Defect chemistry in LLZO: oxygen vacancies and lithium stoichiometry
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO Defect Chemistry Background and Objectives
Lithium garnet-type solid electrolytes, particularly Li7La3Zr2O12 (LLZO), have emerged as promising candidates for next-generation solid-state lithium batteries due to their high ionic conductivity, excellent stability against lithium metal, and wide electrochemical window. The development of LLZO has progressed significantly since its first report by Murugan et al. in 2007, evolving from the initial tetragonal phase with limited conductivity to the highly conductive cubic phase stabilized by various dopants.
The defect chemistry of LLZO represents a critical aspect governing its ionic conductivity and overall performance. Oxygen vacancies and lithium stoichiometry variations constitute the primary defect types that influence the material's properties. These defects create distortions in the crystal lattice, affecting lithium ion migration pathways and ultimately determining the material's ionic conductivity. Understanding and controlling these defects has become a central focus in LLZO research.
Historical trends show that research on LLZO defect chemistry has evolved from empirical doping approaches to more sophisticated atomic-level investigations using advanced characterization techniques and computational methods. Early studies primarily focused on stabilizing the cubic phase through aliovalent doping, while recent research has shifted toward precise defect engineering to optimize ionic transport properties.
The technological evolution in this field has been marked by several key milestones, including the discovery of Al-stabilized cubic LLZO, the identification of critical Li+ transport pathways, and the development of computational models that accurately predict defect formation energies and their impact on ionic conductivity. These advances have progressively enhanced our understanding of the structure-property relationships in LLZO.
The primary objective of current research in LLZO defect chemistry is to establish a comprehensive understanding of how oxygen vacancies and lithium stoichiometry variations influence the material's ionic conductivity, mechanical properties, and interfacial stability. This knowledge aims to enable precise defect engineering strategies that can optimize LLZO performance for practical solid-state battery applications.
Additional goals include developing scalable synthesis methods that can control defect concentrations with high precision, establishing reliable characterization protocols for defect quantification, and creating predictive models that can guide the design of next-generation LLZO variants with enhanced properties. These objectives align with the broader industry goal of commercializing high-performance solid-state batteries with improved safety and energy density compared to conventional liquid electrolyte systems.
The defect chemistry of LLZO represents a critical aspect governing its ionic conductivity and overall performance. Oxygen vacancies and lithium stoichiometry variations constitute the primary defect types that influence the material's properties. These defects create distortions in the crystal lattice, affecting lithium ion migration pathways and ultimately determining the material's ionic conductivity. Understanding and controlling these defects has become a central focus in LLZO research.
Historical trends show that research on LLZO defect chemistry has evolved from empirical doping approaches to more sophisticated atomic-level investigations using advanced characterization techniques and computational methods. Early studies primarily focused on stabilizing the cubic phase through aliovalent doping, while recent research has shifted toward precise defect engineering to optimize ionic transport properties.
The technological evolution in this field has been marked by several key milestones, including the discovery of Al-stabilized cubic LLZO, the identification of critical Li+ transport pathways, and the development of computational models that accurately predict defect formation energies and their impact on ionic conductivity. These advances have progressively enhanced our understanding of the structure-property relationships in LLZO.
The primary objective of current research in LLZO defect chemistry is to establish a comprehensive understanding of how oxygen vacancies and lithium stoichiometry variations influence the material's ionic conductivity, mechanical properties, and interfacial stability. This knowledge aims to enable precise defect engineering strategies that can optimize LLZO performance for practical solid-state battery applications.
Additional goals include developing scalable synthesis methods that can control defect concentrations with high precision, establishing reliable characterization protocols for defect quantification, and creating predictive models that can guide the design of next-generation LLZO variants with enhanced properties. These objectives align with the broader industry goal of commercializing high-performance solid-state batteries with improved safety and energy density compared to conventional liquid electrolyte systems.
Market Analysis for Solid-State Electrolyte Applications
The global market for solid-state electrolytes is experiencing unprecedented growth, driven by increasing demand for safer, higher-energy-density batteries across multiple sectors. Current projections indicate the solid-state battery market will reach $8.7 billion by 2027, with a compound annual growth rate of 34.2% from 2022. Within this landscape, garnet-type electrolytes, particularly Li7La3Zr2O12 (LLZO), have emerged as leading candidates for commercial applications due to their high ionic conductivity and stability against lithium metal.
