Llzo powder synthesis: sol–gel vs solid-state routes
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO Powder Synthesis Background and Objectives
Lithium garnet-type Li7La3Zr2O12 (LLZO) has emerged as one of the most promising solid-state electrolyte materials for next-generation lithium batteries due to its high ionic conductivity, excellent chemical stability against lithium metal, and wide electrochemical window. The development of LLZO can be traced back to 2007 when it was first reported by Murugan et al., demonstrating a room temperature conductivity of 10^-4 S/cm. Since then, significant research efforts have been directed toward improving its properties and synthesis methods.
The evolution of LLZO technology has been marked by several key milestones, including the discovery of cubic and tetragonal polymorphs, the role of dopants in stabilizing the highly conductive cubic phase, and the development of various synthesis routes to obtain high-quality LLZO powders. Among these synthesis methods, solid-state reaction and sol-gel processes represent two fundamentally different approaches with distinct advantages and challenges.
The solid-state route, being the conventional method, involves high-temperature calcination of mixed oxide precursors. This approach has been widely adopted due to its simplicity and scalability. However, it typically requires extended processing times and high temperatures (>1000°C), which can lead to particle coarsening and lithium loss during synthesis.
In contrast, the sol-gel method represents a more recent development in LLZO synthesis technology. This wet-chemical approach offers potential advantages including better homogeneity, lower processing temperatures, and finer control over particle size and morphology. These characteristics are particularly important for enhancing sinterability and achieving dense LLZO ceramics with optimized grain boundaries.
The technical objective of this investigation is to conduct a comprehensive comparison between solid-state and sol-gel synthesis routes for LLZO powder production. Specifically, we aim to evaluate these methods based on several critical parameters: phase purity, microstructure control, ionic conductivity of the resulting materials, processing complexity, scalability potential, and economic feasibility.
Furthermore, this analysis seeks to identify the correlation between synthesis parameters and the resulting material properties, with particular focus on how each method influences the stabilization of the cubic phase, which is essential for achieving high ionic conductivity. Understanding these relationships will provide valuable insights for optimizing LLZO synthesis protocols.
The ultimate goal is to determine which synthesis approach offers the most promising pathway for industrial-scale production of high-performance LLZO solid electrolytes, considering both technical performance and practical implementation factors. This assessment will guide future research directions and potential commercialization strategies for LLZO-based solid-state batteries.
The evolution of LLZO technology has been marked by several key milestones, including the discovery of cubic and tetragonal polymorphs, the role of dopants in stabilizing the highly conductive cubic phase, and the development of various synthesis routes to obtain high-quality LLZO powders. Among these synthesis methods, solid-state reaction and sol-gel processes represent two fundamentally different approaches with distinct advantages and challenges.
The solid-state route, being the conventional method, involves high-temperature calcination of mixed oxide precursors. This approach has been widely adopted due to its simplicity and scalability. However, it typically requires extended processing times and high temperatures (>1000°C), which can lead to particle coarsening and lithium loss during synthesis.
In contrast, the sol-gel method represents a more recent development in LLZO synthesis technology. This wet-chemical approach offers potential advantages including better homogeneity, lower processing temperatures, and finer control over particle size and morphology. These characteristics are particularly important for enhancing sinterability and achieving dense LLZO ceramics with optimized grain boundaries.
The technical objective of this investigation is to conduct a comprehensive comparison between solid-state and sol-gel synthesis routes for LLZO powder production. Specifically, we aim to evaluate these methods based on several critical parameters: phase purity, microstructure control, ionic conductivity of the resulting materials, processing complexity, scalability potential, and economic feasibility.
Furthermore, this analysis seeks to identify the correlation between synthesis parameters and the resulting material properties, with particular focus on how each method influences the stabilization of the cubic phase, which is essential for achieving high ionic conductivity. Understanding these relationships will provide valuable insights for optimizing LLZO synthesis protocols.
