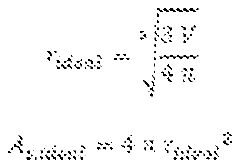Llzo–Li metal interface: dendrite suppression strategies
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO-Li Metal Interface Fundamentals and Objectives
The evolution of solid-state lithium batteries represents a significant advancement in energy storage technology, with LLZO (Li7La3Zr2O12) garnet-type solid electrolytes emerging as promising candidates due to their high ionic conductivity and stability against lithium metal. However, the interface between LLZO and lithium metal presents critical challenges that have hindered commercial implementation of these batteries.
Historically, the development of LLZO electrolytes began in the early 2000s, with researchers identifying their potential as solid electrolytes. The technical trajectory has since focused on improving ionic conductivity, mechanical properties, and most critically, interface stability with lithium metal anodes. The formation of lithium dendrites at this interface has been recognized as a primary failure mechanism, leading to short circuits and compromised battery safety.
The fundamental challenge lies in the poor wettability between LLZO and lithium metal, creating voids and high interfacial resistance. These imperfections become nucleation sites for dendrite growth during cycling. Additionally, the mechanical properties of the interface play a crucial role, as dendrites can propagate through grain boundaries or defects in the LLZO structure.
Recent technological trends have shifted toward engineering this critical interface through various strategies, including surface modifications, interlayers, and pressure application techniques. The field has evolved from simply identifying the dendrite problem to developing sophisticated multi-faceted approaches that address both chemical and mechanical aspects of the interface.
The primary technical objectives for LLZO-Li metal interface research include: reducing interfacial resistance to below 10 Ω·cm², achieving dendrite-free cycling for over 1000 cycles, maintaining stable performance at practical current densities (>1 mA/cm²), and developing scalable manufacturing processes compatible with existing battery production infrastructure.
Future technical goals focus on understanding the fundamental mechanisms of dendrite nucleation and growth at atomic and molecular levels, developing in-situ characterization techniques to monitor interface evolution during cycling, and creating predictive models to accelerate interface optimization. Additionally, researchers aim to design interfaces that self-heal or inherently suppress dendrite formation without external interventions.
The ultimate objective is to enable commercial-scale production of solid-state batteries with LLZO electrolytes that offer energy densities exceeding 400 Wh/kg, cycle life beyond 1000 cycles, and enhanced safety profiles compared to conventional lithium-ion batteries, thereby revolutionizing applications in electric vehicles, grid storage, and portable electronics.
Historically, the development of LLZO electrolytes began in the early 2000s, with researchers identifying their potential as solid electrolytes. The technical trajectory has since focused on improving ionic conductivity, mechanical properties, and most critically, interface stability with lithium metal anodes. The formation of lithium dendrites at this interface has been recognized as a primary failure mechanism, leading to short circuits and compromised battery safety.
The fundamental challenge lies in the poor wettability between LLZO and lithium metal, creating voids and high interfacial resistance. These imperfections become nucleation sites for dendrite growth during cycling. Additionally, the mechanical properties of the interface play a crucial role, as dendrites can propagate through grain boundaries or defects in the LLZO structure.
Recent technological trends have shifted toward engineering this critical interface through various strategies, including surface modifications, interlayers, and pressure application techniques. The field has evolved from simply identifying the dendrite problem to developing sophisticated multi-faceted approaches that address both chemical and mechanical aspects of the interface.
The primary technical objectives for LLZO-Li metal interface research include: reducing interfacial resistance to below 10 Ω·cm², achieving dendrite-free cycling for over 1000 cycles, maintaining stable performance at practical current densities (>1 mA/cm²), and developing scalable manufacturing processes compatible with existing battery production infrastructure.
Future technical goals focus on understanding the fundamental mechanisms of dendrite nucleation and growth at atomic and molecular levels, developing in-situ characterization techniques to monitor interface evolution during cycling, and creating predictive models to accelerate interface optimization. Additionally, researchers aim to design interfaces that self-heal or inherently suppress dendrite formation without external interventions.
