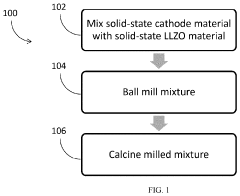
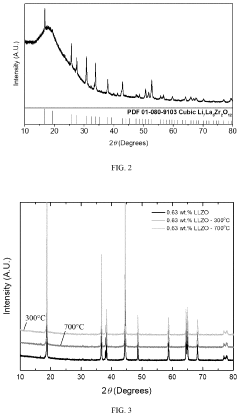
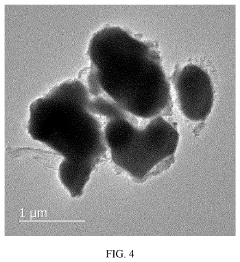
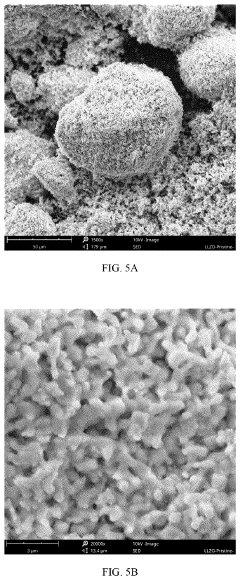
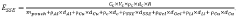Compatibility of LLZO with high-voltage cathodes: coatings that work
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO-Cathode Interface Challenges and Objectives
The development of all-solid-state lithium batteries (ASSBs) represents a significant advancement in energy storage technology, with Li7La3Zr2O12 (LLZO) garnet-type solid electrolytes emerging as promising candidates due to their high ionic conductivity and stability against lithium metal. However, the interface between LLZO and high-voltage cathodes presents substantial challenges that must be addressed to realize the full potential of these batteries.
The primary objective in this technical domain is to develop effective coating strategies that can mitigate the chemical and electrochemical instability at the LLZO-cathode interface. Current research indicates that direct contact between LLZO and high-voltage cathode materials often leads to undesirable interfacial reactions, formation of resistive layers, and consequent capacity fading during cycling.
These interface issues stem from multiple factors: chemical incompatibility between LLZO and cathode active materials, particularly at elevated temperatures during processing; mechanical stress during cycling that can create microcracks and contact loss; and electrochemical decomposition at high operating voltages exceeding the stability window of LLZO. The formation of space charge layers at these interfaces further complicates ion transport mechanisms.
Recent technological evolution has focused on developing protective coatings that can serve as buffer layers between LLZO and cathode materials. These coatings must simultaneously satisfy several critical requirements: chemical stability with both LLZO and cathode materials; sufficient lithium-ion conductivity to minimize interfacial resistance; mechanical flexibility to accommodate volume changes during cycling; and electrochemical stability within the operating voltage window of high-voltage cathodes (often >4.5V vs. Li/Li+).
The geographical distribution of research in this field shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Europe, with significant contributions from both academic institutions and industrial research centers. This global effort underscores the strategic importance of solving the LLZO-cathode interface challenge.
The technical goals for this research area include developing scalable coating methods that can be integrated into existing battery manufacturing processes, achieving interfacial resistance below 100 Ω·cm² at room temperature, and ensuring stable cycling performance for at least 1000 cycles with minimal capacity degradation (<0.01% per cycle) at practical current densities.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and advanced characterization techniques to understand interfacial phenomena at atomic and molecular levels. Success in this domain would represent a significant breakthrough toward commercialization of high-energy-density solid-state batteries with enhanced safety profiles.
The primary objective in this technical domain is to develop effective coating strategies that can mitigate the chemical and electrochemical instability at the LLZO-cathode interface. Current research indicates that direct contact between LLZO and high-voltage cathode materials often leads to undesirable interfacial reactions, formation of resistive layers, and consequent capacity fading during cycling.