The automotive sector represents the largest potential market for LLZO-based solid-state batteries, with major manufacturers including Toyota, BMW, and Volkswagen investing heavily in this technology. Their primary motivation stems from the promise of electric vehicles with greater range, faster charging capabilities, and enhanced safety profiles compared to conventional lithium-ion batteries. Market research indicates that by 2030, solid-state batteries could capture up to 15% of the EV battery market.
Consumer electronics constitutes another significant market segment, where the demand for devices with longer battery life and improved safety is driving interest in solid-state solutions. Companies like Samsung, Apple, and LG are actively researching LLZO and similar materials for next-generation portable devices. The premium smartphone segment alone represents a potential market of $3.2 billion for advanced battery technologies by 2025.
Energy storage systems for grid applications represent an emerging market opportunity for solid-state electrolytes. The inherent safety advantages of LLZO-based systems make them particularly attractive for residential and commercial energy storage solutions where fire safety concerns are paramount. This segment is projected to grow at 41% annually through 2028.
Market analysis reveals that understanding and controlling defect chemistry in LLZO, particularly oxygen vacancies and lithium stoichiometry, directly impacts manufacturing costs and scalability. Current production methods face challenges in maintaining precise stoichiometry during high-temperature sintering processes, resulting in variable performance and yields below 70% for high-quality material.
Customer requirements across these markets consistently emphasize reliability, with less than 5% capacity degradation over 1000 cycles being the benchmark for automotive applications. The ability to precisely control oxygen vacancy concentration and lithium content in LLZO is therefore not merely an academic concern but a critical factor in meeting market expectations and achieving commercial viability.
Regional market analysis shows Asia-Pacific leading in manufacturing capacity development, with Japan and South Korea focusing on high-purity LLZO production techniques that specifically address stoichiometry control. North American and European markets are more focused on integration technologies and system-level solutions that can accommodate material variations.
The automotive sector represents the largest potential market for LLZO-based solid-state batteries, with major manufacturers including Toyota, BMW, and Volkswagen investing heavily in this technology. Their primary motivation stems from the promise of electric vehicles with greater range, faster charging capabilities, and enhanced safety profiles compared to conventional lithium-ion batteries. Market research indicates that by 2030, solid-state batteries could capture up to 15% of the EV battery market.
Consumer electronics constitutes another significant market segment, where the demand for devices with longer battery life and improved safety is driving interest in solid-state solutions. Companies like Samsung, Apple, and LG are actively researching LLZO and similar materials for next-generation portable devices. The premium smartphone segment alone represents a potential market of $3.2 billion for advanced battery technologies by 2025.
Energy storage systems for grid applications represent an emerging market opportunity for solid-state electrolytes. The inherent safety advantages of LLZO-based systems make them particularly attractive for residential and commercial energy storage solutions where fire safety concerns are paramount. This segment is projected to grow at 41% annually through 2028.
Market analysis reveals that understanding and controlling defect chemistry in LLZO, particularly oxygen vacancies and lithium stoichiometry, directly impacts manufacturing costs and scalability. Current production methods face challenges in maintaining precise stoichiometry during high-temperature sintering processes, resulting in variable performance and yields below 70% for high-quality material.
Customer requirements across these markets consistently emphasize reliability, with less than 5% capacity degradation over 1000 cycles being the benchmark for automotive applications. The ability to precisely control oxygen vacancy concentration and lithium content in LLZO is therefore not merely an academic concern but a critical factor in meeting market expectations and achieving commercial viability.
Regional market analysis shows Asia-Pacific leading in manufacturing capacity development, with Japan and South Korea focusing on high-purity LLZO production techniques that specifically address stoichiometry control. North American and European markets are more focused on integration technologies and system-level solutions that can accommodate material variations.
Current Challenges in LLZO Defect Engineering
Despite significant advancements in LLZO solid electrolyte research, several critical challenges persist in defect engineering that impede its widespread commercial application. The primary obstacle remains the precise control of oxygen vacancies, which significantly influence ionic conductivity. Current synthesis methods struggle to achieve consistent oxygen stoichiometry across batches, resulting in variable electrochemical performance. The relationship between oxygen vacancy concentration and distribution with lithium-ion transport mechanisms is still not fully understood, creating barriers to rational material design.