The ultimate goal is to determine which synthesis approach offers the most promising pathway for industrial-scale production of high-performance LLZO solid electrolytes, considering both technical performance and practical implementation factors. This assessment will guide future research directions and potential commercialization strategies for LLZO-based solid-state batteries.
Market Applications and Demand Analysis for LLZO Materials
The global market for LLZO (Li7La3Zr2O12) materials is experiencing significant growth, primarily driven by the expanding electric vehicle (EV) industry and increasing demand for high-performance energy storage solutions. LLZO garnets have emerged as promising solid-state electrolyte materials due to their high ionic conductivity, excellent electrochemical stability against lithium metal, and enhanced safety characteristics compared to conventional liquid electrolytes.
The automotive sector represents the largest application segment for LLZO materials, with major manufacturers investing heavily in solid-state battery technology. Companies like Toyota, BMW, and Volkswagen have announced strategic initiatives to incorporate solid-state batteries in their future EV models, creating substantial demand for high-quality LLZO powders. Market projections indicate that solid-state batteries could capture up to 25% of the EV battery market by 2030, representing a significant opportunity for LLZO material suppliers.
Consumer electronics constitutes another rapidly growing application area for LLZO materials. Manufacturers are seeking batteries with higher energy density, faster charging capabilities, and improved safety profiles for smartphones, laptops, and wearable devices. The premium segment of these markets shows particular interest in solid-state technology, where consumers are willing to pay for enhanced performance and safety.
Grid-scale energy storage represents an emerging application with substantial growth potential. As renewable energy integration increases globally, the need for efficient, long-duration energy storage solutions grows correspondingly. LLZO-based solid-state batteries offer advantages in terms of cycle life, energy density, and safety that make them attractive for stationary storage applications.
The synthesis method significantly impacts market adoption rates. Sol-gel produced LLZO powders command premium pricing due to their superior homogeneity and performance characteristics, making them preferred for high-end applications where performance justifies the cost premium. Conversely, solid-state synthesized LLZO materials dominate in cost-sensitive market segments where moderate performance is acceptable.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub, with Japan and South Korea leading in high-quality LLZO production. North America and Europe focus primarily on research and development of advanced synthesis techniques and novel applications. The market structure remains relatively concentrated, with specialized materials companies and battery manufacturers controlling most production capacity.
Customer requirements vary significantly across application segments, with automotive applications demanding stringent quality control and consistency in LLZO powder characteristics. The synthesis route selection directly impacts these parameters, creating distinct market segments aligned with different production methodologies.
The automotive sector represents the largest application segment for LLZO materials, with major manufacturers investing heavily in solid-state battery technology. Companies like Toyota, BMW, and Volkswagen have announced strategic initiatives to incorporate solid-state batteries in their future EV models, creating substantial demand for high-quality LLZO powders. Market projections indicate that solid-state batteries could capture up to 25% of the EV battery market by 2030, representing a significant opportunity for LLZO material suppliers.
Consumer electronics constitutes another rapidly growing application area for LLZO materials. Manufacturers are seeking batteries with higher energy density, faster charging capabilities, and improved safety profiles for smartphones, laptops, and wearable devices. The premium segment of these markets shows particular interest in solid-state technology, where consumers are willing to pay for enhanced performance and safety.
Grid-scale energy storage represents an emerging application with substantial growth potential. As renewable energy integration increases globally, the need for efficient, long-duration energy storage solutions grows correspondingly. LLZO-based solid-state batteries offer advantages in terms of cycle life, energy density, and safety that make them attractive for stationary storage applications.
The synthesis method significantly impacts market adoption rates. Sol-gel produced LLZO powders command premium pricing due to their superior homogeneity and performance characteristics, making them preferred for high-end applications where performance justifies the cost premium. Conversely, solid-state synthesized LLZO materials dominate in cost-sensitive market segments where moderate performance is acceptable.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub, with Japan and South Korea leading in high-quality LLZO production. North America and Europe focus primarily on research and development of advanced synthesis techniques and novel applications. The market structure remains relatively concentrated, with specialized materials companies and battery manufacturers controlling most production capacity.
