How to reduce grain boundary resistance in sintered LLZO
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO Solid Electrolyte Background and Objectives
Lithium lanthanum zirconate (LLZO) has emerged as one of the most promising solid electrolyte materials for next-generation all-solid-state lithium batteries due to its high ionic conductivity, excellent chemical stability against lithium metal, and wide electrochemical window. The development of LLZO can be traced back to 2007 when Murugan et al. first reported its potential as a solid electrolyte with cubic garnet structure, exhibiting ionic conductivity of 10^-4 S/cm at room temperature.
Over the past decade, significant research efforts have focused on enhancing the ionic conductivity of LLZO through various approaches, including compositional modifications, structural optimizations, and processing techniques. The evolution of LLZO technology has progressed from initial proof-of-concept studies to more sophisticated engineering of its microstructure and interfaces to achieve practical application in commercial batteries.
The primary technical objective in LLZO development is to reduce grain boundary resistance, which represents a major bottleneck limiting overall ionic conductivity in sintered LLZO pellets. Grain boundaries act as barriers to lithium ion transport, significantly reducing the effective conductivity of polycrystalline LLZO compared to its theoretical bulk conductivity. This challenge becomes particularly critical as the industry moves toward practical implementation of LLZO in commercial solid-state batteries.
Current technical goals include achieving total ionic conductivity exceeding 10^-3 S/cm at room temperature in sintered LLZO, with grain boundary resistance contributing less than 20% of the total resistance. Additionally, researchers aim to develop scalable and cost-effective manufacturing processes that can maintain consistent microstructural properties and minimize grain boundary resistance during large-scale production.
The technological trend is moving toward multi-faceted approaches to address grain boundary resistance, including dopant engineering, sintering optimization, and interface modification. Recent advances in analytical techniques have enabled more precise characterization of grain boundary properties, facilitating more targeted strategies for resistance reduction.
Looking forward, the field is trending toward integration of LLZO with practical battery systems, requiring not only high ionic conductivity but also mechanical robustness, long-term stability, and compatibility with electrode materials. The ultimate objective is to enable commercial production of all-solid-state batteries with energy densities exceeding 400 Wh/kg, cycle life beyond 1000 cycles, and enhanced safety compared to conventional lithium-ion batteries.
Over the past decade, significant research efforts have focused on enhancing the ionic conductivity of LLZO through various approaches, including compositional modifications, structural optimizations, and processing techniques. The evolution of LLZO technology has progressed from initial proof-of-concept studies to more sophisticated engineering of its microstructure and interfaces to achieve practical application in commercial batteries.
The primary technical objective in LLZO development is to reduce grain boundary resistance, which represents a major bottleneck limiting overall ionic conductivity in sintered LLZO pellets. Grain boundaries act as barriers to lithium ion transport, significantly reducing the effective conductivity of polycrystalline LLZO compared to its theoretical bulk conductivity. This challenge becomes particularly critical as the industry moves toward practical implementation of LLZO in commercial solid-state batteries.
Current technical goals include achieving total ionic conductivity exceeding 10^-3 S/cm at room temperature in sintered LLZO, with grain boundary resistance contributing less than 20% of the total resistance. Additionally, researchers aim to develop scalable and cost-effective manufacturing processes that can maintain consistent microstructural properties and minimize grain boundary resistance during large-scale production.
The technological trend is moving toward multi-faceted approaches to address grain boundary resistance, including dopant engineering, sintering optimization, and interface modification. Recent advances in analytical techniques have enabled more precise characterization of grain boundary properties, facilitating more targeted strategies for resistance reduction.
Looking forward, the field is trending toward integration of LLZO with practical battery systems, requiring not only high ionic conductivity but also mechanical robustness, long-term stability, and compatibility with electrode materials. The ultimate objective is to enable commercial production of all-solid-state batteries with energy densities exceeding 400 Wh/kg, cycle life beyond 1000 cycles, and enhanced safety compared to conventional lithium-ion batteries.
Market Analysis for Solid-State Battery Technologies
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple industries. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating expansion to $8-10 billion by 2030, representing a compound annual growth rate exceeding 30%. This remarkable growth trajectory is primarily fueled by automotive applications, which account for nearly 60% of market demand.
