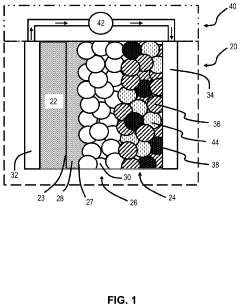
Interfacial layers between LLZO and Li: LIPON, Al₂O₃, and others
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO-Li Interface Technology Background and Objectives
The evolution of solid-state lithium batteries represents a significant advancement in energy storage technology, with the interface between lithium metal anodes and solid electrolytes being a critical area of focus. Garnet-type Li7La3Zr2O12 (LLZO) has emerged as one of the most promising solid electrolytes due to its high ionic conductivity, wide electrochemical stability window, and chemical stability against lithium metal. However, the interface between LLZO and lithium metal presents substantial challenges that have hindered the commercialization of these advanced battery systems.
The development of LLZO-based solid-state batteries can be traced back to 2007 when Murugan et al. first reported the high ionic conductivity of cubic LLZO. Since then, research has intensified on improving the properties of LLZO and addressing the interfacial issues with lithium metal. The primary technical goal in this field is to create stable, low-impedance interfaces between LLZO and lithium that can withstand repeated cycling while maintaining high ionic conductivity.
Interface engineering has become a central focus, with researchers exploring various interlayers to mitigate the challenges at the LLZO-Li interface. These challenges include poor physical contact, chemical instability, and the formation of high-resistance interfacial layers during cycling. The introduction of artificial interlayers such as LIPON (Lithium Phosphorus Oxynitride), Al2O3, and other materials represents a strategic approach to overcome these limitations.
The technical evolution in this field is moving toward multi-functional interlayers that can simultaneously address multiple interface issues. Recent trends indicate a growing interest in composite interlayers that combine the benefits of different materials to achieve optimal performance. Additionally, there is an increasing focus on scalable manufacturing techniques for these interlayers to facilitate the transition from laboratory research to commercial production.
The ultimate objective of research on LLZO-Li interfaces is to enable the development of high-energy-density, long-lasting, and safe solid-state batteries that can outperform conventional lithium-ion batteries. This includes achieving high current densities without dendrite formation, maintaining stable cycling over thousands of cycles, and ensuring compatibility with existing battery manufacturing processes.
As the energy storage landscape continues to evolve, the successful resolution of LLZO-Li interface challenges could revolutionize portable electronics, electric vehicles, and grid-scale energy storage systems. The technical trajectory suggests that breakthroughs in interface engineering will be pivotal in determining the timeline for widespread adoption of solid-state battery technology.
The development of LLZO-based solid-state batteries can be traced back to 2007 when Murugan et al. first reported the high ionic conductivity of cubic LLZO. Since then, research has intensified on improving the properties of LLZO and addressing the interfacial issues with lithium metal. The primary technical goal in this field is to create stable, low-impedance interfaces between LLZO and lithium that can withstand repeated cycling while maintaining high ionic conductivity.
Interface engineering has become a central focus, with researchers exploring various interlayers to mitigate the challenges at the LLZO-Li interface. These challenges include poor physical contact, chemical instability, and the formation of high-resistance interfacial layers during cycling. The introduction of artificial interlayers such as LIPON (Lithium Phosphorus Oxynitride), Al2O3, and other materials represents a strategic approach to overcome these limitations.
The technical evolution in this field is moving toward multi-functional interlayers that can simultaneously address multiple interface issues. Recent trends indicate a growing interest in composite interlayers that combine the benefits of different materials to achieve optimal performance. Additionally, there is an increasing focus on scalable manufacturing techniques for these interlayers to facilitate the transition from laboratory research to commercial production.
The ultimate objective of research on LLZO-Li interfaces is to enable the development of high-energy-density, long-lasting, and safe solid-state batteries that can outperform conventional lithium-ion batteries. This includes achieving high current densities without dendrite formation, maintaining stable cycling over thousands of cycles, and ensuring compatibility with existing battery manufacturing processes.
As the energy storage landscape continues to evolve, the successful resolution of LLZO-Li interface challenges could revolutionize portable electronics, electric vehicles, and grid-scale energy storage systems. The technical trajectory suggests that breakthroughs in interface engineering will be pivotal in determining the timeline for widespread adoption of solid-state battery technology.
