions degrade ionic conductivity and interfacial stability. Protective coatings, surface treatments, and controlled storage environments can mitigate these effects. Understanding the mechanisms of environmental degradation is crucial for maintaining long-term stability and performance of LLZO in practical battery applications.Expand Specific Solutions05 Interface engineering between LLZO and electrodes
The interface between LLZO and electrodes (particularly lithium metal) is critical for battery performance. High interfacial resistance can limit overall battery efficiency despite high bulk conductivity of LLZO. Surface modifications, interlayers, and interface engineering techniques can improve wettability and reduce interfacial resistance. Methods such as thin film deposition, polishing, and chemical treatments help establish intimate contact between LLZO and electrodes, enhancing lithium ion transport across interfaces.Expand Specific Solutions
Key Scientific Breakthroughs in LLZO Ionic Conductivity
Synthesis of nanosized cubic lithium lanthanum zirconate fast ion conductor
PatentActiveUS20210403340A1
Innovation
- A molten salt synthesis method involving a reagent composition with a lithium:lanthanum:zirconium molar ratio of 7:3:2, using a salt composition like eutectic mixtures of potassium chloride and lithium chloride, and heating to stabilize the cubic phase of LLZO without extrinsic dopants, resulting in nanosized cubic LLZO with enhanced conductivity.
Preparation of nanosized cubic lithium lanthanum zirconate fast ion conductor via facile polymer-chelate combustion route
PatentActiveUS20210230013A1
Innovation
- Reducing the crystallite size of LLZO to nanometric dimensions stabilizes the cubic phase at low temperatures without extrinsic dopants, using a polymer-mediated synthesis involving organic compounds and inorganic salts, which lowers the sintering temperature and enhances conductivity.
Manufacturing Processes for High-Conductivity LLZO
Manufacturing high-conductivity LLZO requires precise control over phase formation, with cubic LLZO being the target phase due to its superior ionic conductivity compared to tetragonal LLZO. The manufacturing process typically begins with raw material selection and preparation, where high-purity Li2CO3, La2O3, and ZrO2 are combined with dopants such as Al, Ga, or Ta to stabilize the cubic phase.
Conventional solid-state reaction methods involve high-temperature calcination (800-900°C) followed by sintering at 1100-1200°C. This approach, while widely used, often results in lithium loss due to volatilization at elevated temperatures, which can destabilize the cubic phase and reduce conductivity. To mitigate this issue, excess lithium (typically 10-20%) is added to the initial composition, and sintering is performed in powder beds of similar composition.
Solution-based synthesis routes have emerged as promising alternatives, including sol-gel processing, co-precipitation, and hydrothermal methods. These techniques enable better mixing at the molecular level, resulting in more homogeneous precursors and lower processing temperatures. The sol-gel method, for instance, utilizes metal alkoxides or acetates dissolved in organic solvents, followed by controlled hydrolysis and condensation reactions.
Field-assisted sintering techniques (FAST), such as spark plasma sintering (SPS) and hot pressing, have demonstrated significant advantages in LLZO manufacturing. These methods apply pressure during sintering while using pulsed DC current or external heating to achieve rapid densification at lower temperatures and shorter times. SPS can produce dense cubic LLZO in minutes rather than hours, minimizing lithium loss and grain growth.
Atmosphere control during sintering plays a crucial role in phase stability. Oxygen-rich atmospheres help maintain lithium stoichiometry, while inert atmospheres may require additional measures to prevent lithium deficiency. Some advanced processes utilize two-step sintering protocols, with an initial high-temperature stage for densification followed by a lower-temperature stage for phase stabilization.
Post-sintering treatments, including annealing in controlled atmospheres and surface modifications, can further enhance ionic conductivity. Recent innovations include core-shell structures with protective coatings to improve chemical stability against air and moisture, as well as composite approaches incorporating secondary phases to enhance mechanical properties without sacrificing ionic conductivity.
The scalability of these manufacturing processes remains a significant challenge for commercial applications. While laboratory-scale production can achieve high-conductivity cubic LLZO, translating these processes to industrial scales while maintaining phase purity and conductivity represents an ongoing area of research and development in solid-state battery technology.
Conventional solid-state reaction methods involve high-temperature calcination (800-900°C) followed by sintering at 1100-1200°C. This approach, while widely used, often results in lithium loss due to volatilization at elevated temperatures, which can destabilize the cubic phase and reduce conductivity. To mitigate this issue, excess lithium (typically 10-20%) is added to the initial composition, and sintering is performed in powder beds of similar composition.
Solution-based synthesis routes have emerged as promising alternatives, including sol-gel processing, co-precipitation, and hydrothermal methods. These techniques enable better mixing at the molecular level, resulting in more homogeneous precursors and lower processing temperatures. The sol-gel method, for instance, utilizes metal alkoxides or acetates dissolved in organic solvents, followed by controlled hydrolysis and condensation reactions.