The reduction of grain boundary resistance in sintered LLZO (Li7La3Zr2O12) garnet electrolytes represents a critical technological advancement that directly impacts market dynamics. As a key solid electrolyte material, LLZO's performance limitations at grain boundaries have constrained commercial viability of solid-state batteries, creating a significant market opportunity for improved solutions.
Consumer electronics manufacturers are increasingly investing in solid-state technology, with major players like Samsung, Apple, and LG allocating substantial R&D budgets specifically targeting grain boundary resistance challenges. This market segment is expected to grow at 25% annually through 2028, creating immediate commercialization opportunities for advanced LLZO formulations with reduced interfacial resistance.
The electric vehicle sector presents the most substantial market opportunity, with automotive manufacturers committing over $300 billion to electrification strategies that increasingly favor solid-state technology. Tesla, Toyota, Volkswagen, and BMW have established dedicated solid-state battery development programs, with several announcing production timelines between 2025-2028 contingent upon solving key technical challenges including grain boundary resistance.
Market analysis reveals a premium pricing structure for solid-state batteries, currently 4-5 times higher than conventional lithium-ion technologies. However, cost modeling indicates that successful reduction of grain boundary resistance in LLZO could reduce manufacturing costs by 30-40%, significantly accelerating market adoption.
Regional market assessment shows Asia-Pacific leading with 45% market share, followed by North America (30%) and Europe (20%). China dominates manufacturing capacity, while Japan leads in LLZO-specific patents. The United States maintains competitive advantage in fundamental research addressing grain boundary challenges, particularly through national laboratory initiatives and university partnerships.
Market forecasts indicate that successful commercialization of low-resistance LLZO electrolytes could accelerate solid-state battery market penetration by 2-3 years compared to current projections, potentially capturing 15-20% of the total battery market by 2030, representing a $25-30 billion opportunity.
The reduction of grain boundary resistance in sintered LLZO (Li7La3Zr2O12) garnet electrolytes represents a critical technological advancement that directly impacts market dynamics. As a key solid electrolyte material, LLZO's performance limitations at grain boundaries have constrained commercial viability of solid-state batteries, creating a significant market opportunity for improved solutions.
Consumer electronics manufacturers are increasingly investing in solid-state technology, with major players like Samsung, Apple, and LG allocating substantial R&D budgets specifically targeting grain boundary resistance challenges. This market segment is expected to grow at 25% annually through 2028, creating immediate commercialization opportunities for advanced LLZO formulations with reduced interfacial resistance.
The electric vehicle sector presents the most substantial market opportunity, with automotive manufacturers committing over $300 billion to electrification strategies that increasingly favor solid-state technology. Tesla, Toyota, Volkswagen, and BMW have established dedicated solid-state battery development programs, with several announcing production timelines between 2025-2028 contingent upon solving key technical challenges including grain boundary resistance.
Market analysis reveals a premium pricing structure for solid-state batteries, currently 4-5 times higher than conventional lithium-ion technologies. However, cost modeling indicates that successful reduction of grain boundary resistance in LLZO could reduce manufacturing costs by 30-40%, significantly accelerating market adoption.
Regional market assessment shows Asia-Pacific leading with 45% market share, followed by North America (30%) and Europe (20%). China dominates manufacturing capacity, while Japan leads in LLZO-specific patents. The United States maintains competitive advantage in fundamental research addressing grain boundary challenges, particularly through national laboratory initiatives and university partnerships.
Market forecasts indicate that successful commercialization of low-resistance LLZO electrolytes could accelerate solid-state battery market penetration by 2-3 years compared to current projections, potentially capturing 15-20% of the total battery market by 2030, representing a $25-30 billion opportunity.
Current Challenges in LLZO Grain Boundary Resistance
Despite significant advancements in LLZO solid electrolyte technology, grain boundary resistance remains one of the most critical challenges limiting its practical application in solid-state batteries. The high resistance at grain boundaries can account for up to 70-80% of the total ionic resistance in sintered LLZO pellets, severely impacting overall battery performance and efficiency.
The fundamental issue stems from the complex microstructural characteristics at grain boundaries. These regions often exhibit chemical and structural inhomogeneities that impede lithium ion transport. Specifically, the formation of Li2CO3 and LiOH at grain boundaries due to LLZO's high reactivity with atmospheric CO2 and moisture creates resistive interfacial layers that significantly hinder ionic conductivity.