These interface issues stem from multiple factors: chemical incompatibility between LLZO and cathode active materials, particularly at elevated temperatures during processing; mechanical stress during cycling that can create microcracks and contact loss; and electrochemical decomposition at high operating voltages exceeding the stability window of LLZO. The formation of space charge layers at these interfaces further complicates ion transport mechanisms.
Recent technological evolution has focused on developing protective coatings that can serve as buffer layers between LLZO and cathode materials. These coatings must simultaneously satisfy several critical requirements: chemical stability with both LLZO and cathode materials; sufficient lithium-ion conductivity to minimize interfacial resistance; mechanical flexibility to accommodate volume changes during cycling; and electrochemical stability within the operating voltage window of high-voltage cathodes (often >4.5V vs. Li/Li+).
The geographical distribution of research in this field shows concentration in East Asia (particularly Japan, South Korea, and China), North America, and Europe, with significant contributions from both academic institutions and industrial research centers. This global effort underscores the strategic importance of solving the LLZO-cathode interface challenge.
The technical goals for this research area include developing scalable coating methods that can be integrated into existing battery manufacturing processes, achieving interfacial resistance below 100 Ω·cm² at room temperature, and ensuring stable cycling performance for at least 1000 cycles with minimal capacity degradation (<0.01% per cycle) at practical current densities.
Addressing these challenges requires interdisciplinary approaches combining materials science, electrochemistry, and advanced characterization techniques to understand interfacial phenomena at atomic and molecular levels. Success in this domain would represent a significant breakthrough toward commercialization of high-energy-density solid-state batteries with enhanced safety profiles.
Market Analysis for High-Voltage Solid-State Batteries
The global market for high-voltage solid-state batteries is experiencing significant growth, driven by increasing demand for electric vehicles (EVs), consumer electronics, and renewable energy storage solutions. Current projections indicate the solid-state battery market will reach approximately $8 billion by 2026, with a compound annual growth rate exceeding 34% between 2021 and 2026. High-voltage cathode compatibility solutions represent a critical segment within this expanding market.
The automotive sector constitutes the largest market opportunity, with major manufacturers committing to electrification strategies that require advanced battery technologies. Premium EV manufacturers are particularly interested in solid-state solutions incorporating high-voltage cathodes, as these can deliver superior energy density, faster charging capabilities, and extended range performance that justify premium pricing.
Consumer electronics represents the second most significant market segment, with demand for longer-lasting, faster-charging devices driving interest in solid-state technology. The compatibility solutions for LLZO with high-voltage cathodes could enable next-generation smartphones and laptops with substantially improved battery performance and safety profiles.
Market analysis reveals regional variations in adoption potential. Asia-Pacific leads manufacturing capacity development, with Japan and South Korea hosting the most advanced research facilities focused on LLZO-cathode interface solutions. North America demonstrates strong venture capital investment in startups developing proprietary coating technologies, while European markets show the highest consumer willingness to pay premium prices for enhanced battery performance.
Customer surveys indicate that extended range and faster charging capabilities rank as the top two priorities for EV consumers, directly aligning with the benefits of high-voltage cathode implementation. Safety concerns remain paramount across all market segments, creating additional demand for the enhanced thermal stability offered by properly engineered LLZO-cathode interfaces.
Market penetration faces several barriers, including cost considerations, manufacturing scalability, and competition from incremental improvements to conventional lithium-ion technologies. Current production costs for specialized coating materials compatible with both LLZO and high-voltage cathodes exceed $200 per kWh, significantly above the target threshold for mass-market adoption.
Supply chain analysis indicates potential bottlenecks in specialized coating materials, with limited suppliers capable of meeting quality and volume requirements. Companies that can secure reliable access to these materials while developing cost-effective application methods will likely capture disproportionate market share.
The competitive landscape features both established battery manufacturers investing in solid-state research divisions and specialized startups focused exclusively on interface engineering solutions. Recent partnership announcements between automotive OEMs and technology developers suggest accelerating commercialization timelines, with several pilot production facilities scheduled to begin operations within the next 24 months.