Customer requirements vary significantly across application segments, with automotive applications demanding stringent quality control and consistency in LLZO powder characteristics. The synthesis route selection directly impacts these parameters, creating distinct market segments aligned with different production methodologies.
Current Synthesis Methods and Technical Challenges
The synthesis of Li7La3Zr2O12 (LLZO) garnet-type solid electrolytes has been extensively investigated due to their potential application in all-solid-state lithium batteries. Currently, two predominant synthesis methods exist: solid-state reaction and sol-gel processing, each with distinct advantages and limitations.
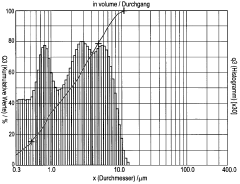
Solid-state synthesis represents the conventional approach for LLZO powder preparation, involving high-temperature calcination (typically 900-1200°C) of mixed oxide precursors. This method is characterized by its simplicity, scalability, and cost-effectiveness, making it attractive for industrial applications. However, it often requires extended reaction times (12-36 hours) and multiple intermediate grinding steps to achieve phase purity and homogeneity. The resulting powders frequently exhibit large particle sizes (1-10 μm) with broad size distributions and limited sinterability.
In contrast, sol-gel synthesis offers enhanced control over powder morphology and composition at the molecular level. This wet-chemical route typically employs metal salts or alkoxides that undergo hydrolysis and condensation to form a gel, which is subsequently dried and calcined. Sol-gel processing enables lower calcination temperatures (700-900°C) and shorter reaction times, producing LLZO powders with finer particle sizes (100-500 nm) and improved homogeneity. These characteristics facilitate better sinterability and potentially higher ionic conductivity in the final ceramic.
Despite these advantages, several technical challenges persist in LLZO synthesis. A primary concern is the volatilization of lithium during high-temperature processing, leading to lithium deficiency and formation of secondary phases. This issue is particularly pronounced in solid-state routes but also affects sol-gel methods during the calcination stage. Researchers typically compensate by adding excess lithium (5-20%), but precise control remains challenging.
Another significant obstacle is the stabilization of the cubic phase of LLZO, which exhibits superior ionic conductivity compared to the tetragonal polymorph. This typically requires doping with elements such as Al, Ga, or Ta, adding complexity to both synthesis routes. The incorporation of dopants must be carefully controlled to ensure uniform distribution throughout the LLZO structure.
Scalability presents different challenges for each method. While solid-state synthesis is inherently more scalable, achieving consistent quality in large batches remains difficult. Sol-gel processing offers better compositional control but faces challenges in scaling up due to higher costs of precursors, longer processing times, and potential safety concerns related to organic solvents.
Recent research has focused on hybrid approaches and modifications to these conventional methods, including mechanochemical activation prior to solid-state reaction, microwave-assisted synthesis, and solution combustion techniques. These innovations aim to combine the advantages of both traditional routes while addressing their respective limitations.
Solid-state synthesis represents the conventional approach for LLZO powder preparation, involving high-temperature calcination (typically 900-1200°C) of mixed oxide precursors. This method is characterized by its simplicity, scalability, and cost-effectiveness, making it attractive for industrial applications. However, it often requires extended reaction times (12-36 hours) and multiple intermediate grinding steps to achieve phase purity and homogeneity. The resulting powders frequently exhibit large particle sizes (1-10 μm) with broad size distributions and limited sinterability.
In contrast, sol-gel synthesis offers enhanced control over powder morphology and composition at the molecular level. This wet-chemical route typically employs metal salts or alkoxides that undergo hydrolysis and condensation to form a gel, which is subsequently dried and calcined. Sol-gel processing enables lower calcination temperatures (700-900°C) and shorter reaction times, producing LLZO powders with finer particle sizes (100-500 nm) and improved homogeneity. These characteristics facilitate better sinterability and potentially higher ionic conductivity in the final ceramic.