Another major challenge is the presence of space charge layers at grain boundaries. These electrically charged regions create potential barriers that lithium ions must overcome, further increasing resistance. The width of these space charge layers typically ranges from 1-5 nm but their cumulative effect across numerous grain boundaries substantially impacts overall conductivity.
Controlling grain size distribution presents another significant hurdle. Current sintering techniques often produce heterogeneous grain structures with varying sizes (1-100 μm), creating inconsistent boundary properties throughout the material. This heterogeneity makes systematic improvement difficult as boundary characteristics vary widely within the same sample.
The densification process itself introduces challenges, as achieving high density (>97%) without excessive grain growth requires precise control of sintering parameters. Conventional sintering approaches often result in a trade-off between density and grain size, both of which affect boundary resistance.
Dopant segregation at grain boundaries further complicates the picture. While dopants like Al, Ga, and Ta are essential for stabilizing the cubic phase of LLZO, they tend to concentrate at grain boundaries, altering local chemistry and potentially creating additional resistance pathways.
The characterization of grain boundaries presents methodological challenges as well. Current analytical techniques have limitations in resolving the nanoscale chemical and structural features of boundaries, making it difficult to fully understand resistance mechanisms and develop targeted solutions.
Finally, the scalability of laboratory solutions to industrial manufacturing remains problematic. Techniques that effectively reduce grain boundary resistance in small-scale samples often face significant implementation barriers when scaled to commercial production volumes, limiting practical application in commercial battery systems.
The fundamental issue stems from the complex microstructural characteristics at grain boundaries. These regions often exhibit chemical and structural inhomogeneities that impede lithium ion transport. Specifically, the formation of Li2CO3 and LiOH at grain boundaries due to LLZO's high reactivity with atmospheric CO2 and moisture creates resistive interfacial layers that significantly hinder ionic conductivity.
Another major challenge is the presence of space charge layers at grain boundaries. These electrically charged regions create potential barriers that lithium ions must overcome, further increasing resistance. The width of these space charge layers typically ranges from 1-5 nm but their cumulative effect across numerous grain boundaries substantially impacts overall conductivity.
Controlling grain size distribution presents another significant hurdle. Current sintering techniques often produce heterogeneous grain structures with varying sizes (1-100 μm), creating inconsistent boundary properties throughout the material. This heterogeneity makes systematic improvement difficult as boundary characteristics vary widely within the same sample.
The densification process itself introduces challenges, as achieving high density (>97%) without excessive grain growth requires precise control of sintering parameters. Conventional sintering approaches often result in a trade-off between density and grain size, both of which affect boundary resistance.
Dopant segregation at grain boundaries further complicates the picture. While dopants like Al, Ga, and Ta are essential for stabilizing the cubic phase of LLZO, they tend to concentrate at grain boundaries, altering local chemistry and potentially creating additional resistance pathways.
The characterization of grain boundaries presents methodological challenges as well. Current analytical techniques have limitations in resolving the nanoscale chemical and structural features of boundaries, making it difficult to fully understand resistance mechanisms and develop targeted solutions.
Finally, the scalability of laboratory solutions to industrial manufacturing remains problematic. Techniques that effectively reduce grain boundary resistance in small-scale samples often face significant implementation barriers when scaled to commercial production volumes, limiting practical application in commercial battery systems.
Current Approaches to Reduce LLZO Grain Boundary Resistance
01 Doping strategies to reduce grain boundary resistance in LLZO
Various dopants can be incorporated into LLZO to reduce grain boundary resistance. Elements such as aluminum, gallium, tantalum, and niobium can be strategically added during the sintering process to modify the grain boundary properties. These dopants can stabilize the cubic phase of LLZO, enhance ionic conductivity across grain boundaries, and reduce interfacial resistance. The optimal doping concentration and combination of dopants can significantly improve the overall performance of LLZO as a solid electrolyte.- Doping strategies to reduce grain boundary resistance: Various dopants can be incorporated into LLZO to reduce grain boundary resistance. Elements such as Al, Ga, Ta, and Nb are commonly used to stabilize the cubic phase of LLZO and improve ionic conductivity across grain boundaries. These dopants can modify the grain boundary structure, reduce lithium ion transport barriers, and enhance overall solid electrolyte performance in lithium batteries.