The ultimate objective is to enable commercial-scale production of solid-state batteries with LLZO electrolytes that offer energy densities exceeding 400 Wh/kg, cycle life beyond 1000 cycles, and enhanced safety profiles compared to conventional lithium-ion batteries, thereby revolutionizing applications in electric vehicles, grid storage, and portable electronics.
Market Analysis for Solid-State Battery Technologies
The solid-state battery market is experiencing unprecedented growth, driven by increasing demand for safer, higher energy density power solutions across multiple industries. Current market valuations place the global solid-state battery sector at approximately $500 million in 2023, with projections indicating potential growth to reach $8-10 billion by 2030, representing a compound annual growth rate (CAGR) of over 34% during this forecast period.
Electric vehicles constitute the primary market driver, with automotive manufacturers investing heavily in solid-state technology to overcome range anxiety and safety concerns associated with conventional lithium-ion batteries. Major automakers including Toyota, Volkswagen, and BMW have announced significant investments totaling over $13.5 billion in solid-state battery development programs specifically targeting LLZO-based technologies.
Consumer electronics represents the second largest application segment, where demand for longer-lasting, safer batteries continues to grow at 25% annually. The aerospace and defense sectors are emerging as significant market players, with investments increasing by 40% year-over-year as these industries seek lightweight, high-performance energy storage solutions.
Regionally, Asia-Pacific dominates the solid-state battery market with approximately 45% market share, led by Japan and South Korea where companies like Toyota, Samsung, and LG are headquartered. North America follows with 30% market share, while Europe accounts for 20% of the global market.
The LLZO-Li metal interface technology segment specifically is projected to grow at a CAGR of 38% through 2030, outpacing the broader solid-state battery market. This accelerated growth reflects the critical importance of dendrite suppression strategies in commercializing viable solid-state batteries.
Key market restraints include high manufacturing costs, with current production expenses 8-10 times higher than conventional lithium-ion batteries. Technical challenges related to LLZO-Li metal interfaces and dendrite formation remain significant barriers to mass commercialization.
Venture capital funding in solid-state battery startups focusing on LLZO interface technologies has reached $2.8 billion in 2023, a 65% increase from the previous year. This investment surge indicates strong market confidence in eventual technical solutions to current dendrite formation challenges.
Market analysts predict that successful commercialization of effective dendrite suppression strategies could accelerate market adoption timelines by 2-3 years, potentially increasing the total addressable market to $15 billion by 2032.
Electric vehicles constitute the primary market driver, with automotive manufacturers investing heavily in solid-state technology to overcome range anxiety and safety concerns associated with conventional lithium-ion batteries. Major automakers including Toyota, Volkswagen, and BMW have announced significant investments totaling over $13.5 billion in solid-state battery development programs specifically targeting LLZO-based technologies.
Consumer electronics represents the second largest application segment, where demand for longer-lasting, safer batteries continues to grow at 25% annually. The aerospace and defense sectors are emerging as significant market players, with investments increasing by 40% year-over-year as these industries seek lightweight, high-performance energy storage solutions.
Regionally, Asia-Pacific dominates the solid-state battery market with approximately 45% market share, led by Japan and South Korea where companies like Toyota, Samsung, and LG are headquartered. North America follows with 30% market share, while Europe accounts for 20% of the global market.
The LLZO-Li metal interface technology segment specifically is projected to grow at a CAGR of 38% through 2030, outpacing the broader solid-state battery market. This accelerated growth reflects the critical importance of dendrite suppression strategies in commercializing viable solid-state batteries.
Key market restraints include high manufacturing costs, with current production expenses 8-10 times higher than conventional lithium-ion batteries. Technical challenges related to LLZO-Li metal interfaces and dendrite formation remain significant barriers to mass commercialization.
Venture capital funding in solid-state battery startups focusing on LLZO interface technologies has reached $2.8 billion in 2023, a 65% increase from the previous year. This investment surge indicates strong market confidence in eventual technical solutions to current dendrite formation challenges.
Market analysts predict that successful commercialization of effective dendrite suppression strategies could accelerate market adoption timelines by 2-3 years, potentially increasing the total addressable market to $15 billion by 2032.