The automotive sector constitutes the largest market opportunity, with major manufacturers committing to electrification strategies that require advanced battery technologies. Premium EV manufacturers are particularly interested in solid-state solutions incorporating high-voltage cathodes, as these can deliver superior energy density, faster charging capabilities, and extended range performance that justify premium pricing.
Consumer electronics represents the second most significant market segment, with demand for longer-lasting, faster-charging devices driving interest in solid-state technology. The compatibility solutions for LLZO with high-voltage cathodes could enable next-generation smartphones and laptops with substantially improved battery performance and safety profiles.
Market analysis reveals regional variations in adoption potential. Asia-Pacific leads manufacturing capacity development, with Japan and South Korea hosting the most advanced research facilities focused on LLZO-cathode interface solutions. North America demonstrates strong venture capital investment in startups developing proprietary coating technologies, while European markets show the highest consumer willingness to pay premium prices for enhanced battery performance.
Customer surveys indicate that extended range and faster charging capabilities rank as the top two priorities for EV consumers, directly aligning with the benefits of high-voltage cathode implementation. Safety concerns remain paramount across all market segments, creating additional demand for the enhanced thermal stability offered by properly engineered LLZO-cathode interfaces.
Market penetration faces several barriers, including cost considerations, manufacturing scalability, and competition from incremental improvements to conventional lithium-ion technologies. Current production costs for specialized coating materials compatible with both LLZO and high-voltage cathodes exceed $200 per kWh, significantly above the target threshold for mass-market adoption.
Supply chain analysis indicates potential bottlenecks in specialized coating materials, with limited suppliers capable of meeting quality and volume requirements. Companies that can secure reliable access to these materials while developing cost-effective application methods will likely capture disproportionate market share.
The competitive landscape features both established battery manufacturers investing in solid-state research divisions and specialized startups focused exclusively on interface engineering solutions. Recent partnership announcements between automotive OEMs and technology developers suggest accelerating commercialization timelines, with several pilot production facilities scheduled to begin operations within the next 24 months.
Current Limitations in LLZO-Cathode Compatibility
Despite significant advancements in solid-state battery technology, the interface between LLZO (Li7La3Zr2O12) solid electrolytes and high-voltage cathodes remains a critical challenge limiting commercial viability. The fundamental incompatibility stems from several interconnected issues that compromise battery performance and longevity. Chemical instability at the LLZO-cathode interface leads to undesirable side reactions, particularly with high-voltage cathodes operating above 4V, resulting in the formation of resistive interlayers that impede lithium ion transport.
Mechanical contact problems further exacerbate these challenges, as the rigid ceramic nature of LLZO creates insufficient contact with cathode materials during cycling. This poor interfacial contact increases impedance and reduces active material utilization. The volume changes during lithiation/delithiation cycles continuously disrupt the interface, creating microcracks and delamination that progressively degrade performance.
Electrochemical stability windows present another significant limitation. While LLZO exhibits excellent stability against lithium metal anodes, its stability against high-voltage cathodes (>4V vs. Li/Li+) remains problematic. Oxidative decomposition occurs at the interface, forming lithium-deficient phases and increasing interfacial resistance over cycling.
Space charge layer effects create additional complications, as the redistribution of lithium ions at the interface forms depletion regions that increase resistance. This phenomenon is particularly pronounced with high-voltage cathode materials due to their strong lithium affinity and electronic structure.
Current manufacturing constraints further limit optimization possibilities. Traditional ceramic processing methods struggle to create ideal interfaces between LLZO and cathode materials. High-temperature co-sintering approaches often trigger undesired elemental interdiffusion and secondary phase formation, while room-temperature methods fail to establish intimate contact necessary for efficient ion transport.