- Sintering process optimization for LLZO: The sintering process significantly affects LLZO grain boundary properties. Parameters such as temperature, duration, atmosphere, and pressure during sintering can be optimized to control grain size, reduce porosity, and minimize grain boundary resistance. Advanced sintering techniques like hot pressing, spark plasma sintering, and two-step sintering processes can produce dense LLZO ceramics with improved grain boundary conductivity.
- Surface modification and coating of LLZO grains: Surface treatments and coatings can be applied to LLZO particles before or after sintering to modify grain boundary properties. These treatments include applying thin layers of lithium salts, polymers, or other ceramic materials at grain boundaries to facilitate lithium ion transport. Such modifications can reduce interfacial resistance, improve wettability with electrodes, and enhance overall ionic conductivity of the sintered material.
- Microstructure engineering of LLZO: Controlling the microstructure of sintered LLZO is crucial for minimizing grain boundary resistance. Techniques to engineer grain size, orientation, and distribution can significantly impact ionic conductivity. Methods such as templated grain growth, addition of sintering aids, and controlled cooling rates can be employed to develop preferred microstructures with reduced grain boundary resistance and enhanced lithium ion transport pathways.
- Composite and hybrid LLZO electrolytes: Incorporating LLZO into composite or hybrid structures can effectively address grain boundary resistance issues. By combining LLZO with polymers, other ceramic materials, or liquid electrolytes, interfaces between LLZO grains can be modified to enhance ionic conductivity. These composite approaches can provide flexible pathways for lithium ion transport while maintaining the mechanical stability and safety advantages of the ceramic component.
02 Sintering process optimization for LLZO grain boundary control
The sintering process parameters significantly impact the grain boundary resistance in LLZO. Factors such as sintering temperature, duration, atmosphere, and pressure can be optimized to control grain growth and boundary formation. Advanced sintering techniques like hot pressing, spark plasma sintering, and two-step sintering processes can be employed to achieve dense LLZO ceramics with minimized grain boundary resistance. Controlled cooling rates after sintering also play a crucial role in determining the final microstructure and grain boundary properties.Expand Specific Solutions03 Surface modification and coating techniques for LLZO grain boundaries
Surface modification and coating techniques can effectively reduce grain boundary resistance in LLZO. Applying thin layers of materials such as lithium salts, polymers, or other ceramic materials at the grain boundaries can improve lithium ion transport. These coatings can fill voids, passivate reactive sites, and create favorable interfaces for ion conduction. Post-sintering treatments including chemical etching, ion exchange, or thermal annealing can also modify grain boundary composition and structure to enhance ionic conductivity.Expand Specific Solutions04 Microstructure engineering of LLZO for reduced grain boundary resistance
Controlling the microstructure of LLZO through engineering approaches can minimize grain boundary resistance. Techniques include manipulating grain size distribution, grain orientation, and porosity. Smaller grain sizes increase the number of grain boundaries but can reduce the total resistance path when properly engineered. Creating textured LLZO with aligned grains can provide preferential pathways for lithium ion transport. Additionally, controlling the density and distribution of secondary phases at grain boundaries can significantly impact the overall ionic conductivity.Expand Specific Solutions05 Composite and hybrid approaches to overcome LLZO grain boundary limitations
Composite and hybrid approaches combine LLZO with other materials to overcome grain boundary resistance limitations. Incorporating polymer electrolytes, other ceramic materials, or conductive fillers at grain boundaries can create alternative ion transport pathways. These composite structures can maintain the mechanical stability of LLZO while improving interfacial properties. Gradient structures with compositional variations from the grain interior to boundaries can also be designed to optimize both bulk and grain boundary conductivity simultaneously.Expand Specific Solutions
Leading Research Groups and Companies in LLZO Development
The solid-state battery market, particularly LLZO grain boundary resistance reduction technology, is in a growth phase with increasing market size driven by electric vehicle demand. The technology maturity varies across players, with academic institutions like University of California and Korea Advanced Institute of Science & Technology conducting foundational research, while commercial entities are advancing toward practical applications. Japanese companies including Murata Manufacturing, Kyocera, and Sumitomo Metal Mining lead in ceramic materials expertise. Battery manufacturers like QuantumScape and SK On are integrating LLZO into commercial products. The competitive landscape shows regional clusters in Japan, South Korea, and the US, with collaboration between academia and industry accelerating technological advancement toward commercialization.