Lithium stoichiometry control presents another major challenge. The high volatility of lithium during high-temperature sintering processes leads to lithium loss, affecting the final Li content in LLZO structures. This variability directly impacts phase stability and ionic conductivity. While researchers have attempted various compensation strategies, such as excess lithium addition during synthesis, these approaches lack precision and reproducibility, particularly when scaling up production.
The interplay between oxygen vacancies and lithium stoichiometry creates complex defect chemistry that remains difficult to characterize accurately. Current analytical techniques have limitations in quantifying defect concentrations at different spatial scales, from atomic to microstructural levels. Advanced characterization methods like neutron diffraction and nuclear magnetic resonance spectroscopy provide valuable insights but are expensive and not widely accessible for routine analysis.
Computational modeling of defect formation energies and migration pathways has advanced significantly but still struggles with accurately representing real-world conditions. The gap between theoretical predictions and experimental observations remains substantial, particularly regarding the dynamic behavior of defects during electrochemical cycling.
Grain boundary engineering represents another frontier challenge. Defects tend to accumulate at grain boundaries, creating resistive interfaces that limit overall ionic conductivity. Current sintering approaches cannot precisely control grain boundary composition and structure, leading to inconsistent performance across different synthesis batches.
The stability of defect structures during electrochemical cycling poses additional concerns. Evidence suggests that defect concentrations and distributions evolve during battery operation, potentially leading to performance degradation over time. Understanding and controlling this evolution remains a significant scientific challenge that requires in-situ characterization techniques still under development.
Finally, scaling up defect-engineered LLZO from laboratory to industrial production presents formidable challenges. Maintaining precise control over defect chemistry in large-batch production environments requires advanced process control systems and quality assurance protocols that have yet to be fully developed for solid electrolyte manufacturing.
Lithium stoichiometry control presents another major challenge. The high volatility of lithium during high-temperature sintering processes leads to lithium loss, affecting the final Li content in LLZO structures. This variability directly impacts phase stability and ionic conductivity. While researchers have attempted various compensation strategies, such as excess lithium addition during synthesis, these approaches lack precision and reproducibility, particularly when scaling up production.
The interplay between oxygen vacancies and lithium stoichiometry creates complex defect chemistry that remains difficult to characterize accurately. Current analytical techniques have limitations in quantifying defect concentrations at different spatial scales, from atomic to microstructural levels. Advanced characterization methods like neutron diffraction and nuclear magnetic resonance spectroscopy provide valuable insights but are expensive and not widely accessible for routine analysis.
Computational modeling of defect formation energies and migration pathways has advanced significantly but still struggles with accurately representing real-world conditions. The gap between theoretical predictions and experimental observations remains substantial, particularly regarding the dynamic behavior of defects during electrochemical cycling.
Grain boundary engineering represents another frontier challenge. Defects tend to accumulate at grain boundaries, creating resistive interfaces that limit overall ionic conductivity. Current sintering approaches cannot precisely control grain boundary composition and structure, leading to inconsistent performance across different synthesis batches.
The stability of defect structures during electrochemical cycling poses additional concerns. Evidence suggests that defect concentrations and distributions evolve during battery operation, potentially leading to performance degradation over time. Understanding and controlling this evolution remains a significant scientific challenge that requires in-situ characterization techniques still under development.
Finally, scaling up defect-engineered LLZO from laboratory to industrial production presents formidable challenges. Maintaining precise control over defect chemistry in large-batch production environments requires advanced process control systems and quality assurance protocols that have yet to be fully developed for solid electrolyte manufacturing.