The kinetic limitations at the interface represent another significant barrier. The charge transfer resistance at LLZO-cathode interfaces is substantially higher than in conventional liquid electrolyte systems, limiting rate capability and power density. This becomes particularly problematic for high-voltage cathodes where redox reactions occur at more extreme potentials.
These multifaceted challenges have prompted extensive research into interface engineering strategies, with protective coatings emerging as the most promising approach. However, developing coatings that simultaneously address chemical stability, mechanical integrity, and ionic conductivity while being compatible with both LLZO and high-voltage cathode materials remains an ongoing challenge requiring innovative materials science solutions.
Mechanical contact problems further exacerbate these challenges, as the rigid ceramic nature of LLZO creates insufficient contact with cathode materials during cycling. This poor interfacial contact increases impedance and reduces active material utilization. The volume changes during lithiation/delithiation cycles continuously disrupt the interface, creating microcracks and delamination that progressively degrade performance.
Electrochemical stability windows present another significant limitation. While LLZO exhibits excellent stability against lithium metal anodes, its stability against high-voltage cathodes (>4V vs. Li/Li+) remains problematic. Oxidative decomposition occurs at the interface, forming lithium-deficient phases and increasing interfacial resistance over cycling.
Space charge layer effects create additional complications, as the redistribution of lithium ions at the interface forms depletion regions that increase resistance. This phenomenon is particularly pronounced with high-voltage cathode materials due to their strong lithium affinity and electronic structure.
Current manufacturing constraints further limit optimization possibilities. Traditional ceramic processing methods struggle to create ideal interfaces between LLZO and cathode materials. High-temperature co-sintering approaches often trigger undesired elemental interdiffusion and secondary phase formation, while room-temperature methods fail to establish intimate contact necessary for efficient ion transport.
The kinetic limitations at the interface represent another significant barrier. The charge transfer resistance at LLZO-cathode interfaces is substantially higher than in conventional liquid electrolyte systems, limiting rate capability and power density. This becomes particularly problematic for high-voltage cathodes where redox reactions occur at more extreme potentials.
These multifaceted challenges have prompted extensive research into interface engineering strategies, with protective coatings emerging as the most promising approach. However, developing coatings that simultaneously address chemical stability, mechanical integrity, and ionic conductivity while being compatible with both LLZO and high-voltage cathode materials remains an ongoing challenge requiring innovative materials science solutions.
Existing Coating Solutions for LLZO-Cathode Integration
01 LLZO coating compatibility with electrode materials
LLZO coatings demonstrate varying levels of compatibility with different electrode materials. The interface between LLZO and electrodes is critical for battery performance. Modifications to improve compatibility include surface treatments, buffer layers, and compositional adjustments. These modifications enhance adhesion, reduce interfacial resistance, and improve electrochemical stability between LLZO and electrode materials such as lithium metal, silicon, and various cathode materials.- LLZO coating compatibility with electrode materials: LLZO coatings can be applied to various electrode materials to improve their electrochemical performance and stability. The compatibility between LLZO and electrode materials such as lithium metal, silicon, and transition metal oxides is crucial for effective battery operation. These coatings help to suppress side reactions at the electrode-electrolyte interface, enhance ionic conductivity, and improve cycling stability. The coating process and thickness need to be optimized to ensure good adhesion and uniform coverage on the electrode surface.
- Interface engineering for LLZO solid electrolyte compatibility: Interface engineering techniques are employed to enhance the compatibility of LLZO coatings with adjacent materials in solid-state batteries. This includes surface modifications, buffer layers, and gradient compositions to reduce interfacial resistance and improve contact between LLZO and electrodes or other electrolyte materials. Various treatments such as annealing, polishing, and chemical modifications can be applied to LLZO surfaces to improve wettability and adhesion. These engineering approaches help to address issues related to mechanical and chemical stability at interfaces.