Market Analysis for Solid-State Battery Applications
The global solid-state battery market is experiencing significant growth, projected to reach $8.7 billion by 2030, with a compound annual growth rate of 34.2% from 2023 to 2030. This remarkable expansion is primarily driven by the increasing demand for electric vehicles (EVs), consumer electronics, and renewable energy storage solutions. The interfacial layer technology between LLZO (Li7La3Zr2O12) and lithium metal represents a critical component in this market's development.
In the automotive sector, major manufacturers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery technology, with particular focus on improving interfacial stability. The EV segment alone is expected to account for approximately 65% of the solid-state battery market by 2028, highlighting the crucial importance of interface engineering solutions like LIPON and Al2O3 coatings.
Consumer electronics represents the second-largest application segment, with companies like Samsung, Apple, and LG actively researching solid-state batteries with enhanced interfacial layers to improve device safety and extend battery life. This segment values the higher energy density and reduced fire risk that stable LLZO-Li interfaces can provide.
The aerospace and defense sectors are emerging as premium markets for advanced solid-state batteries, where the superior safety characteristics of properly engineered interfacial layers command significant price premiums. Though smaller in volume, these applications generate substantial revenue due to their specialized requirements and performance demands.
Geographically, Asia-Pacific dominates the market with approximately 45% share, led by Japan, South Korea, and China, where significant research on interfacial engineering is concentrated. North America follows with 30% market share, with substantial research activities at national laboratories and universities focused specifically on LLZO-Li interfaces.
Market adoption faces several challenges, including high manufacturing costs associated with precision deposition of interfacial layers like LIPON and Al2O3. Current production costs for batteries with engineered interfaces remain 2.5-3 times higher than conventional lithium-ion batteries, though economies of scale are expected to reduce this premium to 1.3-1.5 times by 2027.
Customer willingness to pay for the benefits of solid-state technology varies by application. EV manufacturers have indicated acceptance of a 30-40% price premium for batteries that deliver 80% higher energy density and significantly improved safety through stable interfaces. Consumer electronics manufacturers show similar interest, particularly for premium devices where safety concerns can impact brand reputation.
In the automotive sector, major manufacturers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery technology, with particular focus on improving interfacial stability. The EV segment alone is expected to account for approximately 65% of the solid-state battery market by 2028, highlighting the crucial importance of interface engineering solutions like LIPON and Al2O3 coatings.
Consumer electronics represents the second-largest application segment, with companies like Samsung, Apple, and LG actively researching solid-state batteries with enhanced interfacial layers to improve device safety and extend battery life. This segment values the higher energy density and reduced fire risk that stable LLZO-Li interfaces can provide.
The aerospace and defense sectors are emerging as premium markets for advanced solid-state batteries, where the superior safety characteristics of properly engineered interfacial layers command significant price premiums. Though smaller in volume, these applications generate substantial revenue due to their specialized requirements and performance demands.
Geographically, Asia-Pacific dominates the market with approximately 45% share, led by Japan, South Korea, and China, where significant research on interfacial engineering is concentrated. North America follows with 30% market share, with substantial research activities at national laboratories and universities focused specifically on LLZO-Li interfaces.
Market adoption faces several challenges, including high manufacturing costs associated with precision deposition of interfacial layers like LIPON and Al2O3. Current production costs for batteries with engineered interfaces remain 2.5-3 times higher than conventional lithium-ion batteries, though economies of scale are expected to reduce this premium to 1.3-1.5 times by 2027.
Customer willingness to pay for the benefits of solid-state technology varies by application. EV manufacturers have indicated acceptance of a 30-40% price premium for batteries that deliver 80% higher energy density and significantly improved safety through stable interfaces. Consumer electronics manufacturers show similar interest, particularly for premium devices where safety concerns can impact brand reputation.
Current Challenges in LLZO-Li Interfacial Engineering
Despite significant advancements in solid-state battery technology, the interface between Li metal anodes and LLZO solid electrolytes remains a critical bottleneck for commercial viability. The high interfacial resistance at the LLZO-Li interface, typically ranging from 100-1000 Ω·cm², severely limits battery performance. This resistance stems from multiple factors including poor physical contact, chemical instability, and lithium dendrite formation.