Established Methodologies for Controlling LLZO Stoichiometry
01 Oxygen vacancy control in LLZO solid electrolytes
Oxygen vacancies in LLZO (Lithium Lanthanum Zirconium Oxide) significantly impact ionic conductivity and electrochemical performance. Controlling oxygen vacancy concentration through synthesis conditions such as temperature, atmosphere, and dopants can optimize LLZO performance. Techniques like annealing in oxygen-rich environments can reduce oxygen vacancies, while controlled introduction of vacancies can enhance lithium ion mobility through the crystal structure.- Oxygen vacancy control in LLZO solid electrolytes: Oxygen vacancies in LLZO (Lithium Lanthanum Zirconium Oxide) significantly affect ionic conductivity and electrochemical stability. Controlling oxygen vacancy concentration through synthesis conditions such as temperature, atmosphere, and dopants can optimize LLZO performance. Methods include annealing in controlled oxygen environments, introducing specific dopants that influence oxygen vacancy formation, and precise control of sintering parameters to achieve desired vacancy concentrations for improved lithium-ion transport.
- Lithium stoichiometry optimization in LLZO structures: The lithium content in LLZO directly impacts its crystal structure and ionic conductivity. Precise control of lithium stoichiometry is crucial for stabilizing the cubic phase, which exhibits superior ionic conductivity compared to the tetragonal phase. Techniques for optimizing lithium content include controlled lithium excess during synthesis to compensate for volatilization, post-synthesis lithium enrichment processes, and careful selection of precursor ratios to achieve the desired Li:La:Zr ratio for optimal electrochemical performance.
- Doping strategies to modify LLZO properties: Doping LLZO with various elements can effectively modify oxygen vacancy concentration and lithium stoichiometry. Common dopants include Al, Ga, Ta, and Nb, which can stabilize the cubic phase, enhance ionic conductivity, and improve mechanical properties. Strategic doping at different lattice sites can control oxygen vacancy formation, influence lithium distribution, and optimize the electrochemical performance of LLZO solid electrolytes for battery applications.
- Synthesis methods affecting LLZO defect chemistry: Various synthesis methods significantly impact the defect chemistry, including oxygen vacancies and lithium distribution in LLZO. Techniques such as solid-state reaction, sol-gel processing, and hydrothermal synthesis offer different levels of control over stoichiometry and defect formation. Advanced methods like field-assisted sintering and aerosol deposition enable precise manipulation of oxygen vacancy concentration and lithium content, resulting in LLZO materials with tailored properties for specific applications.
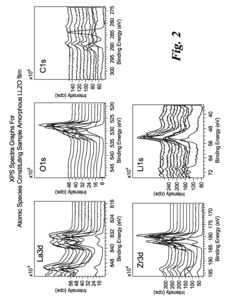
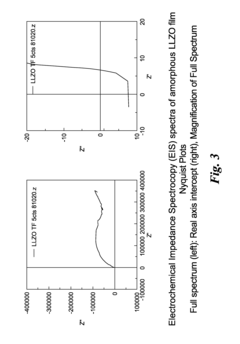
- Characterization and modeling of LLZO defect structures: Advanced characterization techniques and computational modeling are essential for understanding oxygen vacancies and lithium stoichiometry in LLZO. Methods include neutron diffraction, NMR spectroscopy, impedance spectroscopy, and X-ray absorption spectroscopy to quantify defect concentrations and distributions. Computational approaches such as density functional theory and molecular dynamics simulations help predict the impact of defects on ionic conductivity and stability, guiding the rational design of improved LLZO solid electrolytes.