Murata Manufacturing Co. Ltd.
Technical Solution: Murata Manufacturing has developed a proprietary technique for reducing grain boundary resistance in sintered LLZO for solid-state battery applications. Their approach combines precise control of starting powder characteristics with an optimized sintering protocol. Murata's process begins with synthesizing nano-sized LLZO precursor particles (100-300 nm) with carefully controlled stoichiometry and dopant distribution. They employ a unique two-step sintering process: first, a low-temperature densification step (900-950°C) under moderate pressure to achieve initial compaction while minimizing grain growth, followed by a higher temperature (1100-1150°C) sintering step with precisely controlled atmosphere to optimize grain boundary properties. Murata has also developed a proprietary grain boundary modification technique using lithium-rich phases that segregate to grain boundaries during cooling, creating favorable pathways for Li-ion transport. Their LLZO materials demonstrate grain boundary resistance reduced by approximately 65% compared to conventional sintering methods, achieving total ionic conductivity of 0.8-1.0 mS/cm at room temperature with excellent mechanical properties suitable for thin-film electrolyte applications.
Strengths: Their two-step sintering approach achieves high density and low grain boundary resistance while maintaining fine grain structure for improved mechanical properties. The process is compatible with Murata's existing ceramic manufacturing infrastructure. Weaknesses: The precise control of nano-sized precursor particles and sintering atmosphere adds complexity to the manufacturing process, potentially increasing production costs.
Wuhan University of Technology
Technical Solution: Wuhan University of Technology has developed an advanced approach to reducing grain boundary resistance in sintered LLZO through a combination of innovative processing techniques. Their method employs a sol-gel synthesis route with precise control of precursor chemistry, followed by a two-stage sintering protocol. The first stage involves low-temperature calcination (700-800°C) to form phase-pure LLZO powder, while the second stage utilizes spark plasma sintering (SPS) at moderate temperatures (900-1000°C) with applied pressure (50-100 MPa). This approach significantly reduces sintering time to minutes rather than hours, minimizing Li volatilization and maintaining stoichiometry. Their research demonstrates that controlling the grain boundary composition through targeted dopants (including Ga, Ta, and Al in specific ratios) creates favorable Li-ion transport pathways at grain interfaces. The resulting LLZO exhibits reduced grain boundary resistance with total ionic conductivity reaching 1.2-1.5 mS/cm at room temperature, among the highest reported values for polycrystalline LLZO.
Strengths: Their SPS approach achieves high density and excellent ionic conductivity while significantly reducing processing time and energy consumption. The multi-dopant strategy effectively addresses multiple aspects of grain boundary resistance. Weaknesses: The specialized SPS equipment requirements may limit widespread adoption, and precise control of multiple dopants increases process complexity.
Key Patents and Innovations in LLZO Sintering Technology
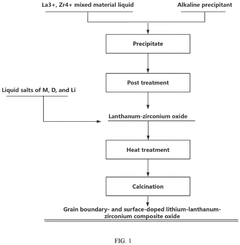
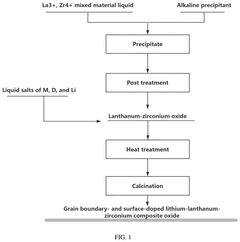
Grain boundary- and surface-doped lithium-lanthanum-zirconium composite oxide electrolyte, preparation method therefor, and application thereof
PatentPendingEP4451406A1
Innovation
- A lithium-lanthanum-zirconium composite oxide is doped at the surface and grain boundary with specific cationic and anionic elements, reducing grain boundary resistance and enhancing ionic conductivity through a method involving co-precipitation and heat treatment processes.
Grain boundary- and surface-doped lithium-lanthanum-zirconium composite oxide electrolyte, preparation method therefor, and application thereof
PatentPendingUS20240363896A1
Innovation
- A grain boundary- and surface-doped lithium-lanthanum-zirconium composite oxide solid electrolyte is developed, where doping elements are strategically located at grain boundaries and surfaces to reduce resistance and enhance ionic conductivity, using a method that includes mixing aqueous solutions of lanthanum and zirconium compounds, precipitation reactions, and heat treatments to achieve a garnet-type structure with improved doping distribution.