Physical contact issues arise from LLZO's high surface energy and lithium's high reactivity with atmospheric components, creating contamination layers that impede ion transfer. Even with polished surfaces, achieving uniform contact across the entire interface remains challenging due to microscopic roughness and the rigid nature of ceramic electrolytes.
Chemical instability presents another significant challenge. When LLZO contacts lithium metal, interfacial reactions can form resistive layers containing Li₂CO₃, LiOH, and other compounds that impede ion transport. Additionally, the reduction of Zr⁴⁺ to lower valence states at the interface can create electronic conductivity pathways that compromise the electrolyte's function.
Lithium dendrite propagation through LLZO grain boundaries represents a persistent safety concern. These dendrites can eventually create short circuits, particularly at high current densities, limiting the practical charging rates of solid-state batteries. The mechanisms behind dendrite initiation and growth remain incompletely understood, complicating mitigation strategies.
Current interfacial engineering approaches face scalability challenges. Laboratory-scale techniques like vacuum deposition of interlayers or controlled atmosphere processing are difficult to implement in mass production environments. The cost-effectiveness of these solutions at industrial scale remains questionable, particularly for consumer electronics applications where price sensitivity is high.
Long-term stability of engineered interfaces presents another obstacle. While initial performance improvements have been demonstrated with various interlayers, their ability to maintain low resistance over hundreds or thousands of cycles under realistic operating conditions remains largely unproven. Degradation mechanisms at these modified interfaces are still being investigated.
The characterization of these buried interfaces presents methodological challenges. In-situ and operando techniques capable of monitoring interfacial evolution during battery operation are limited, making it difficult to fully understand degradation mechanisms and optimize interface designs. Advanced characterization methods that can probe these interfaces without disrupting them are urgently needed.
Physical contact issues arise from LLZO's high surface energy and lithium's high reactivity with atmospheric components, creating contamination layers that impede ion transfer. Even with polished surfaces, achieving uniform contact across the entire interface remains challenging due to microscopic roughness and the rigid nature of ceramic electrolytes.
Chemical instability presents another significant challenge. When LLZO contacts lithium metal, interfacial reactions can form resistive layers containing Li₂CO₃, LiOH, and other compounds that impede ion transport. Additionally, the reduction of Zr⁴⁺ to lower valence states at the interface can create electronic conductivity pathways that compromise the electrolyte's function.
Lithium dendrite propagation through LLZO grain boundaries represents a persistent safety concern. These dendrites can eventually create short circuits, particularly at high current densities, limiting the practical charging rates of solid-state batteries. The mechanisms behind dendrite initiation and growth remain incompletely understood, complicating mitigation strategies.
Current interfacial engineering approaches face scalability challenges. Laboratory-scale techniques like vacuum deposition of interlayers or controlled atmosphere processing are difficult to implement in mass production environments. The cost-effectiveness of these solutions at industrial scale remains questionable, particularly for consumer electronics applications where price sensitivity is high.
Long-term stability of engineered interfaces presents another obstacle. While initial performance improvements have been demonstrated with various interlayers, their ability to maintain low resistance over hundreds or thousands of cycles under realistic operating conditions remains largely unproven. Degradation mechanisms at these modified interfaces are still being investigated.
The characterization of these buried interfaces presents methodological challenges. In-situ and operando techniques capable of monitoring interfacial evolution during battery operation are limited, making it difficult to fully understand degradation mechanisms and optimize interface designs. Advanced characterization methods that can probe these interfaces without disrupting them are urgently needed.
Comparative Analysis of LIPON and Al₂O₃ Interfacial Solutions
01 LIPON as interfacial layer between LLZO and lithium
Lithium phosphorous oxynitride (LIPON) serves as an effective interfacial layer between LLZO solid electrolyte and lithium metal. This thin film coating helps mitigate interfacial resistance and prevents unwanted reactions at the LLZO-Li interface. LIPON provides excellent ionic conductivity while acting as a protective barrier, enhancing the overall stability and performance of solid-state batteries with LLZO electrolytes.- LIPON as interfacial layer between LLZO and lithium: Lithium phosphorous oxynitride (LIPON) serves as an effective interfacial layer between LLZO solid electrolyte and lithium metal. This thin film coating helps mitigate interfacial resistance and prevents direct contact between LLZO and lithium, enhancing electrochemical stability. LIPON creates a stable interface that prevents lithium dendrite formation and improves the cycling performance of solid-state batteries by facilitating smooth lithium ion transport across the interface.
