Measuring true ionic conductivity in LLZO: avoiding contact artifacts
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
LLZO Ionic Conductivity Measurement Background and Objectives
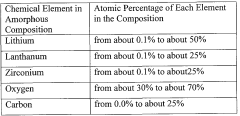
Lithium-ion conducting solid electrolytes have emerged as critical components in the development of next-generation energy storage technologies, particularly all-solid-state batteries (ASSBs). Among these solid electrolytes, Li7La3Zr2O12 (LLZO) garnet-type ceramics have attracted significant attention due to their high ionic conductivity, excellent chemical stability against lithium metal, and wide electrochemical window. Since its discovery in 2007 by Murugan et al., LLZO has been extensively studied as a promising candidate for enabling safer and higher energy density batteries.
The evolution of LLZO research has progressed through several key phases. Initially, the focus was on synthesizing and stabilizing the cubic phase of LLZO, which exhibits significantly higher ionic conductivity compared to its tetragonal counterpart. This was followed by efforts to enhance the ionic conductivity through various doping strategies, with elements such as Al, Ga, and Ta being incorporated to stabilize the cubic structure and increase lithium vacancy concentration.
A critical challenge in the LLZO development trajectory has been the accurate measurement of its true ionic conductivity. Early reports showed considerable variations in conductivity values, ranging from 10^-4 to 10^-3 S/cm at room temperature. These discrepancies have been attributed to various factors including sample preparation methods, microstructural differences, and most significantly, measurement artifacts arising from electrode-electrolyte interfaces.
The technical objective of this investigation is to establish reliable methodologies for measuring the intrinsic ionic conductivity of LLZO while eliminating or accounting for contact artifacts. These artifacts primarily stem from the formation of resistive interlayers at electrode-electrolyte interfaces, non-uniform current distribution, and partial contact between electrodes and the ceramic surface. Such phenomena can significantly skew conductivity measurements, leading to underestimated values and inconsistent results across different research groups.
Understanding and addressing these measurement challenges is crucial for several reasons. First, accurate conductivity values are essential for realistic modeling and design of LLZO-based battery systems. Second, reliable measurement techniques enable meaningful comparison between different material compositions and processing methods. Finally, distinguishing between bulk, grain boundary, and interfacial contributions to the total resistance provides valuable insights for targeted material optimization.
Recent technological advancements in impedance spectroscopy, microelectrode techniques, and in-situ characterization methods have opened new avenues for more precise conductivity measurements. These developments, coupled with improved understanding of interfacial phenomena, are paving the way toward standardized testing protocols that can yield reproducible and accurate assessments of LLZO's ionic transport properties.
The evolution of LLZO research has progressed through several key phases. Initially, the focus was on synthesizing and stabilizing the cubic phase of LLZO, which exhibits significantly higher ionic conductivity compared to its tetragonal counterpart. This was followed by efforts to enhance the ionic conductivity through various doping strategies, with elements such as Al, Ga, and Ta being incorporated to stabilize the cubic structure and increase lithium vacancy concentration.
A critical challenge in the LLZO development trajectory has been the accurate measurement of its true ionic conductivity. Early reports showed considerable variations in conductivity values, ranging from 10^-4 to 10^-3 S/cm at room temperature. These discrepancies have been attributed to various factors including sample preparation methods, microstructural differences, and most significantly, measurement artifacts arising from electrode-electrolyte interfaces.
The technical objective of this investigation is to establish reliable methodologies for measuring the intrinsic ionic conductivity of LLZO while eliminating or accounting for contact artifacts. These artifacts primarily stem from the formation of resistive interlayers at electrode-electrolyte interfaces, non-uniform current distribution, and partial contact between electrodes and the ceramic surface. Such phenomena can significantly skew conductivity measurements, leading to underestimated values and inconsistent results across different research groups.
Understanding and addressing these measurement challenges is crucial for several reasons. First, accurate conductivity values are essential for realistic modeling and design of LLZO-based battery systems. Second, reliable measurement techniques enable meaningful comparison between different material compositions and processing methods. Finally, distinguishing between bulk, grain boundary, and interfacial contributions to the total resistance provides valuable insights for targeted material optimization.
Recent technological advancements in impedance spectroscopy, microelectrode techniques, and in-situ characterization methods have opened new avenues for more precise conductivity measurements. These developments, coupled with improved understanding of interfacial phenomena, are paving the way toward standardized testing protocols that can yield reproducible and accurate assessments of LLZO's ionic transport properties.