Current Challenges in LLZO-Li Metal Interface Engineering
Despite significant advancements in solid-state battery technology, the LLZO-Li metal interface presents several persistent challenges that impede commercial viability. The primary issue remains the formation of lithium dendrites at the interface, which can penetrate through the solid electrolyte, causing short circuits and potential safety hazards. This dendrite growth is exacerbated by the inherent high interfacial impedance between LLZO and lithium metal, resulting in non-uniform lithium deposition during cycling.
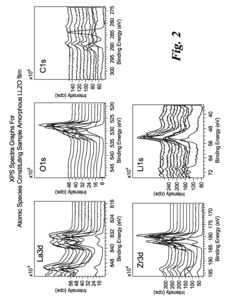
Surface contamination of LLZO represents another critical challenge. When exposed to air, LLZO readily reacts with atmospheric components, forming lithium carbonate (Li₂CO₃) and lithium hydroxide (LiOH) layers. These reaction products significantly increase interfacial resistance and hinder lithium ion transport across the interface, ultimately degrading battery performance and cycle life.
The mechanical stability of the LLZO-Li interface poses additional difficulties. During battery operation, volume changes in the lithium metal anode can create physical gaps at the interface, leading to contact loss and increased impedance. This dynamic interface behavior complicates the maintenance of stable electrochemical performance over extended cycling periods.
Current manufacturing processes also present substantial hurdles. Achieving and maintaining intimate contact between LLZO and lithium metal during battery assembly requires precise control of temperature, pressure, and surface conditions. The scalability of these processes for mass production remains questionable, particularly considering the reactive nature of lithium metal and the sensitivity of LLZO surfaces.
The characterization of the LLZO-Li interface presents methodological challenges as well. In-situ and operando techniques for monitoring interface evolution during cycling are limited, making it difficult to fully understand degradation mechanisms and evaluate improvement strategies in real-time conditions.
From a materials perspective, the intrinsic properties of LLZO, including its relatively low electronic conductivity and high grain boundary resistance, contribute to non-uniform current distribution at the interface. This heterogeneity promotes preferential lithium deposition at high-current density sites, accelerating dendrite nucleation and growth.
The development of effective interface engineering strategies is further complicated by the trade-off between different performance metrics. For instance, approaches that enhance wetting and reduce interfacial resistance may simultaneously compromise mechanical strength or chemical stability, necessitating careful optimization of multiple parameters simultaneously.
Surface contamination of LLZO represents another critical challenge. When exposed to air, LLZO readily reacts with atmospheric components, forming lithium carbonate (Li₂CO₃) and lithium hydroxide (LiOH) layers. These reaction products significantly increase interfacial resistance and hinder lithium ion transport across the interface, ultimately degrading battery performance and cycle life.
The mechanical stability of the LLZO-Li interface poses additional difficulties. During battery operation, volume changes in the lithium metal anode can create physical gaps at the interface, leading to contact loss and increased impedance. This dynamic interface behavior complicates the maintenance of stable electrochemical performance over extended cycling periods.
Current manufacturing processes also present substantial hurdles. Achieving and maintaining intimate contact between LLZO and lithium metal during battery assembly requires precise control of temperature, pressure, and surface conditions. The scalability of these processes for mass production remains questionable, particularly considering the reactive nature of lithium metal and the sensitivity of LLZO surfaces.
The characterization of the LLZO-Li interface presents methodological challenges as well. In-situ and operando techniques for monitoring interface evolution during cycling are limited, making it difficult to fully understand degradation mechanisms and evaluate improvement strategies in real-time conditions.
From a materials perspective, the intrinsic properties of LLZO, including its relatively low electronic conductivity and high grain boundary resistance, contribute to non-uniform current distribution at the interface. This heterogeneity promotes preferential lithium deposition at high-current density sites, accelerating dendrite nucleation and growth.
The development of effective interface engineering strategies is further complicated by the trade-off between different performance metrics. For instance, approaches that enhance wetting and reduce interfacial resistance may simultaneously compromise mechanical strength or chemical stability, necessitating careful optimization of multiple parameters simultaneously.