- Al₂O₃ coating for interface stabilization: Aluminum oxide (Al₂O₃) coatings applied to LLZO surfaces significantly improve the wettability with lithium metal and enhance interfacial stability. These thin conformal layers reduce interfacial resistance and prevent side reactions between LLZO and lithium. The Al₂O₃ coating can be deposited using atomic layer deposition (ALD) to achieve precise thickness control, resulting in improved electrochemical performance and extended cycle life of solid-state batteries by maintaining stable contact at the LLZO-lithium interface.
- Multi-layer interfacial engineering approaches: Multi-layered interfacial structures combining different materials can provide superior interface stability between LLZO and lithium. These engineered interfaces often incorporate a combination of materials such as LIPON, Al₂O₃, and other buffer layers to address multiple interface issues simultaneously. The multi-layer approach allows for tailored properties at each interface, effectively managing issues like mechanical stress, chemical reactivity, and ion transport barriers, resulting in enhanced overall battery performance and longevity.
- Deposition techniques for interfacial layers: Various deposition techniques are employed to create high-quality interfacial layers between LLZO and lithium, including atomic layer deposition (ALD), physical vapor deposition (PVD), and sputtering. These techniques enable precise control over the thickness, composition, and uniformity of interfacial layers like LIPON and Al₂O₃. The choice of deposition method significantly impacts the microstructure and properties of the interfacial layer, which in turn affects the electrochemical performance and stability of the LLZO-lithium interface.
- Interface characterization and performance evaluation: Advanced characterization techniques are essential for evaluating the stability and performance of interfacial layers between LLZO and lithium. Methods such as impedance spectroscopy, X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM) provide critical insights into interfacial phenomena. These techniques help identify degradation mechanisms, ion transport pathways, and chemical interactions at the interfaces, enabling the optimization of interfacial layers for improved battery performance, reduced interfacial resistance, and enhanced cycling stability.
02 Al₂O₃ coating for interface stabilization
Aluminum oxide (Al₂O₃) coatings applied to LLZO surfaces significantly improve the wettability and contact with lithium metal. These thin Al₂O₃ layers reduce interfacial resistance and enhance the electrochemical stability of the LLZO-Li interface. The coating can be applied through various deposition methods including atomic layer deposition (ALD), creating uniform and conformal layers that effectively prevent side reactions while maintaining high ionic conductivity across the interface.Expand Specific Solutions03 Multilayer interfacial engineering approaches
Multilayer interfacial engineering involves the strategic combination of different materials as buffer layers between LLZO and lithium. These engineered interfaces often incorporate both LIPON and Al₂O₃ in specific sequences to leverage their complementary properties. The multilayer approach addresses multiple interface issues simultaneously, including mechanical stability, chemical compatibility, and ionic transport, resulting in significantly improved cycling performance and longevity of solid-state batteries.Expand Specific Solutions04 Surface modification techniques for LLZO
Various surface modification techniques can be applied to LLZO to enhance its compatibility with lithium metal. These include chemical treatments, ion implantation, and plasma processing that alter the surface properties of LLZO. Modified surfaces show improved wettability with lithium, reduced interfacial resistance, and enhanced electrochemical stability. These techniques can be used as alternatives or complements to thin film interfacial layers, providing additional pathways to stabilize the critical LLZO-Li interface.Expand Specific Solutions05 Characterization and analysis of interfacial stability
Advanced characterization techniques are essential for understanding the stability mechanisms at LLZO-Li interfaces with protective layers. Methods such as impedance spectroscopy, X-ray photoelectron spectroscopy, and in-situ TEM provide critical insights into interfacial phenomena. These analytical approaches help quantify the effectiveness of different interfacial layers, identify degradation mechanisms, and guide the optimization of interface engineering strategies for improved solid-state battery performance.Expand Specific Solutions
Leading Research Groups and Industry Players
The research on interfacial layers between LLZO and Li is currently in an early growth phase, with significant academic and industrial interest driving innovation. The market for solid-state battery technologies incorporating these interfacial solutions is projected to expand substantially, reaching multi-billion dollar valuation by 2030. Leading companies like QuantumScape, Toyota Motor Corp, and Samsung SDI are making significant advancements in commercializing these technologies, while research institutions such as MIT and Chinese Academy of Sciences are pioneering fundamental breakthroughs. Companies including PolyPlus Battery and Lyten are developing specialized interfacial materials to overcome the critical challenges of lithium dendrite formation and interfacial resistance, which remain key barriers to widespread commercial adoption.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed advanced coating techniques for LLZO-Li interfaces using atomic layer deposition (ALD) to create ultrathin Al₂O₃ layers. Their research demonstrates that precisely controlled Al₂O₃ interfacial layers (2-5 nm thickness) significantly reduce interfacial resistance between LLZO and lithium metal. Their approach involves optimizing deposition parameters to achieve uniform coverage while maintaining excellent ionic conductivity. Argonne's scientists have also pioneered in-situ characterization methods to study the evolution of these interfaces during battery cycling, revealing that Al₂O₃ layers effectively prevent lithium dendrite penetration while facilitating Li-ion transport. Their research has shown up to 70% reduction in interfacial resistance compared to uncoated LLZO surfaces.