Market Analysis for Solid-State Electrolyte Technologies
The global market for solid-state electrolytes is experiencing robust growth, driven primarily by the increasing demand for safer and higher energy density batteries. The market size for solid-state battery technologies was valued at approximately $52 million in 2021 and is projected to reach $314 million by 2028, representing a compound annual growth rate (CAGR) of 25.2% during the forecast period.
Lithium garnet-type solid electrolytes, particularly Li7La3Zr2O12 (LLZO), have emerged as one of the most promising materials for solid-state battery applications due to their high ionic conductivity and stability against lithium metal. The accurate measurement of ionic conductivity in these materials is crucial for product development and quality control, making the issue of contact artifacts in LLZO conductivity measurements a significant market concern.
The automotive sector represents the largest end-use market for solid-state electrolyte technologies, accounting for approximately 38% of the total market share. Major automotive manufacturers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery research, with particular focus on garnet-type electrolytes like LLZO. Toyota alone has committed over $13.5 billion toward battery technology development through 2030.
Consumer electronics constitutes the second-largest market segment at 29%, followed by energy storage systems at 18%. These applications demand increasingly accurate characterization methods for solid electrolytes, creating a growing market for advanced measurement technologies that can eliminate contact artifacts and provide true ionic conductivity values.
Regionally, Asia Pacific dominates the market with 42% share, led by Japan, South Korea, and China. North America follows at 31%, with significant research activities concentrated in the United States. Europe accounts for 24% of the market, with particularly strong growth in Germany and the UK.
The market for measurement and characterization equipment specific to solid-state electrolytes was valued at approximately $78 million in 2022, with impedance spectroscopy systems representing the largest segment. Equipment manufacturers are increasingly focusing on developing specialized solutions that address the contact artifact issue in LLZO and similar materials, as accurate conductivity measurements directly impact commercial viability of end products.
Industry analysts predict that solving the contact artifact problem in conductivity measurements could accelerate market adoption of LLZO-based technologies by 15-18 months, potentially unlocking an additional $120 million in market value by 2025 through improved product performance and manufacturing yield.
Lithium garnet-type solid electrolytes, particularly Li7La3Zr2O12 (LLZO), have emerged as one of the most promising materials for solid-state battery applications due to their high ionic conductivity and stability against lithium metal. The accurate measurement of ionic conductivity in these materials is crucial for product development and quality control, making the issue of contact artifacts in LLZO conductivity measurements a significant market concern.
The automotive sector represents the largest end-use market for solid-state electrolyte technologies, accounting for approximately 38% of the total market share. Major automotive manufacturers including Toyota, BMW, and Volkswagen have announced substantial investments in solid-state battery research, with particular focus on garnet-type electrolytes like LLZO. Toyota alone has committed over $13.5 billion toward battery technology development through 2030.
Consumer electronics constitutes the second-largest market segment at 29%, followed by energy storage systems at 18%. These applications demand increasingly accurate characterization methods for solid electrolytes, creating a growing market for advanced measurement technologies that can eliminate contact artifacts and provide true ionic conductivity values.
Regionally, Asia Pacific dominates the market with 42% share, led by Japan, South Korea, and China. North America follows at 31%, with significant research activities concentrated in the United States. Europe accounts for 24% of the market, with particularly strong growth in Germany and the UK.
The market for measurement and characterization equipment specific to solid-state electrolytes was valued at approximately $78 million in 2022, with impedance spectroscopy systems representing the largest segment. Equipment manufacturers are increasingly focusing on developing specialized solutions that address the contact artifact issue in LLZO and similar materials, as accurate conductivity measurements directly impact commercial viability of end products.
Industry analysts predict that solving the contact artifact problem in conductivity measurements could accelerate market adoption of LLZO-based technologies by 15-18 months, potentially unlocking an additional $120 million in market value by 2025 through improved product performance and manufacturing yield.
Current Challenges in LLZO Conductivity Measurement
The accurate measurement of ionic conductivity in Li7La3Zr2O12 (LLZO) solid electrolytes presents significant challenges that have led to inconsistent reporting in scientific literature. One of the primary issues is the presence of contact artifacts that can substantially distort conductivity measurements. These artifacts arise from the interface between the LLZO sample and the electrodes used in measurement setups, creating additional resistance that is often mistakenly attributed to the bulk material properties.