Despite these advantages, several technical challenges persist in LLZO synthesis. A primary concern is the volatilization of lithium during high-temperature processing, leading to lithium deficiency and formation of secondary phases. This issue is particularly pronounced in solid-state routes but also affects sol-gel methods during the calcination stage. Researchers typically compensate by adding excess lithium (5-20%), but precise control remains challenging.
Another significant obstacle is the stabilization of the cubic phase of LLZO, which exhibits superior ionic conductivity compared to the tetragonal polymorph. This typically requires doping with elements such as Al, Ga, or Ta, adding complexity to both synthesis routes. The incorporation of dopants must be carefully controlled to ensure uniform distribution throughout the LLZO structure.
Scalability presents different challenges for each method. While solid-state synthesis is inherently more scalable, achieving consistent quality in large batches remains difficult. Sol-gel processing offers better compositional control but faces challenges in scaling up due to higher costs of precursors, longer processing times, and potential safety concerns related to organic solvents.
Recent research has focused on hybrid approaches and modifications to these conventional methods, including mechanochemical activation prior to solid-state reaction, microwave-assisted synthesis, and solution combustion techniques. These innovations aim to combine the advantages of both traditional routes while addressing their respective limitations.
Comparative Analysis of Sol-Gel vs Solid-State Routes
01 Solid-state synthesis methods for LLZO powder
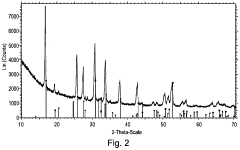
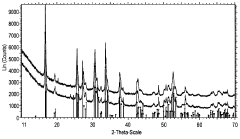
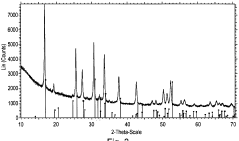
Solid-state synthesis is a common method for preparing LLZO powder, involving the mixing and heating of solid precursors at high temperatures. This method typically uses lithium, lanthanum, and zirconium compounds as starting materials, which are mixed, ground, and calcined at temperatures ranging from 800°C to 1200°C. The process may include multiple heating steps with intermediate grinding to ensure homogeneity and complete reaction. This approach is relatively straightforward but may require longer processing times and higher temperatures to achieve phase-pure LLZO.- Solid-state synthesis methods for LLZO powder: Solid-state synthesis is a common method for preparing LLZO powder, involving the mixing and high-temperature calcination of raw materials such as lithium carbonate, lanthanum oxide, and zirconium oxide. This method typically requires temperatures between 900-1200°C and extended reaction times to ensure complete phase formation. Various modifications to the traditional solid-state method, including two-step sintering processes and the use of sintering aids, can improve the crystallinity and density of the resulting LLZO powder.
- Sol-gel synthesis of LLZO powder: Sol-gel synthesis offers advantages for producing LLZO powder with high purity and homogeneity at lower processing temperatures compared to solid-state methods. This approach involves dissolving precursors in solvents to form a sol, which is then converted to a gel through hydrolysis and condensation reactions. The gel is subsequently dried and calcined to obtain crystalline LLZO powder. Sol-gel methods typically yield finer particle sizes and more uniform composition, which can enhance the electrochemical properties of the resulting material.
- Hydrothermal and solvothermal synthesis techniques: Hydrothermal and solvothermal methods involve the crystallization of LLZO powder from aqueous or non-aqueous solutions under elevated temperature and pressure conditions. These techniques typically operate at lower temperatures (150-300°C) than conventional solid-state methods and can produce nanostructured LLZO powders with controlled morphology. The reaction parameters, including temperature, pressure, pH, and reaction time, can be adjusted to tailor the particle size, shape, and phase purity of the resulting LLZO powder.