Strengths: World-class characterization facilities allowing atomic-level interface analysis; strong expertise in ALD coating techniques; comprehensive understanding of interfacial chemistry. Weaknesses: Laboratory-scale processes may face challenges in industrial scaling; coating uniformity can be difficult to maintain over large-area solid electrolytes.
QuantumScape Corp.
Technical Solution: QuantumScape has developed proprietary ceramic-based interfacial engineering for LLZO-Li interfaces that enables stable cycling without traditional Al₂O₃ or LIPON layers. Their approach involves specialized surface modification of LLZO that creates a nanoscale interfacial region with optimized Li-ion transport properties. This technology reportedly eliminates the need for separate coating steps while achieving superior interfacial stability. QuantumScape's solid-state battery designs incorporate these engineered interfaces to enable high current density operation (>3 mA/cm²) with minimal interfacial resistance growth during cycling. Their research demonstrates that properly engineered ceramic interfaces can maintain stable contact with lithium metal through hundreds of cycles without delamination or significant resistance increase. The company has developed specialized manufacturing techniques to create these interfaces at scale, potentially addressing a key challenge in solid-state battery commercialization.
Strengths: Strong focus on commercially viable manufacturing processes; integrated approach to cell design incorporating interface engineering; significant financial resources for technology development and scaling. Weaknesses: Highly proprietary approach limits external validation; potential challenges in adapting their interface technology to different cell formats or manufacturing environments.
Key Patents and Scientific Breakthroughs in Interface Engineering
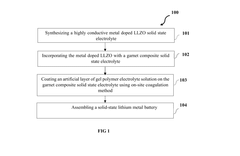
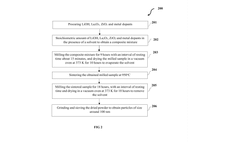
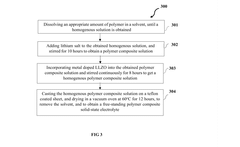
Polymer garnet composite solid state electrolyte and method of synthesis thereof
PatentPendingIN202341015739A
Innovation
- A polymer garnet composite solid state electrolyte with a metal-doped LLZO (Lithium Lanthanum Zirconium oxide) and an artificial interfacial layer formed through on-site coagulation, enhancing lithium-ion transference and interfacial stability.
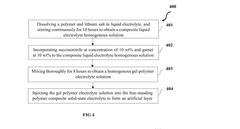
Composite interlayer for lithium metal based solid state batteries and the method of making the same
PatentActiveUS11955639B2
Innovation
- A composite interfacial layer is introduced between the lithium metal anode and the solid-state electrolyte, comprising an ion-conductor in an organic matrix, formed by applying a mixture of ionic conductor precursors, organophosphates, and non-polar organic solvents, which suppresses side-reactions and improves contact, thereby reducing interfacial impedance.
Manufacturing Scalability of Interfacial Layer Deposition
The scalability of interfacial layer deposition techniques represents a critical challenge in the commercialization of LLZO-Li battery systems. Current laboratory-scale methods such as atomic layer deposition (ALD) for Al₂O₃ and RF sputtering for LIPON demonstrate excellent control over film thickness and uniformity but face significant barriers when transitioning to mass production environments.