Electrode-electrolyte interfacial resistance represents a major challenge, as poor contact between metallic electrodes and the ceramic LLZO surface can introduce significant measurement errors. This issue is exacerbated by surface roughness, chemical instability at the interface, and the formation of passivation layers that contribute to artificially high impedance readings.
Sample preparation inconsistencies further complicate conductivity measurements. Variations in sintering conditions, density, grain size, and microstructural features directly impact ionic transport properties. Additionally, many researchers fail to adequately characterize sample density and microstructure, making cross-study comparisons problematic and contributing to the wide range of reported conductivity values for seemingly identical LLZO compositions.
Environmental factors introduce another layer of complexity. LLZO is highly sensitive to atmospheric conditions, particularly moisture and CO2, which can lead to surface degradation through Li+ exchange with H+ and the formation of Li2CO3 surface layers. These reactions significantly alter surface conductivity and create measurement artifacts if not properly controlled during testing.
Temperature control and calibration errors represent technical challenges that are often overlooked. Precise temperature regulation is critical for accurate conductivity measurements, as ionic conductivity is highly temperature-dependent. Even small temperature gradients across the sample or discrepancies between the measured and actual sample temperature can lead to significant errors in reported values.
Measurement technique limitations also contribute to the challenge. Impedance spectroscopy, the most common method for conductivity determination, requires careful interpretation and appropriate equivalent circuit modeling. Improper frequency ranges, electrode configurations, or data fitting approaches can lead to misinterpretation of the electrical response and inaccurate conductivity values.
Standardization issues persist across the research community, with variations in sample geometry, electrode materials, measurement protocols, and data analysis methods making direct comparisons between studies difficult. This lack of standardized approaches has contributed to the wide scatter in reported LLZO conductivity values, ranging from 10^-4 to 10^-3 S/cm at room temperature for nominally identical compositions.
Electrode-electrolyte interfacial resistance represents a major challenge, as poor contact between metallic electrodes and the ceramic LLZO surface can introduce significant measurement errors. This issue is exacerbated by surface roughness, chemical instability at the interface, and the formation of passivation layers that contribute to artificially high impedance readings.
Sample preparation inconsistencies further complicate conductivity measurements. Variations in sintering conditions, density, grain size, and microstructural features directly impact ionic transport properties. Additionally, many researchers fail to adequately characterize sample density and microstructure, making cross-study comparisons problematic and contributing to the wide range of reported conductivity values for seemingly identical LLZO compositions.
Environmental factors introduce another layer of complexity. LLZO is highly sensitive to atmospheric conditions, particularly moisture and CO2, which can lead to surface degradation through Li+ exchange with H+ and the formation of Li2CO3 surface layers. These reactions significantly alter surface conductivity and create measurement artifacts if not properly controlled during testing.
Temperature control and calibration errors represent technical challenges that are often overlooked. Precise temperature regulation is critical for accurate conductivity measurements, as ionic conductivity is highly temperature-dependent. Even small temperature gradients across the sample or discrepancies between the measured and actual sample temperature can lead to significant errors in reported values.
Measurement technique limitations also contribute to the challenge. Impedance spectroscopy, the most common method for conductivity determination, requires careful interpretation and appropriate equivalent circuit modeling. Improper frequency ranges, electrode configurations, or data fitting approaches can lead to misinterpretation of the electrical response and inaccurate conductivity values.
Standardization issues persist across the research community, with variations in sample geometry, electrode materials, measurement protocols, and data analysis methods making direct comparisons between studies difficult. This lack of standardized approaches has contributed to the wide scatter in reported LLZO conductivity values, ranging from 10^-4 to 10^-3 S/cm at room temperature for nominally identical compositions.
Established Methods for Eliminating Contact Artifacts
01 Doping strategies to enhance LLZO ionic conductivity
Various doping strategies can be employed to enhance the ionic conductivity of LLZO. Doping with elements such as aluminum, gallium, or tantalum can stabilize the cubic phase of LLZO, which exhibits higher ionic conductivity compared to the tetragonal phase. These dopants can occupy lithium sites or substitute for zirconium in the crystal structure, creating additional lithium vacancies that facilitate lithium ion transport through the material.- Doping strategies to enhance LLZO ionic conductivity: Various doping strategies can be employed to enhance the ionic conductivity of LLZO. Substituting elements like Al, Ga, Ta, or Nb into the LLZO structure can stabilize the cubic phase, which has higher ionic conductivity than the tetragonal phase. These dopants can modify the lithium ion transport pathways, reduce grain boundary resistance, and create additional lithium vacancies that facilitate faster ion movement through the material.