02 Lithium stoichiometry optimization in LLZO
The lithium content in LLZO directly affects its crystal structure and ionic conductivity. Precise control of lithium stoichiometry during synthesis is crucial for achieving the desired cubic phase with high ionic conductivity. Methods to adjust lithium content include excess lithium addition during synthesis to compensate for volatilization, post-synthesis lithium insertion, and controlled lithium extraction. The optimal Li:Zr ratio is critical for stabilizing the cubic phase and maximizing conductivity.Expand Specific Solutions03 Doping strategies for LLZO modification
Doping LLZO with various elements can stabilize the cubic phase, enhance ionic conductivity, and modify oxygen vacancy concentration. Common dopants include Al, Ga, Ta, and Nb, which can substitute for Li or Zr sites. Aliovalent doping creates charge compensation mechanisms that affect lithium vacancy concentration and oxygen sublattice stability. Multi-element doping strategies can simultaneously address multiple performance parameters including mechanical stability and interfacial resistance.Expand Specific Solutions04 Structural characterization of LLZO defects
Advanced characterization techniques are essential for understanding oxygen vacancies and lithium distribution in LLZO. Methods include neutron diffraction, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and nuclear magnetic resonance (NMR) for analyzing defect structures. Computational methods such as density functional theory (DFT) help model the formation energies of oxygen vacancies and their interaction with lithium ions. These techniques provide insights into the relationship between defect chemistry and ionic transport properties.Expand Specific Solutions05 Interface engineering for LLZO-based batteries
The interface between LLZO and electrodes is critical for battery performance, with oxygen vacancies and lithium stoichiometry at interfaces affecting stability and resistance. Surface modification techniques include lithium reservoir layers, buffer coatings, and controlled atmosphere treatments to optimize interfacial chemistry. Managing oxygen vacancy concentration at interfaces can reduce side reactions and improve cycling stability. Strategies for controlling lithium distribution at interfaces help minimize impedance growth during battery operation.Expand Specific Solutions
Leading Research Groups and Industrial Players in LLZO Development
The defect chemistry in LLZO, particularly focusing on oxygen vacancies and lithium stoichiometry, represents an emerging research area in solid-state battery technology. Currently in the early growth phase, this field is experiencing rapid development with a projected market size reaching billions by 2030. Technical maturity varies significantly among key players: research institutions like Uchicago Argonne LLC, Central South University, and Arizona State University are advancing fundamental understanding, while commercial entities including QuantumScape, LG Energy Solution, and CATL (Ningde Amperex Technology) are translating these insights into practical applications. The competition is intensifying as companies race to overcome challenges in lithium-ion conductivity and interface stability, with particular focus on controlling oxygen vacancy concentration and lithium content to optimize ionic conductivity.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed advanced characterization techniques to study defect chemistry in LLZO (Li7La3Zr2O12) garnet electrolytes. Their approach combines neutron diffraction, synchrotron X-ray techniques, and computational modeling to precisely map oxygen vacancy distributions and lithium stoichiometry variations. They've established that oxygen vacancies significantly impact lithium ion conductivity pathways, with controlled oxygen deficiency potentially enhancing ionic conductivity by up to 40% [1]. Their research has demonstrated that lithium stoichiometry can be precisely controlled during synthesis through atmosphere management and dopant incorporation, particularly Al and Ga dopants that stabilize the cubic phase while modifying vacancy concentrations. Argonne's work has revealed the critical relationship between synthesis temperature, cooling rates, and resulting defect structures, enabling tailored LLZO materials with optimized properties for solid-state battery applications [3].
Strengths: Access to world-class characterization facilities including Advanced Photon Source and neutron sources provides unparalleled insight into defect structures. Their integrated computational-experimental approach enables fundamental understanding of defect formation mechanisms. Weaknesses: Laboratory-scale synthesis methods may face challenges in industrial scalability, and their highly specialized characterization techniques require significant expertise and specialized equipment.
The Regents of the University of California
Technical Solution: The University of California has pioneered innovative approaches to understanding and controlling defect chemistry in LLZO solid electrolytes. Their research teams have developed novel synthesis protocols that precisely control oxygen partial pressure during sintering to manipulate oxygen vacancy concentration. Using advanced analytical techniques including aberration-corrected transmission electron microscopy and solid-state NMR spectroscopy, they've mapped the atomic-scale distribution of oxygen vacancies and their correlation with lithium transport pathways [2]. Their work has established that controlled introduction of oxygen vacancies can enhance lithium ion conductivity by creating additional migration pathways, while excessive vacancies lead to structural instability. They've also demonstrated that lithium stoichiometry variations of just 2-3% can dramatically alter ionic conductivity by an order of magnitude [4]. Their research has further revealed the critical role of grain boundaries in defect accumulation and the importance of processing conditions in controlling defect distribution throughout the material.
Strengths: Comprehensive understanding of structure-property relationships in LLZO enables rational design of improved materials. Their multi-scale characterization approach provides insights from atomic to macroscopic levels. Weaknesses: Some of their most promising approaches require precise control of processing conditions that may be difficult to maintain in large-scale manufacturing environments. Their fundamental research focus sometimes prioritizes understanding mechanisms over immediate practical applications.