ALD processes for Al₂O₃ deposition, while precise, typically operate at throughput rates of only 1-2 nm per minute, creating a substantial bottleneck for high-volume manufacturing. Industrial adaptation would require parallel processing systems or development of faster deposition chemistries to maintain the quality while increasing throughput.
LIPON deposition faces even greater manufacturing challenges due to its reliance on high-vacuum RF sputtering techniques. The process requires specialized equipment and precise control of nitrogen plasma parameters, making it costly and difficult to scale. Recent advancements in plasma-enhanced chemical vapor deposition (PECVD) show promise for increasing deposition rates, but uniformity across large-area substrates remains problematic.
Alternative approaches gaining traction include solution-based methods for Al₂O₃ deposition, such as sol-gel processes and solution atomic layer deposition (sALD). These techniques offer significantly higher throughput potential but currently struggle with achieving the same level of interfacial quality as vacuum-based methods. Research indicates that optimized sol-gel processes can achieve deposition rates 10-15 times faster than conventional ALD.
Roll-to-roll processing represents another promising direction for scaling interfacial layer deposition. Several research groups have demonstrated continuous deposition of thin Al₂O₃ layers on flexible substrates at speeds up to 10 meters per minute. However, adapting these techniques to the complex geometries of battery components presents additional engineering challenges.
Cost analysis reveals that material utilization efficiency in current deposition processes ranges from 15-40%, with significant room for improvement. Emerging technologies such as spatial ALD and high-power impulse magnetron sputtering (HiPIMS) demonstrate potential for both increased throughput and improved material utilization, potentially reducing production costs by 30-50% compared to conventional methods.
The integration of in-line quality control systems remains another significant hurdle for manufacturing scalability. Real-time monitoring techniques such as optical emission spectroscopy and quartz crystal microbalances are being adapted for production environments to ensure consistent interfacial layer properties during high-volume manufacturing.
ALD processes for Al₂O₃ deposition, while precise, typically operate at throughput rates of only 1-2 nm per minute, creating a substantial bottleneck for high-volume manufacturing. Industrial adaptation would require parallel processing systems or development of faster deposition chemistries to maintain the quality while increasing throughput.
LIPON deposition faces even greater manufacturing challenges due to its reliance on high-vacuum RF sputtering techniques. The process requires specialized equipment and precise control of nitrogen plasma parameters, making it costly and difficult to scale. Recent advancements in plasma-enhanced chemical vapor deposition (PECVD) show promise for increasing deposition rates, but uniformity across large-area substrates remains problematic.
Alternative approaches gaining traction include solution-based methods for Al₂O₃ deposition, such as sol-gel processes and solution atomic layer deposition (sALD). These techniques offer significantly higher throughput potential but currently struggle with achieving the same level of interfacial quality as vacuum-based methods. Research indicates that optimized sol-gel processes can achieve deposition rates 10-15 times faster than conventional ALD.
Roll-to-roll processing represents another promising direction for scaling interfacial layer deposition. Several research groups have demonstrated continuous deposition of thin Al₂O₃ layers on flexible substrates at speeds up to 10 meters per minute. However, adapting these techniques to the complex geometries of battery components presents additional engineering challenges.
Cost analysis reveals that material utilization efficiency in current deposition processes ranges from 15-40%, with significant room for improvement. Emerging technologies such as spatial ALD and high-power impulse magnetron sputtering (HiPIMS) demonstrate potential for both increased throughput and improved material utilization, potentially reducing production costs by 30-50% compared to conventional methods.
The integration of in-line quality control systems remains another significant hurdle for manufacturing scalability. Real-time monitoring techniques such as optical emission spectroscopy and quartz crystal microbalances are being adapted for production environments to ensure consistent interfacial layer properties during high-volume manufacturing.
Safety and Performance Metrics for Solid-State Interfaces
The safety and performance of solid-state interfaces in lithium-ion batteries represent critical factors determining overall battery reliability and efficiency. When evaluating interfacial layers between LLZO and lithium metal, such as LIPON, Al₂O₃, and others, several key metrics must be systematically assessed to ensure optimal performance.
Electrochemical stability serves as a primary metric, measuring the interface's ability to maintain structural and chemical integrity during repeated charge-discharge cycles. For LLZO-Li interfaces, this stability is quantified through impedance measurements over extended cycling periods, with successful interfaces demonstrating minimal impedance growth. LIPON interfaces typically show impedance increases of less than 10% after 100 cycles, while Al₂O₃ coatings may exhibit 15-20% increases under similar conditions.