- Chemical stability and reactivity of LLZO coatings: The chemical stability of LLZO coatings in different environments and with various battery components is essential for long-term battery performance. LLZO can undergo reactions with atmospheric components (moisture, CO2), electrode materials, or other electrolyte components, potentially forming undesirable interfacial products. Strategies to enhance chemical stability include doping with elements like Al, Ga, or Ta, surface passivation treatments, and protective overlayers. Understanding and controlling these chemical interactions is crucial for maintaining the integrity and functionality of LLZO coatings throughout battery operation.
- Processing techniques for LLZO coating deposition: Various processing techniques can be employed to deposit LLZO coatings with optimal compatibility properties. These include physical vapor deposition, chemical vapor deposition, sol-gel methods, atomic layer deposition, and sputtering. Each technique offers different advantages in terms of coating thickness, uniformity, density, and adhesion. The processing parameters, such as temperature, pressure, and precursor composition, significantly influence the crystallinity, grain structure, and defect concentration in LLZO coatings, which in turn affect their compatibility with other battery components.
- Composite structures with LLZO for enhanced compatibility: Composite structures incorporating LLZO with polymers, other ceramic materials, or conductive additives can enhance compatibility in battery applications. These composites combine the high ionic conductivity of LLZO with the flexibility of polymers or the specific properties of other materials. The composite approach helps to address issues such as brittleness, poor contact, and processing challenges associated with pure LLZO coatings. By creating these hybrid structures, improved mechanical properties, better interfacial contact, and enhanced electrochemical performance can be achieved while maintaining compatibility with other battery components.
02 LLZO coating methods and their impact on compatibility
Various coating methods for LLZO significantly affect its compatibility with other battery components. Techniques include atomic layer deposition, sputtering, sol-gel processing, and chemical vapor deposition. Each method produces coatings with different morphologies, densities, and crystallinities, which directly influence interfacial properties. Optimized coating processes can minimize defects, ensure uniform coverage, and enhance adhesion, resulting in improved electrochemical performance and compatibility.Expand Specific Solutions03 Chemical stability and interface reactions of LLZO coatings
The chemical stability of LLZO coatings and their interface reactions with adjacent materials are crucial for long-term battery performance. LLZO can react with atmospheric components like moisture and CO2, forming lithium carbonate and hydroxide layers that affect ionic conductivity. Additionally, high-temperature processing can trigger reactions with neighboring components. Protective layers, dopants, and controlled processing environments can mitigate these reactions and enhance chemical compatibility.Expand Specific Solutions04 Doping strategies to enhance LLZO coating compatibility
Doping LLZO with various elements improves its compatibility with other battery components. Common dopants include aluminum, gallium, tantalum, and niobium, which stabilize the cubic phase of LLZO and enhance ionic conductivity. These dopants can modify surface properties, reduce grain boundary resistance, and improve wettability with electrodes. Optimized doping strategies lead to better mechanical properties, reduced interfacial resistance, and enhanced electrochemical performance.Expand Specific Solutions05 LLZO composite coatings for enhanced compatibility
LLZO-based composite coatings offer improved compatibility with battery components compared to pure LLZO. These composites incorporate polymers, other ceramic materials, or conductive additives to create multifunctional interfaces. The composite approach can address multiple compatibility issues simultaneously, such as mechanical mismatch, chemical reactivity, and ionic/electronic conductivity gaps. These engineered interfaces facilitate better ion transport, reduce interfacial resistance, and enhance overall battery performance.Expand Specific Solutions
Key Industry Players in Solid-State Battery Development
The solid-state battery market is in an early growth phase, with LLZO (Lithium Lanthanum Zirconium Oxide) compatibility with high-voltage cathodes representing a critical technical challenge. The market is projected to reach significant scale by 2030, driven by electric vehicle adoption. Technical maturity varies across players: research institutions like Argonne, Northwestern University, and University of Michigan are advancing fundamental solutions, while commercial entities including QuantumScape, LG Energy Solution, and A123 Systems are developing proprietary coating technologies to address the LLZO-cathode interface issues. Automotive manufacturers such as BMW and GM are strategically investing in this technology to secure competitive advantages in next-generation battery systems, recognizing the potential for higher energy density and improved safety profiles.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed innovative coating strategies for LLZO solid electrolytes to enhance compatibility with high-voltage cathodes. Their approach involves applying ultrathin ALD (Atomic Layer Deposition) coatings of Al2O3 and other metal oxides to create a protective interface between LLZO and high-voltage cathode materials. The laboratory has demonstrated that these nanoscale coatings (typically 5-20 nm thick) effectively mitigate interfacial reactions while maintaining ionic conductivity. Their research shows that Al2O3 coatings can reduce interfacial resistance by up to 70% when used with NMC811 cathodes, while ZrO2 coatings have shown promise for stabilizing interfaces with high-voltage LNMO cathodes. Argonne has also pioneered the use of lithium-containing coating materials that can act as artificial SEI layers, preventing transition metal dissolution from cathodes during cycling.