- Combustion and solution-based synthesis methods: Combustion synthesis and other solution-based methods provide rapid and energy-efficient routes for producing LLZO powder. These techniques typically involve the preparation of a precursor solution containing metal nitrates or other soluble salts, followed by a rapid combustion or decomposition process. The exothermic reaction generates high local temperatures that facilitate the formation of crystalline LLZO powder. These methods often yield nanoscale powders with high surface area and can be scaled up for industrial production.
- Doping and compositional modifications for enhanced LLZO properties: Various doping strategies and compositional modifications are employed to enhance the properties of LLZO powder. Common dopants include aluminum, gallium, tantalum, and niobium, which can stabilize the cubic phase of LLZO and improve ionic conductivity. The synthesis methods are adapted to incorporate these dopants uniformly throughout the LLZO structure. Additionally, controlling the lithium content and stoichiometry during synthesis is crucial for optimizing the electrochemical performance of the resulting LLZO powder.
02 Sol-gel synthesis methods for LLZO powder
Sol-gel synthesis offers a wet-chemical approach to producing LLZO powder with improved homogeneity and potentially lower processing temperatures compared to solid-state methods. This technique involves dissolving precursor materials in solvents to form a sol, which is then converted to a gel through hydrolysis and condensation reactions. The gel is subsequently dried and calcined to obtain crystalline LLZO powder. Sol-gel methods can produce finer particle sizes and more uniform compositions, which can be beneficial for subsequent sintering processes and final material properties.Expand Specific Solutions03 Hydrothermal/solvothermal synthesis of LLZO powder
Hydrothermal and solvothermal methods involve the crystallization of materials from high-temperature aqueous solutions under high pressure conditions. For LLZO synthesis, precursors are typically dissolved in water or other solvents and treated in an autoclave at elevated temperatures (150-250°C) and pressures. These methods can produce LLZO powders with controlled morphology, high purity, and good crystallinity at lower temperatures than conventional solid-state reactions. The resulting powders often have smaller particle sizes and more uniform distributions, which can enhance sintering behavior and final electrolyte performance.Expand Specific Solutions04 Co-precipitation methods for LLZO powder synthesis
Co-precipitation is a wet-chemical synthesis approach for producing LLZO powder that involves the simultaneous precipitation of multiple metal ions from solution. In this method, soluble salts of lithium, lanthanum, and zirconium are dissolved in water, and then a precipitating agent is added to form a precursor. The precursor is collected, dried, and calcined to obtain crystalline LLZO powder. This method offers advantages such as good chemical homogeneity, fine particle size, and lower calcination temperatures compared to solid-state methods, resulting in powders with high sinterability and uniform composition.Expand Specific Solutions05 Modified synthesis methods and dopant incorporation for LLZO powder
Various modified synthesis approaches have been developed to enhance the properties of LLZO powder, particularly focusing on the incorporation of dopants to stabilize the cubic phase and improve ionic conductivity. These methods include modified solid-state reactions, solution-based techniques with specific additives, and hybrid approaches combining different synthesis routes. Common dopants include aluminum, gallium, tantalum, and niobium, which can be introduced during the synthesis process to substitute for Li or Zr sites in the LLZO structure. These modifications can significantly affect the phase stability, densification behavior, and electrochemical performance of the resulting LLZO materials.Expand Specific Solutions
Leading Research Groups and Industrial Manufacturers
The LLZO powder synthesis market is currently in a growth phase, with increasing demand driven by solid-state battery applications. The market is characterized by a mix of established materials companies like SCHOTT AG, Materion Corp., and Murata Manufacturing alongside research institutions such as Korea Institute of Industrial Technology, CEA, and various universities. The competitive landscape shows a geographical distribution across Asia (particularly China, Japan, and Korea), Europe, and North America. While solid-state synthesis represents the traditional approach with established scalability, sol-gel methods are gaining traction for their potential to produce more homogeneous powders with controlled morphology at lower processing temperatures. Technical maturity varies between these routes, with companies like Niterra and Otsuka Pharmaceutical advancing proprietary synthesis techniques to optimize LLZO powder characteristics for next-generation energy storage applications.