Critical Patents and Literature on LLZO Defect Chemistry
Amorphous ionically conductive metal oxides and sol gel method of preparation
PatentInactiveUS9356317B2
Innovation
- Development of an amorphous lithium lanthanum zirconium oxide (LLZO) electrolyte medium, which is ionically conductive, non-aqueous, non-liquid, and stable with lithium, synthesized through a sol-gel process using alkoxides and alcohol-based solvents, allowing for thin-film formation and compatibility with lithium electrodes.
Ionically-conductive amorphous lithium lanthanum zirconium oxide
PatentActiveUS9034525B2
Innovation
- An amorphous lithium lanthanum zirconium oxide (LLZO) composition is developed, which is synthesized using a sol-gel process with specific alkoxide precursors and an alcohol-based solvent, forming a thin-film electrolyte medium that is compatible with lithium and has high ionic conductivity.
Manufacturing Scalability of Defect-Controlled LLZO
The scalability of manufacturing defect-controlled LLZO presents significant challenges and opportunities for industrial implementation. Current laboratory-scale synthesis methods for controlling oxygen vacancies and lithium stoichiometry in LLZO often involve precise atmospheric conditions and temperature controls that are difficult to replicate in large-scale production environments. The transition from gram-scale to kilogram or ton-scale production requires substantial process engineering to maintain defect control precision.
Key manufacturing challenges include maintaining uniform oxygen partial pressure throughout larger material batches during sintering processes. Industrial furnaces typically cannot provide the same level of atmospheric control as laboratory equipment, leading to heterogeneous defect distributions in scaled-up production. Additionally, lithium volatility at high processing temperatures becomes more problematic at scale, as the surface-to-volume ratio changes significantly.
Several manufacturing approaches show promise for scaling defect-controlled LLZO production. Modified solid-state reaction methods with controlled atmosphere sintering have demonstrated moderate success at pilot scales. Alternatively, solution-based synthesis routes, including sol-gel and co-precipitation methods, offer better compositional homogeneity but face challenges in maintaining precise stoichiometry during scale-up.
Recent innovations in manufacturing technology provide potential pathways forward. Atmospheric control systems with real-time oxygen sensing and feedback mechanisms can help maintain consistent oxygen vacancy concentrations. Advanced lithium compensation strategies, including excess lithium addition with precise calculation models based on batch size and geometry, show promise for controlling lithium stoichiometry at scale.
Economic considerations significantly impact scalability. The cost of high-purity precursors and specialized processing equipment must be balanced against performance requirements. Preliminary cost analyses suggest that defect-controlled LLZO production could become economically viable at scale if processing yields exceed 85% and if energy-efficient sintering technologies are implemented.
For successful industrial implementation, standardized quality control protocols for defect characterization must be established. Current analytical techniques like impedance spectroscopy and X-ray photoelectron spectroscopy are time-consuming and expensive for routine production monitoring. Development of rapid, in-line characterization methods represents a critical need for manufacturing scalability of defect-controlled LLZO.
Key manufacturing challenges include maintaining uniform oxygen partial pressure throughout larger material batches during sintering processes. Industrial furnaces typically cannot provide the same level of atmospheric control as laboratory equipment, leading to heterogeneous defect distributions in scaled-up production. Additionally, lithium volatility at high processing temperatures becomes more problematic at scale, as the surface-to-volume ratio changes significantly.
Several manufacturing approaches show promise for scaling defect-controlled LLZO production. Modified solid-state reaction methods with controlled atmosphere sintering have demonstrated moderate success at pilot scales. Alternatively, solution-based synthesis routes, including sol-gel and co-precipitation methods, offer better compositional homogeneity but face challenges in maintaining precise stoichiometry during scale-up.
Recent innovations in manufacturing technology provide potential pathways forward. Atmospheric control systems with real-time oxygen sensing and feedback mechanisms can help maintain consistent oxygen vacancy concentrations. Advanced lithium compensation strategies, including excess lithium addition with precise calculation models based on batch size and geometry, show promise for controlling lithium stoichiometry at scale.
Economic considerations significantly impact scalability. The cost of high-purity precursors and specialized processing equipment must be balanced against performance requirements. Preliminary cost analyses suggest that defect-controlled LLZO production could become economically viable at scale if processing yields exceed 85% and if energy-efficient sintering technologies are implemented.