Material Characterization Techniques for LLZO Interfaces
Effective characterization of LLZO interfaces is crucial for understanding and mitigating grain boundary resistance in sintered LLZO solid electrolytes. Advanced microscopy techniques, particularly scanning electron microscopy (SEM) and transmission electron microscopy (TEM), provide essential morphological and structural information about grain boundaries. SEM offers valuable insights into grain size distribution, boundary morphology, and porosity, while TEM enables atomic-level examination of interfacial structures and defects that contribute to resistance.
Spectroscopic methods complement microscopic analysis by revealing chemical compositions and bonding states at interfaces. X-ray photoelectron spectroscopy (XPS) can detect elemental compositions and chemical states at grain boundaries, while Raman spectroscopy identifies local structural variations and phase distributions. Energy-dispersive X-ray spectroscopy (EDX) mapping, often coupled with electron microscopy, visualizes elemental segregation at boundaries—a critical factor affecting ionic conductivity.
Electrical characterization techniques provide direct measurements of grain boundary resistance contributions. Electrochemical impedance spectroscopy (EIS) distinguishes between bulk and grain boundary resistances through frequency-dependent measurements. Temperature-dependent conductivity measurements help determine activation energies for ion transport across boundaries. Four-point probe measurements can isolate boundary resistance from contact resistance, offering more accurate assessments.
Advanced synchrotron-based techniques offer non-destructive analysis of LLZO interfaces. X-ray absorption spectroscopy (XAS) provides information about local coordination environments and oxidation states, while small-angle X-ray scattering (SAXS) characterizes nanoscale features at interfaces. These techniques are particularly valuable for in-situ studies during sintering or electrochemical cycling.
Computational modeling approaches increasingly complement experimental characterization. Density functional theory (DFT) calculations predict interfacial energetics and ion transport mechanisms, while molecular dynamics simulations model ion movement across boundaries under various conditions. These computational methods help interpret experimental results and guide interface engineering strategies.
Emerging techniques like atom probe tomography (APT) and in-situ TEM during heating or biasing provide unprecedented insights into dynamic processes at grain boundaries. Time-of-flight secondary ion mass spectrometry (ToF-SIMS) offers high-sensitivity detection of trace impurities that may segregate to boundaries and increase resistance. These advanced characterization methods collectively form a powerful toolkit for understanding and ultimately reducing grain boundary resistance in sintered LLZO.
Spectroscopic methods complement microscopic analysis by revealing chemical compositions and bonding states at interfaces. X-ray photoelectron spectroscopy (XPS) can detect elemental compositions and chemical states at grain boundaries, while Raman spectroscopy identifies local structural variations and phase distributions. Energy-dispersive X-ray spectroscopy (EDX) mapping, often coupled with electron microscopy, visualizes elemental segregation at boundaries—a critical factor affecting ionic conductivity.
Electrical characterization techniques provide direct measurements of grain boundary resistance contributions. Electrochemical impedance spectroscopy (EIS) distinguishes between bulk and grain boundary resistances through frequency-dependent measurements. Temperature-dependent conductivity measurements help determine activation energies for ion transport across boundaries. Four-point probe measurements can isolate boundary resistance from contact resistance, offering more accurate assessments.
Advanced synchrotron-based techniques offer non-destructive analysis of LLZO interfaces. X-ray absorption spectroscopy (XAS) provides information about local coordination environments and oxidation states, while small-angle X-ray scattering (SAXS) characterizes nanoscale features at interfaces. These techniques are particularly valuable for in-situ studies during sintering or electrochemical cycling.
Computational modeling approaches increasingly complement experimental characterization. Density functional theory (DFT) calculations predict interfacial energetics and ion transport mechanisms, while molecular dynamics simulations model ion movement across boundaries under various conditions. These computational methods help interpret experimental results and guide interface engineering strategies.
Emerging techniques like atom probe tomography (APT) and in-situ TEM during heating or biasing provide unprecedented insights into dynamic processes at grain boundaries. Time-of-flight secondary ion mass spectrometry (ToF-SIMS) offers high-sensitivity detection of trace impurities that may segregate to boundaries and increase resistance. These advanced characterization methods collectively form a powerful toolkit for understanding and ultimately reducing grain boundary resistance in sintered LLZO.