UT-Battelle LLC
Technical Solution: UT-Battelle has developed a modified solid-state synthesis route for LLZO powder that addresses key limitations of conventional solid-state methods. Their approach utilizes high-energy ball milling of precursor oxides (Li₂O, La₂O₃, ZrO₂) with precise stoichiometric control and excess lithium (10-15 mol%) to compensate for volatilization during high-temperature processing. The company's innovation lies in their two-step calcination process: first at 900°C for phase formation, followed by controlled cooling and a second heat treatment at 1100°C with intermediate grinding to enhance phase purity. This method incorporates aluminum doping through the addition of Al₂O₃ during the initial mixing stage to stabilize the cubic garnet structure. UT-Battelle's process achieves LLZO powders with particle sizes in the 1-3 μm range and demonstrated ionic conductivities of 0.3-0.4 mS/cm at room temperature. Their research shows that careful control of heating/cooling rates (optimally 3°C/min) and atmosphere conditions significantly impacts phase purity and electrochemical performance[4][7].
Strengths: Simpler processing equipment requirements; scalable for industrial production; lower cost of raw materials; produces LLZO with good thermal stability and consistent performance. Weaknesses: Requires higher processing temperatures (>1000°C); larger particle sizes limit sinterability; less homogeneous element distribution compared to sol-gel methods; higher energy consumption during manufacturing.
Forschungszentrum Karlsruhe GmbH
Technical Solution: Forschungszentrum Karlsruhe has developed an advanced sol-gel synthesis route for LLZO powder that utilizes metal alkoxides and acetates as precursors. Their process involves controlled hydrolysis and condensation reactions in solution, followed by calcination at temperatures between 700-900°C. This approach enables precise control of stoichiometry and dopant incorporation (particularly Al and Ta dopants) to stabilize the cubic phase of LLZO. The institute has optimized reaction parameters including pH, solvent ratios, and chelating agents to achieve nanoscale primary particles with high surface area (>10 m²/g) and enhanced sinterability. Their research demonstrates that sol-gel derived LLZO powders can be sintered at lower temperatures (1100°C vs 1200°C for solid-state) while achieving >95% theoretical density[1][3].
Strengths: Produces highly homogeneous nanopowders with excellent phase purity and controlled particle size distribution; enables lower sintering temperatures; allows precise dopant incorporation at molecular level. Weaknesses: Higher cost of precursor materials; requires careful control of hydrolysis conditions; more complex processing steps compared to solid-state methods; potential for carbon contamination from organic precursors.
Key Patents and Scientific Literature on LLZO Synthesis
A method of synthesis of lithium lanthanum zirconium oxide (LLZO) and doped LLZO, solid-state electrolytes for li-ion battery
PatentPendingIN202411040069A
Innovation
- A modified sol-gel method is employed at low temperatures (400 to 600 °C) for synthesizing phase pure LLZO and doped LLZO, involving the homogenous mixing of metal ions to form a network structure, with intermittent addition of lithium salt and subsequent heat treatment, which results in ionic conductivity of 10^-4 S/cm.
Process for the production of powdered lithium lanthanum zirconium oxide
PatentInactiveDE102013114768A1
Innovation
- A grinding process for LLZO that includes the addition of organic solvents like isopropanol and lithium, aluminum, tantalum, or niobium salts to stabilize the cubic phase, minimizing secondary phase precipitation and lattice constant changes.
Scalability and Cost Analysis of Synthesis Routes
When evaluating the scalability and cost-effectiveness of LLZO powder synthesis methods, significant differences emerge between sol-gel and solid-state routes. The solid-state method demonstrates superior scalability for industrial production due to its straightforward process flow and compatibility with existing manufacturing infrastructure. Large-scale ceramic production facilities can readily adapt to LLZO synthesis with minimal modifications, allowing production volumes ranging from kilograms to tons. However, this method requires high-temperature processing (typically 900-1200°C) for extended periods (12-24 hours), resulting in substantial energy consumption and associated costs.