State-of-the-Art Dendrite Suppression Approaches
01 LLZO solid electrolyte interface modifications
Various interface modifications between LLZO solid electrolytes and lithium metal can effectively suppress dendrite formation. These modifications include surface treatments, interlayers, and coatings that improve wetting and contact between the lithium metal and the LLZO electrolyte. By enhancing the interfacial stability and reducing interfacial resistance, these modifications help prevent lithium dendrite growth and improve the overall performance of solid-state batteries.- Surface modification of LLZO for improved interface stability: Surface modification techniques can be applied to LLZO solid electrolytes to improve the interface stability with lithium metal anodes. These modifications can include coating with various materials, surface treatments, or chemical modifications that enhance the wettability and reduce the interfacial resistance between LLZO and lithium metal. Such modifications help prevent dendrite formation by creating a more uniform and stable interface that suppresses lithium dendrite growth during cycling.
- Composite electrolyte structures with LLZO: Composite electrolyte structures incorporating LLZO with other materials can effectively suppress dendrite formation at the lithium metal interface. These composites may combine LLZO with polymers, other ceramic materials, or additives to create hybrid electrolytes with enhanced mechanical properties and ionic conductivity. The composite structure provides physical barriers against dendrite penetration while maintaining high ionic conductivity, thus improving the overall performance and safety of lithium metal batteries.
- Microstructure engineering of LLZO: Engineering the microstructure of LLZO solid electrolytes, including grain size control, porosity management, and crystal structure optimization, can significantly improve dendrite suppression capabilities. By controlling the grain boundaries and reducing defects in the LLZO structure, lithium ion transport pathways can be optimized while physical barriers to dendrite propagation are strengthened. These microstructural modifications create more uniform lithium deposition and reduce the likelihood of dendrite formation at the interface.
- Interfacial layer engineering between LLZO and lithium metal: Introducing engineered interfacial layers between LLZO and lithium metal can effectively suppress dendrite formation. These interfacial layers can be composed of various materials that facilitate uniform lithium ion transport while preventing direct contact between lithium metal and LLZO. The engineered interfaces help distribute current density evenly across the surface, reduce local hotspots for dendrite nucleation, and provide additional mechanical resistance against dendrite penetration.
- Doping strategies for LLZO to enhance dendrite resistance: Doping LLZO with various elements can enhance its dendrite resistance properties when interfacing with lithium metal. Dopants can modify the crystal structure, improve ionic conductivity, enhance mechanical properties, and stabilize the interface with lithium metal. Common dopants include aluminum, tantalum, gallium, and other elements that can occupy specific sites in the LLZO structure. These doping strategies help create more stable and dendrite-resistant interfaces by modifying the fundamental properties of the LLZO electrolyte.
02 Doping strategies for LLZO electrolytes
Doping LLZO with various elements can enhance its ionic conductivity and mechanical properties, which are crucial for dendrite suppression. Common dopants include aluminum, gallium, tantalum, and niobium, which can stabilize the cubic phase of LLZO and improve grain boundary properties. These doping strategies help create a more uniform lithium ion distribution at the interface, reducing the likelihood of dendrite formation and improving the electrochemical stability of the LLZO-Li metal interface.Expand Specific Solutions03 Microstructure engineering of LLZO
Controlling the microstructure of LLZO through grain size optimization, porosity reduction, and densification techniques can significantly impact dendrite suppression. High-density LLZO with optimized grain boundaries provides fewer pathways for dendrite penetration. Advanced processing methods such as hot pressing, spark plasma sintering, and controlled crystallization can produce LLZO electrolytes with superior mechanical properties that effectively resist dendrite growth at the interface with lithium metal.Expand Specific Solutions04 Composite and hybrid electrolyte systems
Composite and hybrid electrolyte systems combining LLZO with polymers, other ceramic materials, or liquid electrolytes can create synergistic effects for dendrite suppression. These systems often feature improved mechanical flexibility, enhanced interfacial contact, and reduced interfacial resistance. The composite approach allows for customization of the electrolyte properties to address specific dendrite formation mechanisms while maintaining high ionic conductivity and electrochemical stability at the LLZO-Li metal interface.Expand Specific Solutions05 Advanced interface engineering techniques
Advanced interface engineering techniques such as atomic layer deposition, plasma treatment, and nanoscale coatings can create specialized interfaces between LLZO and lithium metal. These techniques modify the surface chemistry and structure of LLZO to promote uniform lithium deposition and prevent dendrite nucleation. Some approaches include creating artificial SEI layers, introducing buffer layers with gradient properties, or developing self-healing interfaces that can accommodate volume changes during cycling while maintaining dendrite suppression capabilities.Expand Specific Solutions
Leading Research Groups and Companies in LLZO Technology
The LLZO-Li metal interface dendrite suppression market is in an early growth phase, characterized by intensive research and development activities. The market is expanding as solid-state batteries gain traction, with projections indicating significant growth potential due to increasing demand for safer, higher-energy-density batteries. Technologically, this field remains in development with varying maturity levels across approaches. Academic institutions like Chinese Academy of Sciences, Rice University, University of Michigan, and Drexel University are leading fundamental research, while companies including Shenzhen Solid New Material Technology, TSMC, Corning, and Panasonic are advancing commercial applications. The competition landscape features collaboration between research institutions and industry players working to overcome interface stability challenges and scale manufacturing processes for commercial viability.