- Synthesis methods affecting LLZO conductivity: Different synthesis methods significantly impact the ionic conductivity of LLZO. Techniques such as sol-gel processing, solid-state reaction, co-precipitation, and hydrothermal synthesis can produce LLZO with varying grain sizes, densities, and phase purities. Advanced processing methods like spark plasma sintering can achieve higher densification at lower temperatures, reducing lithium loss during sintering and resulting in improved ionic conductivity.
- Interface engineering for LLZO-based solid electrolytes: Interface engineering is crucial for optimizing LLZO ionic conductivity in solid-state batteries. Treatments to reduce interfacial resistance between LLZO and electrodes include surface coatings, buffer layers, and chemical modifications. These approaches can minimize lithium dendrite formation, improve wettability with lithium metal anodes, and enhance the overall electrochemical performance of LLZO-based solid electrolytes.
- Composite LLZO electrolytes with enhanced properties: Composite approaches combine LLZO with other materials to overcome limitations of pure LLZO electrolytes. Incorporating polymers, other ceramic materials, or conductive additives can improve mechanical properties, reduce grain boundary resistance, and enhance overall ionic conductivity. These composite structures can provide flexibility while maintaining high ionic conductivity, making them suitable for various battery configurations.
- Microstructure control for optimized LLZO performance: Controlling the microstructure of LLZO is essential for achieving high ionic conductivity. Parameters such as grain size, porosity, grain boundary characteristics, and crystallographic orientation significantly affect lithium ion transport. Techniques to optimize these features include controlled sintering profiles, grain growth inhibitors, and templated growth methods. Reducing the grain boundary resistance through microstructure engineering can lead to substantial improvements in overall ionic conductivity.
02 Synthesis methods for high-conductivity LLZO
Different synthesis methods can significantly impact the ionic conductivity of LLZO. Techniques such as solid-state reaction, sol-gel processing, and hydrothermal synthesis can be optimized to produce LLZO with enhanced ionic conductivity. Parameters including calcination temperature, sintering time, and cooling rate play crucial roles in determining the phase purity, grain size, and ultimately the ionic conductivity of the final LLZO product.Expand Specific Solutions03 Interface engineering for LLZO-based solid electrolytes
Interface engineering is critical for improving the performance of LLZO-based solid electrolytes. The interfaces between LLZO and electrodes often suffer from high resistance and poor contact, limiting overall ionic conductivity. Various approaches including surface modification, buffer layer introduction, and composite formation can be employed to reduce interfacial resistance and enhance lithium ion transport across interfaces, resulting in improved overall ionic conductivity of LLZO-based battery systems.Expand Specific Solutions04 Microstructure control for optimized LLZO conductivity
The microstructure of LLZO significantly influences its ionic conductivity. Controlling grain size, porosity, and grain boundary characteristics can optimize lithium ion transport pathways. Dense LLZO ceramics with minimized grain boundary resistance typically exhibit higher ionic conductivity. Various processing techniques can be employed to tailor the microstructure, including pressure-assisted sintering, spark plasma sintering, and controlled grain growth approaches.Expand Specific Solutions05 Composite LLZO electrolytes for enhanced performance
Composite approaches combining LLZO with other materials can enhance overall ionic conductivity and mechanical properties. Incorporating polymers, other ceramic electrolytes, or conductive additives into LLZO can create synergistic effects that improve lithium ion transport. These composite strategies can address challenges such as brittleness of pure LLZO while maintaining or enhancing ionic conductivity, making them promising for practical solid-state battery applications.Expand Specific Solutions
Leading Research Groups and Companies in LLZO Development
The field of measuring true ionic conductivity in LLZO is currently in a growth phase, with increasing market interest driven by solid-state battery development. The market is expanding rapidly as companies seek to overcome contact artifacts that have historically skewed conductivity measurements. Technologically, the field is approaching maturity with several key players making significant advances. Samsung Electronics and LG Energy Solution are leading commercial development, leveraging their battery expertise to improve measurement accuracy. Academic institutions like Tsinghua University, Arizona State University, and Shanghai University are contributing fundamental research to standardize methodologies. Companies such as Mitsui Mining & Smelting and Sumitomo Metal Mining are developing specialized materials and testing equipment, while Energy Exploration Technologies is focusing on innovative measurement techniques to eliminate contact-related errors.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has pioneered an integrated measurement platform for accurate ionic conductivity assessment in LLZO that combines electrochemical impedance spectroscopy (EIS) with advanced surface characterization techniques. Their system employs gold-sputtered blocking electrodes with precisely controlled pressure contacts to minimize interfacial resistance while maintaining sample integrity. Samsung's approach incorporates in-situ X-ray diffraction during impedance measurements to correlate structural changes with conductivity variations, particularly important for detecting phase transitions in LLZO that affect ion transport. Their methodology includes specialized equivalent circuit models that account for grain boundary contributions and electrode polarization effects, allowing for separation of bulk, grain boundary, and interface components. Samsung researchers have demonstrated that surface contamination by Li2CO3 can create significant measurement artifacts, and have developed proprietary surface cleaning protocols using controlled atmosphere gloveboxes with <0.1 ppm water and oxygen levels to prevent these issues[3][4].