Strengths: Precise nanoscale control over coating thickness and composition; demonstrated significant reduction in interfacial resistance; expertise in ALD techniques allows for conformal coatings on complex geometries. Weaknesses: ALD processes can be time-consuming and expensive for large-scale production; some coating materials may reduce overall ionic conductivity; optimization required for each specific cathode chemistry.
QuantumScape Corp.
Technical Solution: QuantumScape has developed a proprietary ceramic separator technology that addresses compatibility issues between solid electrolytes and high-voltage cathodes. While not specifically focused on LLZO, their approach to solid-state battery interfaces provides valuable insights. Their technology employs a multilayer design with engineered interlayers that manage the chemical and mechanical interactions between cathode materials and the solid electrolyte. QuantumScape's solution includes specialized coatings that form stable interfaces with high-voltage cathodes while maintaining high ionic conductivity. Their testing has demonstrated stable cycling with NMC cathodes at high voltages (>4.3V) without significant capacity fade over hundreds of cycles. The company has reported that their interface engineering approach enables fast charging capabilities (80% charge in less than 15 minutes) while maintaining compatibility with conventional cathode manufacturing processes.
Strengths: Demonstrated stable cycling with high-voltage cathodes; multilayer approach addresses both chemical and mechanical interface issues; compatible with existing cathode manufacturing. Weaknesses: Proprietary nature limits detailed technical understanding; may require specialized manufacturing processes; scaling to mass production remains challenging.
Critical Innovations in Interface Engineering
Solvent-free processing of lithium lanthanum zirconium oxide coated-cathodes
PatentActiveUS11821091B2
Innovation
- A cubic phase, aluminum-doped lithium lanthanum zirconium oxide (LLZO) coating is applied using flame spray pyrolysis and calcination at low temperatures, forming micron- or sub-micron-sized particles for a uniform, ionically conductive coating on Ni-rich cathodes without damaging the cathode particles.
Solid-state battery cathodes and methods thereof
PatentWO2021126998A1
Innovation
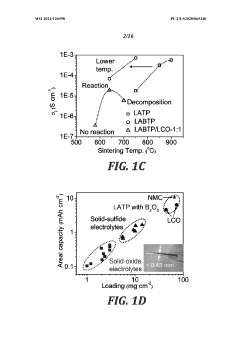
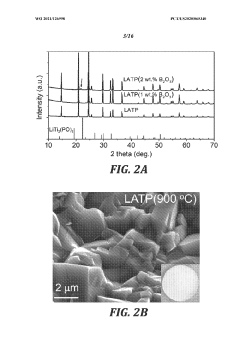
- The use of low-melting-point additives, such as B2O3, for liquid-phase sintering to create mixed conductive interphases at lower temperatures, reducing sintering temperatures and minimizing side reactions, while achieving high electronic and ionic conductivity, mechanical deformability, and high oxidation potential, thereby enhancing cathode performance.