Chinese Academy of Sciences Institute of Physics
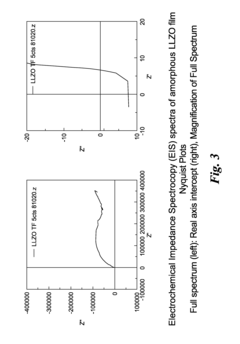
Technical Solution: The Chinese Academy of Sciences Institute of Physics has developed a multi-faceted approach to LLZO-Li metal interface dendrite suppression. Their strategy involves surface modification of LLZO with ultrathin Al2O3 layers deposited via atomic layer deposition (ALD), creating a buffer zone that improves wettability and reduces interfacial resistance. They've pioneered the use of pressure-assisted sintering techniques to achieve high-density LLZO with minimized grain boundaries, reducing potential dendrite nucleation sites. Additionally, they've implemented lithiophilic interlayers containing metals like indium and tin that form alloys with lithium, promoting uniform lithium deposition. Their research has demonstrated that controlling the surface chemistry and microstructure of LLZO can significantly enhance the critical current density before dendrite formation, achieving values exceeding 0.5 mA/cm² at room temperature.
Strengths: Advanced expertise in surface modification techniques and atomic-level interface engineering; comprehensive understanding of lithium transport mechanisms. Weaknesses: Laboratory-scale demonstrations may face challenges in scaling to commercial production; some approaches require expensive equipment like ALD systems.
The Regents of the University of Michigan
Technical Solution: The University of Michigan has developed innovative strategies for LLZO-Li metal interface dendrite suppression focusing on interface engineering and microstructure control. Their approach includes creating artificial interlayers between LLZO and lithium metal using thin films of metals (such as Al, Au) that react with lithium to form stable interphases. They've pioneered a technique involving the application of a molten lithium process that significantly improves wetting and contact at the LLZO-Li interface, reducing interfacial resistance to as low as 2 Ω·cm². Their research has demonstrated that controlling the surface chemistry through hydrogen annealing creates oxygen vacancies that enhance lithium wettability. Additionally, they've developed composite LLZO structures with polymer components that provide mechanical flexibility while maintaining ionic conductivity, allowing for accommodation of volume changes during cycling. Their work has shown that these strategies can increase critical current densities to over 1 mA/cm² and extend cycle life beyond 1000 hours without dendrite penetration.
Strengths: Comprehensive approach combining surface chemistry, interface engineering, and composite structures; strong focus on practical implementation and performance metrics. Weaknesses: Some techniques require precise control of processing conditions that may be difficult to maintain in mass production environments.
Critical Patents and Publications on LLZO Interface Modification
Ionically-conductive amorphous lithium lanthanum zirconium oxide
PatentActiveUS9034525B2
Innovation
- An amorphous lithium lanthanum zirconium oxide (LLZO) composition is developed, which is synthesized using a sol-gel process with specific alkoxide precursors and an alcohol-based solvent, forming a thin-film electrolyte medium that is compatible with lithium and has high ionic conductivity.