Strengths: Exceptional instrumentation capabilities with custom-designed measurement systems that integrate multiple characterization techniques. Strong materials science expertise allows for comprehensive understanding of structure-property relationships in LLZO. Weaknesses: Their approach requires sophisticated and expensive equipment that may not be accessible to all researchers, potentially limiting reproducibility across the broader scientific community.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive approach to measuring true ionic conductivity in LLZO solid electrolytes that addresses contact artifacts through multi-electrode impedance spectroscopy techniques. Their methodology employs a four-point probe measurement system that effectively separates bulk and interfacial resistances, eliminating the influence of electrode-electrolyte interfaces on conductivity measurements. The company utilizes specialized sample preparation protocols including precise polishing techniques and controlled atmosphere handling to maintain sample integrity. LG's approach incorporates temperature-dependent measurements (typically between 25-100°C) to calculate activation energies, providing deeper insights into ion transport mechanisms. Their research has demonstrated that proper surface treatment of LLZO with Li3BO3 or Li2CO3 buffer layers significantly reduces interfacial resistance, allowing for more accurate bulk conductivity measurements[1][2].
Strengths: Advanced manufacturing capabilities allow for precise control of LLZO synthesis parameters and consistent sample quality. Their extensive battery production experience provides practical insights into how measurement techniques translate to actual device performance. Weaknesses: Their proprietary nature limits full disclosure of methodological details, and their focus on commercialization may prioritize practical solutions over fundamental scientific understanding.
Critical Patents and Literature on LLZO Characterization
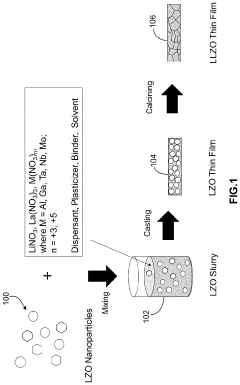
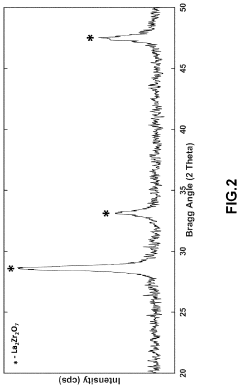
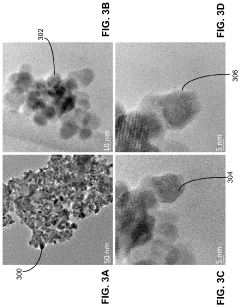
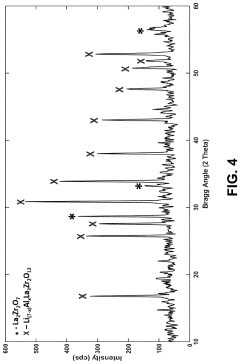
Synthesis of lithium lanthanum zirconate from nanocrystalline lanthanum zirconate
PatentActiveUS20230391633A1
Innovation
- The synthesis of LLZO thin films from lanthanum zirconate nanocrystals, using a slurry with lithium and lanthanum precursors, calcined and sintered at lower temperatures, enabling fine-grain structure and enhanced mechanical properties without the need for extrinsic dopants, facilitating scalable and cost-effective production.