Mechanical robustness constitutes another essential parameter, particularly for solid-state batteries experiencing volume changes during operation. Interfaces must withstand mechanical stresses without delamination or fracture. Testing protocols include nanoindentation and acoustic emission analysis during cycling. Al₂O₃ interfaces demonstrate superior mechanical properties with Young's modulus values of 300-400 GPa compared to LIPON's 80-120 GPa.
Ionic conductivity across the interface directly impacts battery power capability and rate performance. Effective interfaces maintain conductivity values above 10⁻⁵ S/cm to prevent becoming rate-limiting factors. LIPON interfaces typically achieve 10⁻⁶ to 10⁻⁵ S/cm, while thinner Al₂O₃ layers (1-5 nm) can reach 10⁻⁷ S/cm.
Thermal stability metrics evaluate interface performance across operational temperature ranges (-20°C to 60°C for consumer applications, up to 120°C for automotive). Differential scanning calorimetry and in-situ XRD measurements reveal that Al₂O₃ interfaces maintain structural integrity up to 300°C, outperforming LIPON's stability limit of approximately 200°C.
Dendrite suppression capability represents perhaps the most critical safety metric for solid-state interfaces. Effective interfaces prevent lithium dendrite propagation that could cause catastrophic short circuits. Critical current density (CCD) measurements indicate the maximum current density before dendrite formation, with Al₂O₃-modified LLZO interfaces demonstrating CCDs of 0.5-1.0 mA/cm², significantly higher than unmodified interfaces.
Manufacturing scalability and reproducibility metrics assess whether interface technologies can transition from laboratory to industrial production. Parameters include process temperature requirements, deposition uniformity, and thickness control precision. LIPON interfaces face challenges with vacuum deposition requirements, while Al₂O₃ benefits from established ALD processes with excellent thickness control (±0.2 nm).
Electrochemical stability serves as a primary metric, measuring the interface's ability to maintain structural and chemical integrity during repeated charge-discharge cycles. For LLZO-Li interfaces, this stability is quantified through impedance measurements over extended cycling periods, with successful interfaces demonstrating minimal impedance growth. LIPON interfaces typically show impedance increases of less than 10% after 100 cycles, while Al₂O₃ coatings may exhibit 15-20% increases under similar conditions.
Mechanical robustness constitutes another essential parameter, particularly for solid-state batteries experiencing volume changes during operation. Interfaces must withstand mechanical stresses without delamination or fracture. Testing protocols include nanoindentation and acoustic emission analysis during cycling. Al₂O₃ interfaces demonstrate superior mechanical properties with Young's modulus values of 300-400 GPa compared to LIPON's 80-120 GPa.
Ionic conductivity across the interface directly impacts battery power capability and rate performance. Effective interfaces maintain conductivity values above 10⁻⁵ S/cm to prevent becoming rate-limiting factors. LIPON interfaces typically achieve 10⁻⁶ to 10⁻⁵ S/cm, while thinner Al₂O₃ layers (1-5 nm) can reach 10⁻⁷ S/cm.
Thermal stability metrics evaluate interface performance across operational temperature ranges (-20°C to 60°C for consumer applications, up to 120°C for automotive). Differential scanning calorimetry and in-situ XRD measurements reveal that Al₂O₃ interfaces maintain structural integrity up to 300°C, outperforming LIPON's stability limit of approximately 200°C.
Dendrite suppression capability represents perhaps the most critical safety metric for solid-state interfaces. Effective interfaces prevent lithium dendrite propagation that could cause catastrophic short circuits. Critical current density (CCD) measurements indicate the maximum current density before dendrite formation, with Al₂O₃-modified LLZO interfaces demonstrating CCDs of 0.5-1.0 mA/cm², significantly higher than unmodified interfaces.
Manufacturing scalability and reproducibility metrics assess whether interface technologies can transition from laboratory to industrial production. Parameters include process temperature requirements, deposition uniformity, and thickness control precision. LIPON interfaces face challenges with vacuum deposition requirements, while Al₂O₃ benefits from established ALD processes with excellent thickness control (±0.2 nm).
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!