For successful industrial implementation, standardized quality control protocols for defect characterization must be established. Current analytical techniques like impedance spectroscopy and X-ray photoelectron spectroscopy are time-consuming and expensive for routine production monitoring. Development of rapid, in-line characterization methods represents a critical need for manufacturing scalability of defect-controlled LLZO.
Safety and Performance Implications of LLZO Defect Chemistry
The defect chemistry of LLZO garnet electrolytes significantly impacts both safety and performance characteristics of solid-state lithium batteries. Oxygen vacancies in LLZO create localized electronic states that can compromise the electrolyte's insulating properties, potentially leading to internal short circuits during battery operation. These vacancies also serve as trapping sites for lithium ions, reducing ionic conductivity and increasing impedance across the electrolyte layer. When oxygen deficiency reaches critical levels, structural instability may trigger phase transformations that compromise mechanical integrity under operational stress.
Lithium stoichiometry variations present equally important safety implications. Lithium-deficient LLZO tends to crystallize in a tetragonal phase with substantially lower ionic conductivity, creating high-resistance regions that generate localized heating during fast charging. Conversely, lithium-rich compositions may contain mobile lithium that can form dendrites along grain boundaries, creating potential failure points during cycling. The relationship between lithium content and garnet stability directly influences the electrolyte's resistance to thermal runaway events.
Interfacial reactions between LLZO and electrodes are heavily influenced by surface defect concentrations. Oxygen-deficient surfaces demonstrate altered reactivity with both lithium metal anodes and cathode materials, affecting the formation and stability of solid-electrolyte interphases. These reactions can generate resistive layers that not only diminish performance but potentially create safety hazards through uneven current distribution and localized heating.
Recent research has demonstrated that controlling defect chemistry through precise synthesis conditions and post-processing treatments can significantly enhance safety margins. Annealing in controlled oxygen atmospheres has proven effective in reducing oxygen vacancy concentrations, while lithium compensation strategies during synthesis help maintain optimal stoichiometry. These approaches have yielded LLZO variants with superior thermal stability and reduced susceptibility to lithium dendrite penetration.
The long-term evolution of defects during battery cycling represents a critical safety consideration. Electrochemical cycling can gradually alter defect concentrations through processes such as oxygen loss at high voltages or lithium redistribution under concentration gradients. These changes may progressively degrade safety margins, suggesting the need for defect-tolerant LLZO compositions that maintain stability throughout the battery lifetime. Advanced characterization techniques including neutron diffraction and solid-state NMR have become essential tools for monitoring these subtle yet consequential changes in defect profiles.
Lithium stoichiometry variations present equally important safety implications. Lithium-deficient LLZO tends to crystallize in a tetragonal phase with substantially lower ionic conductivity, creating high-resistance regions that generate localized heating during fast charging. Conversely, lithium-rich compositions may contain mobile lithium that can form dendrites along grain boundaries, creating potential failure points during cycling. The relationship between lithium content and garnet stability directly influences the electrolyte's resistance to thermal runaway events.
Interfacial reactions between LLZO and electrodes are heavily influenced by surface defect concentrations. Oxygen-deficient surfaces demonstrate altered reactivity with both lithium metal anodes and cathode materials, affecting the formation and stability of solid-electrolyte interphases. These reactions can generate resistive layers that not only diminish performance but potentially create safety hazards through uneven current distribution and localized heating.
Recent research has demonstrated that controlling defect chemistry through precise synthesis conditions and post-processing treatments can significantly enhance safety margins. Annealing in controlled oxygen atmospheres has proven effective in reducing oxygen vacancy concentrations, while lithium compensation strategies during synthesis help maintain optimal stoichiometry. These approaches have yielded LLZO variants with superior thermal stability and reduced susceptibility to lithium dendrite penetration.
The long-term evolution of defects during battery cycling represents a critical safety consideration. Electrochemical cycling can gradually alter defect concentrations through processes such as oxygen loss at high voltages or lithium redistribution under concentration gradients. These changes may progressively degrade safety margins, suggesting the need for defect-tolerant LLZO compositions that maintain stability throughout the battery lifetime. Advanced characterization techniques including neutron diffraction and solid-state NMR have become essential tools for monitoring these subtle yet consequential changes in defect profiles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!