Scalability and Manufacturing Considerations for LLZO Electrolytes
The scalability of LLZO electrolyte production represents a critical challenge for the commercialization of solid-state batteries. Current laboratory-scale synthesis methods for LLZO, while effective for research purposes, face significant barriers when transitioning to industrial-scale manufacturing. These challenges primarily stem from the complex sintering processes required to achieve high ionic conductivity while minimizing grain boundary resistance.
Traditional solid-state reaction methods for LLZO synthesis typically require high temperatures (>1000°C) and extended sintering times (>12 hours), resulting in energy-intensive processes that are difficult to scale economically. Additionally, these conditions often lead to lithium volatilization, which creates compositional inhomogeneities and increases grain boundary resistance in the final product.
Recent advancements in manufacturing techniques have focused on reducing sintering temperatures and times while maintaining or improving ionic conductivity. Solution-based synthesis methods, including sol-gel and co-precipitation approaches, have demonstrated promise for producing more homogeneous LLZO precursors, which can subsequently be sintered at lower temperatures with reduced grain boundary resistance.
Cold sintering processes and field-assisted sintering techniques (FAST), such as spark plasma sintering (SPS), represent promising alternatives to conventional high-temperature sintering. These methods apply pressure during the sintering process, enabling densification at lower temperatures and shorter times, which helps preserve the optimal LLZO microstructure and minimize grain boundary resistance.
The economic viability of LLZO electrolytes also depends on raw material considerations. The high cost of lithium salts and other precursors necessitates efficient material utilization and potentially the development of recycling processes for production waste. Furthermore, quality control measures must be implemented to ensure consistent ionic conductivity across large-scale production batches.
Equipment scaling presents another significant challenge. Current laboratory equipment for LLZO synthesis cannot simply be enlarged for industrial production. Instead, continuous or semi-continuous processing methods need to be developed to replace batch processes, potentially incorporating in-line monitoring techniques to ensure consistent product quality and minimize grain boundary resistance.
Environmental considerations also play a crucial role in scaling LLZO production. The high energy consumption of traditional sintering processes contributes significantly to the carbon footprint of LLZO electrolytes. Developing more energy-efficient manufacturing methods will be essential for sustainable large-scale production, aligning with global trends toward greener manufacturing practices in the battery industry.
Traditional solid-state reaction methods for LLZO synthesis typically require high temperatures (>1000°C) and extended sintering times (>12 hours), resulting in energy-intensive processes that are difficult to scale economically. Additionally, these conditions often lead to lithium volatilization, which creates compositional inhomogeneities and increases grain boundary resistance in the final product.
Recent advancements in manufacturing techniques have focused on reducing sintering temperatures and times while maintaining or improving ionic conductivity. Solution-based synthesis methods, including sol-gel and co-precipitation approaches, have demonstrated promise for producing more homogeneous LLZO precursors, which can subsequently be sintered at lower temperatures with reduced grain boundary resistance.
Cold sintering processes and field-assisted sintering techniques (FAST), such as spark plasma sintering (SPS), represent promising alternatives to conventional high-temperature sintering. These methods apply pressure during the sintering process, enabling densification at lower temperatures and shorter times, which helps preserve the optimal LLZO microstructure and minimize grain boundary resistance.
The economic viability of LLZO electrolytes also depends on raw material considerations. The high cost of lithium salts and other precursors necessitates efficient material utilization and potentially the development of recycling processes for production waste. Furthermore, quality control measures must be implemented to ensure consistent ionic conductivity across large-scale production batches.
Equipment scaling presents another significant challenge. Current laboratory equipment for LLZO synthesis cannot simply be enlarged for industrial production. Instead, continuous or semi-continuous processing methods need to be developed to replace batch processes, potentially incorporating in-line monitoring techniques to ensure consistent product quality and minimize grain boundary resistance.
Environmental considerations also play a crucial role in scaling LLZO production. The high energy consumption of traditional sintering processes contributes significantly to the carbon footprint of LLZO electrolytes. Developing more energy-efficient manufacturing methods will be essential for sustainable large-scale production, aligning with global trends toward greener manufacturing practices in the battery industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!