Manufacturing Scalability of Coating Technologies
The scalability of coating technologies for LLZO solid electrolytes represents a critical factor in their commercial viability for next-generation high-voltage batteries. Current laboratory-scale coating methods such as atomic layer deposition (ALD) and pulsed laser deposition (PLD) demonstrate excellent control over coating thickness and composition but face significant challenges when scaled to industrial production volumes.
Conventional ALD processes, while providing exceptional conformality and precision, typically operate at deposition rates of only 1-2 nm per hour, making them prohibitively slow for mass production. Recent advancements in spatial ALD and batch processing have improved throughput by approximately 10-20 times, but still fall short of the production speeds required for gigawatt-scale battery manufacturing.
Solution-based coating methods, including sol-gel and wet chemical approaches, offer more promising scalability pathways. These techniques can process larger batches of LLZO particles simultaneously and utilize existing industrial equipment. However, they currently struggle with coating uniformity and thickness control when applied to the complex geometries of LLZO particles, resulting in inconsistent interfacial properties with high-voltage cathodes.
Roll-to-roll processing represents another promising avenue for scaling LLZO coating technologies. This continuous manufacturing approach has demonstrated coating speeds of up to 100 meters per minute in other industries. Adapting these systems for LLZO coating requires significant engineering modifications to maintain the precise atmospheric conditions needed for high-quality protective layers that prevent unwanted reactions with high-voltage cathode materials.
Economic analysis indicates that coating costs must be reduced by approximately 70-80% from current laboratory-scale processes to meet commercial battery price targets. This necessitates not only faster deposition rates but also higher material utilization efficiency, as many current coating processes waste 40-60% of precursor materials.
Several industrial partnerships between solid-state battery developers and equipment manufacturers are actively addressing these scalability challenges. Notable progress has been made in hybrid approaches that combine the precision of vapor deposition techniques with the throughput advantages of solution processing, potentially offering a viable pathway to industrial-scale production of coated LLZO compatible with high-voltage cathodes.
Conventional ALD processes, while providing exceptional conformality and precision, typically operate at deposition rates of only 1-2 nm per hour, making them prohibitively slow for mass production. Recent advancements in spatial ALD and batch processing have improved throughput by approximately 10-20 times, but still fall short of the production speeds required for gigawatt-scale battery manufacturing.
Solution-based coating methods, including sol-gel and wet chemical approaches, offer more promising scalability pathways. These techniques can process larger batches of LLZO particles simultaneously and utilize existing industrial equipment. However, they currently struggle with coating uniformity and thickness control when applied to the complex geometries of LLZO particles, resulting in inconsistent interfacial properties with high-voltage cathodes.
Roll-to-roll processing represents another promising avenue for scaling LLZO coating technologies. This continuous manufacturing approach has demonstrated coating speeds of up to 100 meters per minute in other industries. Adapting these systems for LLZO coating requires significant engineering modifications to maintain the precise atmospheric conditions needed for high-quality protective layers that prevent unwanted reactions with high-voltage cathode materials.
Economic analysis indicates that coating costs must be reduced by approximately 70-80% from current laboratory-scale processes to meet commercial battery price targets. This necessitates not only faster deposition rates but also higher material utilization efficiency, as many current coating processes waste 40-60% of precursor materials.
Several industrial partnerships between solid-state battery developers and equipment manufacturers are actively addressing these scalability challenges. Notable progress has been made in hybrid approaches that combine the precision of vapor deposition techniques with the throughput advantages of solution processing, potentially offering a viable pathway to industrial-scale production of coated LLZO compatible with high-voltage cathodes.
Safety and Performance Metrics for Coated Interfaces
The evaluation of safety and performance metrics for coated interfaces in LLZO-based solid-state batteries requires systematic assessment frameworks that balance protection capabilities with electrochemical functionality. When developing protective coatings for high-voltage cathode interfaces with LLZO electrolytes, several critical metrics must be established to ensure both safety enhancement and performance optimization.