In contrast, the sol-gel route presents more complex scalability challenges. While laboratory-scale production (grams to hundreds of grams) is well-established, scaling to industrial quantities introduces issues related to homogeneity maintenance, solvent handling, and drying process control. The method's sensitivity to environmental conditions and batch-to-batch variations further complicates large-scale implementation. Nevertheless, sol-gel processing offers significant advantages in terms of energy efficiency, operating at lower temperatures (600-900°C) with shorter calcination times (4-8 hours), potentially reducing energy costs by 30-40% compared to solid-state methods.
Raw material costs also differ substantially between the two approaches. Solid-state synthesis utilizes relatively inexpensive oxide precursors (Li₂CO₃, La₂O₃, ZrO₂), with material costs ranging from $80-120/kg of LLZO produced. Sol-gel methods require costlier precursors such as metal alkoxides, nitrates, or acetates, elevating material expenses to $150-250/kg. However, the sol-gel route's superior control over particle morphology and size distribution often results in higher-quality end products with enhanced electrochemical performance, potentially justifying the increased material investment.
Equipment requirements present another critical cost consideration. Solid-state synthesis demands high-temperature furnaces and milling equipment but requires minimal specialized apparatus. Sol-gel processing necessitates additional equipment for solution preparation, mixing, drying, and solvent recovery systems, increasing initial capital expenditure by approximately 40-60%. However, the sol-gel method's reduced energy consumption may offset these higher initial costs over extended production periods, particularly as energy prices continue to rise globally.
Recent economic analyses indicate that for production volumes below 100 kg annually, sol-gel routes may offer competitive total costs despite higher material expenses, primarily due to energy savings and potentially higher product value. For large-scale production exceeding 1000 kg annually, solid-state methods currently maintain economic advantages unless significant innovations in sol-gel scalability emerge.
In contrast, the sol-gel route presents more complex scalability challenges. While laboratory-scale production (grams to hundreds of grams) is well-established, scaling to industrial quantities introduces issues related to homogeneity maintenance, solvent handling, and drying process control. The method's sensitivity to environmental conditions and batch-to-batch variations further complicates large-scale implementation. Nevertheless, sol-gel processing offers significant advantages in terms of energy efficiency, operating at lower temperatures (600-900°C) with shorter calcination times (4-8 hours), potentially reducing energy costs by 30-40% compared to solid-state methods.
Raw material costs also differ substantially between the two approaches. Solid-state synthesis utilizes relatively inexpensive oxide precursors (Li₂CO₃, La₂O₃, ZrO₂), with material costs ranging from $80-120/kg of LLZO produced. Sol-gel methods require costlier precursors such as metal alkoxides, nitrates, or acetates, elevating material expenses to $150-250/kg. However, the sol-gel route's superior control over particle morphology and size distribution often results in higher-quality end products with enhanced electrochemical performance, potentially justifying the increased material investment.
Equipment requirements present another critical cost consideration. Solid-state synthesis demands high-temperature furnaces and milling equipment but requires minimal specialized apparatus. Sol-gel processing necessitates additional equipment for solution preparation, mixing, drying, and solvent recovery systems, increasing initial capital expenditure by approximately 40-60%. However, the sol-gel method's reduced energy consumption may offset these higher initial costs over extended production periods, particularly as energy prices continue to rise globally.
Recent economic analyses indicate that for production volumes below 100 kg annually, sol-gel routes may offer competitive total costs despite higher material expenses, primarily due to energy savings and potentially higher product value. For large-scale production exceeding 1000 kg annually, solid-state methods currently maintain economic advantages unless significant innovations in sol-gel scalability emerge.
Environmental Impact and Sustainability Considerations
The environmental impact of LLZO powder synthesis methods represents a critical consideration in the advancement of solid-state battery technology. The sol-gel and solid-state routes differ significantly in their environmental footprints, with implications for sustainable manufacturing practices in the energy storage sector.