Lithium lanthanum zirconium oxide (LLZO) materials
PatentWO2023009380A2
Innovation
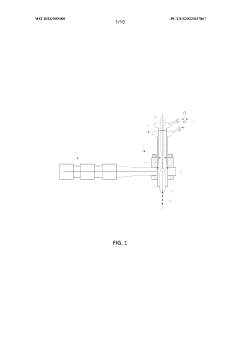
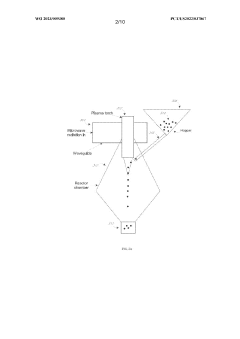
- A process involving heating a multiphase material comprising lithium carbonate in the presence of hydrogen gas at a temperature below its melting point, followed by further heating to crystallize lithium lanthanum zirconium oxide (LLZO) particles, utilizing a microwave plasma process to control particle size and reduce lithium loss.
Safety and Performance Implications of Dendrite Suppression
The effective suppression of lithium dendrites at the LLZO-Li metal interface carries profound implications for both safety and performance of solid-state batteries. From a safety perspective, dendrite suppression directly addresses the critical risk of internal short circuits that have historically plagued lithium metal battery systems. When dendrites penetrate through the solid electrolyte, they create conductive pathways between electrodes, potentially triggering thermal runaway events that can lead to catastrophic battery failure, including fire or explosion hazards.
Performance benefits of successful dendrite suppression are equally significant. By maintaining interface integrity, batteries can sustain higher current densities without degradation, enabling faster charging capabilities that are essential for practical applications like electric vehicles. The elimination of dendrite formation pathways also substantially extends cycle life, as each charge-discharge cycle no longer contributes to progressive interface deterioration.
Energy density advantages emerge as another crucial benefit of effective dendrite suppression strategies. When the LLZO-Li metal interface remains stable, battery designs can incorporate thinner solid electrolytes without compromising safety margins, directly increasing the overall energy density of the cell. This translates to lighter, more compact battery systems with greater range capabilities in mobile applications.
The operational temperature window of solid-state batteries also expands with robust dendrite suppression. Interface stability at elevated temperatures allows for more efficient thermal management systems, while low-temperature performance improves as dendrite-free interfaces maintain consistent ion transport pathways even under challenging thermal conditions.
From a commercial viability standpoint, dendrite suppression strategies that can be implemented using scalable manufacturing processes significantly reduce production costs while enhancing product reliability. The elimination of complex safety mechanisms required to mitigate dendrite-related failures simplifies battery pack design and reduces system complexity.
Long-term storage characteristics also benefit substantially, as dendrite-free interfaces resist the degradation mechanisms that typically occur during extended idle periods. This translates to longer shelf life and more predictable performance in applications where batteries may remain dormant for extended periods before deployment.
Performance benefits of successful dendrite suppression are equally significant. By maintaining interface integrity, batteries can sustain higher current densities without degradation, enabling faster charging capabilities that are essential for practical applications like electric vehicles. The elimination of dendrite formation pathways also substantially extends cycle life, as each charge-discharge cycle no longer contributes to progressive interface deterioration.
Energy density advantages emerge as another crucial benefit of effective dendrite suppression strategies. When the LLZO-Li metal interface remains stable, battery designs can incorporate thinner solid electrolytes without compromising safety margins, directly increasing the overall energy density of the cell. This translates to lighter, more compact battery systems with greater range capabilities in mobile applications.
The operational temperature window of solid-state batteries also expands with robust dendrite suppression. Interface stability at elevated temperatures allows for more efficient thermal management systems, while low-temperature performance improves as dendrite-free interfaces maintain consistent ion transport pathways even under challenging thermal conditions.
From a commercial viability standpoint, dendrite suppression strategies that can be implemented using scalable manufacturing processes significantly reduce production costs while enhancing product reliability. The elimination of complex safety mechanisms required to mitigate dendrite-related failures simplifies battery pack design and reduces system complexity.
Long-term storage characteristics also benefit substantially, as dendrite-free interfaces resist the degradation mechanisms that typically occur during extended idle periods. This translates to longer shelf life and more predictable performance in applications where batteries may remain dormant for extended periods before deployment.