Electrochemical stability represents a primary metric, measured through cyclic voltammetry and impedance spectroscopy to determine the coating's ability to prevent parasitic reactions at high voltages (>4.3V). Effective coatings must demonstrate minimal impedance growth during extended cycling while maintaining a stable electrochemical window that accommodates high-voltage cathode operation.
Mechanical integrity metrics focus on adhesion strength between the coating and both the cathode and LLZO surfaces. Nanoindentation testing and cross-sectional electron microscopy provide quantitative data on interfacial bonding quality. Superior coatings exhibit elastic modulus values that balance rigidity with flexibility to accommodate volume changes during cycling without delamination or fracture formation.
Thermal stability represents another crucial safety parameter, with coatings required to maintain structural and chemical integrity across operating temperature ranges (typically -20°C to 60°C) and during thermal runaway scenarios. Differential scanning calorimetry and thermogravimetric analysis help quantify thermal decomposition thresholds and exothermic reaction potentials.
Ion transport efficiency metrics evaluate the coating's ability to facilitate lithium-ion movement while blocking transition metal dissolution. Techniques such as galvanostatic intermittent titration and electrochemical impedance spectroscopy provide quantitative measurements of lithium-ion conductivity through the coating layer, with optimal values exceeding 10^-5 S/cm at room temperature.
Chemical compatibility assessment involves monitoring interfacial reactions between the coating and adjacent materials using techniques like X-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectrometry. Effective coatings demonstrate minimal interdiffusion of elements and formation of resistive interlayers during both processing and battery operation.
Standardized accelerated aging protocols are essential for predicting long-term coating performance, including high-temperature storage tests and rapid charge-discharge cycling. These tests generate quantitative degradation rates that can be used to estimate coating lifetimes under normal operating conditions, with premium coatings maintaining 80% of initial performance metrics after equivalent aging to 1000+ cycles.
Electrochemical stability represents a primary metric, measured through cyclic voltammetry and impedance spectroscopy to determine the coating's ability to prevent parasitic reactions at high voltages (>4.3V). Effective coatings must demonstrate minimal impedance growth during extended cycling while maintaining a stable electrochemical window that accommodates high-voltage cathode operation.
Mechanical integrity metrics focus on adhesion strength between the coating and both the cathode and LLZO surfaces. Nanoindentation testing and cross-sectional electron microscopy provide quantitative data on interfacial bonding quality. Superior coatings exhibit elastic modulus values that balance rigidity with flexibility to accommodate volume changes during cycling without delamination or fracture formation.
Thermal stability represents another crucial safety parameter, with coatings required to maintain structural and chemical integrity across operating temperature ranges (typically -20°C to 60°C) and during thermal runaway scenarios. Differential scanning calorimetry and thermogravimetric analysis help quantify thermal decomposition thresholds and exothermic reaction potentials.
Ion transport efficiency metrics evaluate the coating's ability to facilitate lithium-ion movement while blocking transition metal dissolution. Techniques such as galvanostatic intermittent titration and electrochemical impedance spectroscopy provide quantitative measurements of lithium-ion conductivity through the coating layer, with optimal values exceeding 10^-5 S/cm at room temperature.
Chemical compatibility assessment involves monitoring interfacial reactions between the coating and adjacent materials using techniques like X-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectrometry. Effective coatings demonstrate minimal interdiffusion of elements and formation of resistive interlayers during both processing and battery operation.
Standardized accelerated aging protocols are essential for predicting long-term coating performance, including high-temperature storage tests and rapid charge-discharge cycling. These tests generate quantitative degradation rates that can be used to estimate coating lifetimes under normal operating conditions, with premium coatings maintaining 80% of initial performance metrics after equivalent aging to 1000+ cycles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!