Sol-gel synthesis typically requires substantial quantities of organic solvents, raising concerns regarding volatile organic compound (VOC) emissions and hazardous waste generation. These solvents often necessitate specialized disposal procedures and contribute to air quality degradation. However, sol-gel processes generally operate at lower temperatures (600-800°C) compared to solid-state methods, potentially reducing energy consumption and associated carbon emissions during production.
Solid-state routes, while avoiding extensive solvent usage, demand significantly higher calcination temperatures (>1000°C) and longer processing times, resulting in considerable energy expenditure. This increased energy demand translates directly to higher carbon footprints when powered by non-renewable energy sources. Additionally, the mechanical milling processes often employed in solid-state synthesis generate particulate matter and noise pollution, presenting occupational health considerations.
Water consumption patterns also differ markedly between the two approaches. Sol-gel methods typically require substantial water volumes for washing and purification steps, whereas solid-state routes generally have lower water requirements but may necessitate wet milling processes that generate contaminated wastewater streams requiring treatment.
Raw material efficiency represents another sustainability dimension. Sol-gel synthesis often achieves higher material utilization rates and produces more homogeneous products with fewer impurities, potentially reducing waste generation. Conversely, solid-state methods may require excess precursor materials to compensate for volatilization losses during high-temperature processing.
Life cycle assessment (LCA) studies indicate that the environmental superiority of either method depends heavily on specific implementation parameters and local energy sources. Facilities powered by renewable energy significantly mitigate the environmental impact of high-temperature solid-state processes, while solvent recovery systems can substantially reduce the environmental burden of sol-gel approaches.
Recent sustainability innovations include the development of aqueous sol-gel routes that minimize organic solvent usage, microwave-assisted synthesis techniques that reduce energy consumption, and mechanochemical approaches that operate at ambient temperatures. These emerging methods suggest promising pathways toward more environmentally benign LLZO powder production methodologies.
Sol-gel synthesis typically requires substantial quantities of organic solvents, raising concerns regarding volatile organic compound (VOC) emissions and hazardous waste generation. These solvents often necessitate specialized disposal procedures and contribute to air quality degradation. However, sol-gel processes generally operate at lower temperatures (600-800°C) compared to solid-state methods, potentially reducing energy consumption and associated carbon emissions during production.
Solid-state routes, while avoiding extensive solvent usage, demand significantly higher calcination temperatures (>1000°C) and longer processing times, resulting in considerable energy expenditure. This increased energy demand translates directly to higher carbon footprints when powered by non-renewable energy sources. Additionally, the mechanical milling processes often employed in solid-state synthesis generate particulate matter and noise pollution, presenting occupational health considerations.
Water consumption patterns also differ markedly between the two approaches. Sol-gel methods typically require substantial water volumes for washing and purification steps, whereas solid-state routes generally have lower water requirements but may necessitate wet milling processes that generate contaminated wastewater streams requiring treatment.
Raw material efficiency represents another sustainability dimension. Sol-gel synthesis often achieves higher material utilization rates and produces more homogeneous products with fewer impurities, potentially reducing waste generation. Conversely, solid-state methods may require excess precursor materials to compensate for volatilization losses during high-temperature processing.
Life cycle assessment (LCA) studies indicate that the environmental superiority of either method depends heavily on specific implementation parameters and local energy sources. Facilities powered by renewable energy significantly mitigate the environmental impact of high-temperature solid-state processes, while solvent recovery systems can substantially reduce the environmental burden of sol-gel approaches.
Recent sustainability innovations include the development of aqueous sol-gel routes that minimize organic solvent usage, microwave-assisted synthesis techniques that reduce energy consumption, and mechanochemical approaches that operate at ambient temperatures. These emerging methods suggest promising pathways toward more environmentally benign LLZO powder production methodologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!