Manufacturing Scalability of LLZO-Based Solid Electrolytes
The scalability of LLZO-based solid electrolytes represents a critical challenge in transitioning from laboratory-scale demonstrations to commercial production of solid-state batteries incorporating lithium metal anodes. Current manufacturing processes for LLZO ceramics typically involve complex multi-step procedures including powder synthesis, consolidation, sintering, and surface treatments that are difficult to scale efficiently.
Traditional ceramic processing methods such as tape casting and dry pressing have been adapted for LLZO production, but these approaches face significant hurdles when scaled up. The high sintering temperatures (>1100°C) required for LLZO densification lead to substantial energy costs and potential lithium loss during processing. Additionally, maintaining phase purity and controlling grain boundaries at industrial scales remains problematic, directly impacting dendrite suppression capabilities at the LLZO-Li metal interface.
Recent advances in manufacturing techniques show promising directions for improved scalability. Cold sintering processes and field-assisted sintering technology (FAST) have demonstrated potential to reduce processing temperatures and times while maintaining the critical cubic phase structure necessary for optimal ionic conductivity. These methods could significantly reduce production costs and energy requirements while preserving the interfacial properties needed for dendrite suppression.
Thin film deposition techniques including pulsed laser deposition and sputtering offer alternative routes for manufacturing LLZO electrolytes with precisely controlled interfaces. However, these approaches currently face throughput limitations that restrict their application to specialized or small-format batteries rather than large-format automotive cells where dendrite suppression is particularly crucial.
The integration of LLZO with polymer components to form composite electrolytes presents another pathway toward manufacturing scalability. These composites can potentially be processed using conventional polymer processing equipment, significantly reducing capital investment requirements compared to specialized ceramic processing lines.
Supply chain considerations also impact scalability, with limited availability of high-purity precursors for LLZO synthesis. The cost and availability of lanthanum and zirconium precursors at industrial scales may become bottlenecks as production volumes increase. Developing robust supply chains and potentially identifying alternative dopants could mitigate these risks.
Ultimately, achieving manufacturing scalability for LLZO-based solid electrolytes will require parallel advances in materials processing, interface engineering, and equipment design. The successful translation of laboratory-scale dendrite suppression strategies to mass production environments will determine the commercial viability of LLZO-based solid-state batteries with lithium metal anodes.
Traditional ceramic processing methods such as tape casting and dry pressing have been adapted for LLZO production, but these approaches face significant hurdles when scaled up. The high sintering temperatures (>1100°C) required for LLZO densification lead to substantial energy costs and potential lithium loss during processing. Additionally, maintaining phase purity and controlling grain boundaries at industrial scales remains problematic, directly impacting dendrite suppression capabilities at the LLZO-Li metal interface.
Recent advances in manufacturing techniques show promising directions for improved scalability. Cold sintering processes and field-assisted sintering technology (FAST) have demonstrated potential to reduce processing temperatures and times while maintaining the critical cubic phase structure necessary for optimal ionic conductivity. These methods could significantly reduce production costs and energy requirements while preserving the interfacial properties needed for dendrite suppression.
Thin film deposition techniques including pulsed laser deposition and sputtering offer alternative routes for manufacturing LLZO electrolytes with precisely controlled interfaces. However, these approaches currently face throughput limitations that restrict their application to specialized or small-format batteries rather than large-format automotive cells where dendrite suppression is particularly crucial.
The integration of LLZO with polymer components to form composite electrolytes presents another pathway toward manufacturing scalability. These composites can potentially be processed using conventional polymer processing equipment, significantly reducing capital investment requirements compared to specialized ceramic processing lines.
Supply chain considerations also impact scalability, with limited availability of high-purity precursors for LLZO synthesis. The cost and availability of lanthanum and zirconium precursors at industrial scales may become bottlenecks as production volumes increase. Developing robust supply chains and potentially identifying alternative dopants could mitigate these risks.
Ultimately, achieving manufacturing scalability for LLZO-based solid electrolytes will require parallel advances in materials processing, interface engineering, and equipment design. The successful translation of laboratory-scale dendrite suppression strategies to mass production environments will determine the commercial viability of LLZO-based solid-state batteries with lithium metal anodes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!