Ionically-conductive amorphous lithium lanthanum zirconium oxide
PatentWO2012018831A1
Innovation
- An amorphous lithium lanthanum zirconium oxide (LLZO) composition is developed as a non-aqueous, non-liquid, ionically-conductive electrolyte medium, synthesized through a sol-gel process using alkoxides in an alcohol-based solvent, which is compatible with lithium and can be manufactured in thin-film configurations, preventing dendritic growth and ensuring safety.
Standardization Efforts in Solid Electrolyte Characterization
The field of solid-state electrolytes, particularly lithium lanthanum zirconium oxide (LLZO), has seen significant growth in research and development over the past decade. However, inconsistencies in measurement techniques and reporting standards have created challenges in comparing results across different research groups and institutions. This has highlighted the urgent need for standardization efforts in solid electrolyte characterization.
Several international organizations have begun addressing this issue through collaborative initiatives. The International Electrotechnical Commission (IEC) has established working groups specifically focused on developing standardized testing protocols for solid electrolytes. These protocols aim to eliminate variations in sample preparation, measurement conditions, and data analysis that can significantly affect reported conductivity values.
The Materials Measurement Laboratory at NIST (National Institute of Standards and Technology) has proposed reference materials and measurement protocols specifically for garnet-type solid electrolytes like LLZO. Their guidelines include detailed specifications for electrode materials, surface preparation techniques, and temperature control parameters to minimize contact artifacts that often lead to erroneous conductivity measurements.
Academic consortia, including the Battery500 Consortium and the Joint Center for Energy Storage Research (JCESR), have published best practices for ionic conductivity measurements. These include recommendations for using ion-blocking electrodes, implementing four-probe measurement techniques, and accounting for grain boundary effects in polycrystalline samples.
Industry stakeholders have also recognized the importance of standardization. Companies like Toyota Research Institute, Samsung Advanced Institute of Technology, and Solid Power have participated in round-robin testing programs to validate measurement techniques across different laboratories. These efforts have revealed significant variations in reported conductivity values for identical materials, underscoring the need for standardized approaches.
Recent progress includes the development of certified reference materials for LLZO with well-characterized ionic conductivity values. These reference materials allow researchers to calibrate their measurement systems and validate their experimental procedures. Additionally, open-source software tools have been developed to analyze impedance spectroscopy data consistently, reducing variations in data interpretation.
The Battery Interface Genome/Materials Acceleration Platform (BIG-MAP) initiative in Europe has incorporated machine learning approaches to standardize characterization data and extract meaningful patterns from diverse measurement techniques. This computational approach helps bridge gaps between different experimental methodologies and establishes more reliable benchmarks for solid electrolyte performance.
Several international organizations have begun addressing this issue through collaborative initiatives. The International Electrotechnical Commission (IEC) has established working groups specifically focused on developing standardized testing protocols for solid electrolytes. These protocols aim to eliminate variations in sample preparation, measurement conditions, and data analysis that can significantly affect reported conductivity values.
The Materials Measurement Laboratory at NIST (National Institute of Standards and Technology) has proposed reference materials and measurement protocols specifically for garnet-type solid electrolytes like LLZO. Their guidelines include detailed specifications for electrode materials, surface preparation techniques, and temperature control parameters to minimize contact artifacts that often lead to erroneous conductivity measurements.
Academic consortia, including the Battery500 Consortium and the Joint Center for Energy Storage Research (JCESR), have published best practices for ionic conductivity measurements. These include recommendations for using ion-blocking electrodes, implementing four-probe measurement techniques, and accounting for grain boundary effects in polycrystalline samples.
Industry stakeholders have also recognized the importance of standardization. Companies like Toyota Research Institute, Samsung Advanced Institute of Technology, and Solid Power have participated in round-robin testing programs to validate measurement techniques across different laboratories. These efforts have revealed significant variations in reported conductivity values for identical materials, underscoring the need for standardized approaches.
Recent progress includes the development of certified reference materials for LLZO with well-characterized ionic conductivity values. These reference materials allow researchers to calibrate their measurement systems and validate their experimental procedures. Additionally, open-source software tools have been developed to analyze impedance spectroscopy data consistently, reducing variations in data interpretation.
The Battery Interface Genome/Materials Acceleration Platform (BIG-MAP) initiative in Europe has incorporated machine learning approaches to standardize characterization data and extract meaningful patterns from diverse measurement techniques. This computational approach helps bridge gaps between different experimental methodologies and establishes more reliable benchmarks for solid electrolyte performance.