Field-assisted sintering techniques (FAST), such as spark plasma sintering (SPS) and hot pressing, have demonstrated significant advantages in LLZO manufacturing. These methods apply pressure during sintering while using pulsed DC current or external heating to achieve rapid densification at lower temperatures and shorter times. SPS can produce dense cubic LLZO in minutes rather than hours, minimizing lithium loss and grain growth.
Atmosphere control during sintering plays a crucial role in phase stability. Oxygen-rich atmospheres help maintain lithium stoichiometry, while inert atmospheres may require additional measures to prevent lithium deficiency. Some advanced processes utilize two-step sintering protocols, with an initial high-temperature stage for densification followed by a lower-temperature stage for phase stabilization.
Post-sintering treatments, including annealing in controlled atmospheres and surface modifications, can further enhance ionic conductivity. Recent innovations include core-shell structures with protective coatings to improve chemical stability against air and moisture, as well as composite approaches incorporating secondary phases to enhance mechanical properties without sacrificing ionic conductivity.
The scalability of these manufacturing processes remains a significant challenge for commercial applications. While laboratory-scale production can achieve high-conductivity cubic LLZO, translating these processes to industrial scales while maintaining phase purity and conductivity represents an ongoing area of research and development in solid-state battery technology.
Safety and Performance Standards for LLZO Electrolytes
The establishment of comprehensive safety and performance standards for LLZO electrolytes is critical for their successful implementation in solid-state batteries. Current standards focus primarily on liquid electrolytes, creating a significant gap in regulatory frameworks for solid-state alternatives like LLZO.
For cubic and tetragonal LLZO phases, distinct safety standards must be developed due to their different ionic conductivity properties and phase stability characteristics. The cubic phase, with its superior ionic conductivity, requires standards that ensure maintenance of this phase during battery operation across varying temperature ranges and mechanical stress conditions.
Performance standards for LLZO electrolytes should address minimum ionic conductivity thresholds, with cubic LLZO typically requiring conductivity values above 10^-4 S/cm at room temperature to be commercially viable. Tetragonal LLZO, with its lower conductivity, may be suitable for specific applications with different performance requirements.
Mechanical integrity standards are particularly important, as LLZO electrolytes must maintain structural stability during thermal cycling and under pressure. Testing protocols should evaluate resistance to fracture, deformation, and interfacial degradation, with cubic LLZO generally demonstrating superior mechanical properties compared to its tetragonal counterpart.
Chemical stability standards must address LLZO's interactions with electrode materials and atmospheric components. The cubic phase typically exhibits better chemical stability against lithium metal anodes, but standardized testing is needed to quantify this advantage across different operating conditions.
Manufacturing quality control standards should specify acceptable levels of impurities and dopants, as these significantly impact phase stability between cubic and tetragonal structures. Aluminum and gallium doping, which stabilize the cubic phase, require precise concentration standards to ensure consistent performance.
Accelerated aging tests must be standardized to predict long-term stability of both phases, with particular attention to phase transformation triggers that could convert cubic to tetragonal LLZO during operation, potentially causing catastrophic conductivity loss.
International harmonization of these standards is essential, with organizations like IEC, ISO, and ASTM developing unified testing protocols that specifically address the unique properties of cubic and tetragonal LLZO, enabling reliable comparison of research results and commercial products across the global battery industry.
For cubic and tetragonal LLZO phases, distinct safety standards must be developed due to their different ionic conductivity properties and phase stability characteristics. The cubic phase, with its superior ionic conductivity, requires standards that ensure maintenance of this phase during battery operation across varying temperature ranges and mechanical stress conditions.
Performance standards for LLZO electrolytes should address minimum ionic conductivity thresholds, with cubic LLZO typically requiring conductivity values above 10^-4 S/cm at room temperature to be commercially viable. Tetragonal LLZO, with its lower conductivity, may be suitable for specific applications with different performance requirements.
Mechanical integrity standards are particularly important, as LLZO electrolytes must maintain structural stability during thermal cycling and under pressure. Testing protocols should evaluate resistance to fracture, deformation, and interfacial degradation, with cubic LLZO generally demonstrating superior mechanical properties compared to its tetragonal counterpart.
Chemical stability standards must address LLZO's interactions with electrode materials and atmospheric components. The cubic phase typically exhibits better chemical stability against lithium metal anodes, but standardized testing is needed to quantify this advantage across different operating conditions.
Manufacturing quality control standards should specify acceptable levels of impurities and dopants, as these significantly impact phase stability between cubic and tetragonal structures. Aluminum and gallium doping, which stabilize the cubic phase, require precise concentration standards to ensure consistent performance.
Accelerated aging tests must be standardized to predict long-term stability of both phases, with particular attention to phase transformation triggers that could convert cubic to tetragonal LLZO during operation, potentially causing catastrophic conductivity loss.
International harmonization of these standards is essential, with organizations like IEC, ISO, and ASTM developing unified testing protocols that specifically address the unique properties of cubic and tetragonal LLZO, enabling reliable comparison of research results and commercial products across the global battery industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!