Materials Interface Science for Improved Electrode Contacts
The interface between solid electrolytes and electrodes represents a critical frontier in the development of all-solid-state batteries, particularly when utilizing LLZO (Li7La3Zr2O12) garnet electrolytes. Understanding and optimizing these interfaces is essential for accurate conductivity measurements and overall battery performance.
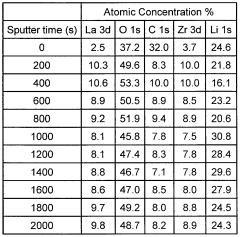
Contact resistance at the electrode-electrolyte interface often introduces significant artifacts in conductivity measurements of LLZO. These artifacts can lead to underestimation of the true ionic conductivity, complicating efforts to develop high-performance solid-state batteries. Recent research has demonstrated that poor interfacial contact can contribute up to 70-80% of the total cell resistance in some cases.
Several approaches have emerged to improve electrode contacts with LLZO. Physical modification techniques include surface polishing to atomic-level smoothness, which maximizes contact area, and thin-film deposition of interlayers such as Au, Pt, or Al to enhance wetting and reduce interfacial resistance. Chemical treatments involving Li3BO3, Li2CO3, or other lithium-containing compounds have shown promise in creating favorable interfacial chemistry.
Temperature-controlled processing has proven particularly effective, with researchers developing protocols for controlled heating during electrode application. This approach facilitates better wetting of molten lithium on LLZO surfaces, significantly reducing contact resistance. Some studies report reduction in interfacial resistance by over an order of magnitude using optimized thermal processing.
Advanced characterization techniques have been instrumental in understanding these interfaces. In-situ X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) have revealed the formation of interfacial layers and their evolution during cycling. Electrochemical impedance spectroscopy (EIS) with careful equivalent circuit modeling has enabled researchers to separate bulk and interfacial contributions to resistance.
Computational studies using density functional theory (DFT) and molecular dynamics simulations have provided atomic-level insights into interfacial phenomena, guiding experimental approaches. These simulations have identified promising coating materials and surface modifications that minimize energy barriers for lithium ion transport across interfaces.
Recent innovations include plasma treatment of LLZO surfaces to create lithium-deficient layers that enhance wetting, and atomic layer deposition (ALD) of nanometer-thick buffer layers that simultaneously improve contact and protect against side reactions. These approaches have demonstrated significant improvements in measured conductivity values, approaching the theoretical limits for LLZO electrolytes.
Contact resistance at the electrode-electrolyte interface often introduces significant artifacts in conductivity measurements of LLZO. These artifacts can lead to underestimation of the true ionic conductivity, complicating efforts to develop high-performance solid-state batteries. Recent research has demonstrated that poor interfacial contact can contribute up to 70-80% of the total cell resistance in some cases.
Several approaches have emerged to improve electrode contacts with LLZO. Physical modification techniques include surface polishing to atomic-level smoothness, which maximizes contact area, and thin-film deposition of interlayers such as Au, Pt, or Al to enhance wetting and reduce interfacial resistance. Chemical treatments involving Li3BO3, Li2CO3, or other lithium-containing compounds have shown promise in creating favorable interfacial chemistry.
Temperature-controlled processing has proven particularly effective, with researchers developing protocols for controlled heating during electrode application. This approach facilitates better wetting of molten lithium on LLZO surfaces, significantly reducing contact resistance. Some studies report reduction in interfacial resistance by over an order of magnitude using optimized thermal processing.
Advanced characterization techniques have been instrumental in understanding these interfaces. In-situ X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) have revealed the formation of interfacial layers and their evolution during cycling. Electrochemical impedance spectroscopy (EIS) with careful equivalent circuit modeling has enabled researchers to separate bulk and interfacial contributions to resistance.
Computational studies using density functional theory (DFT) and molecular dynamics simulations have provided atomic-level insights into interfacial phenomena, guiding experimental approaches. These simulations have identified promising coating materials and surface modifications that minimize energy barriers for lithium ion transport across interfaces.
Recent innovations include plasma treatment of LLZO surfaces to create lithium-deficient layers that enhance wetting, and atomic layer deposition (ALD) of nanometer-thick buffer layers that simultaneously improve contact and protect against side reactions. These approaches have demonstrated significant improvements in measured conductivity values, approaching the theoretical limits for LLZO electrolytes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!