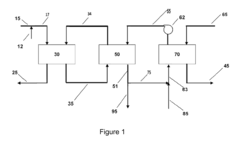
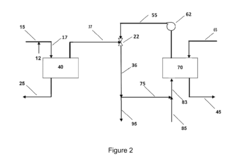
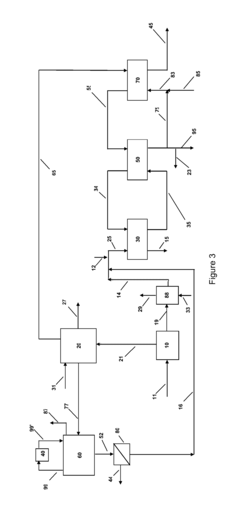
Ammonium hydroxide's role in wastewater nutrient recovery
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NH4OH in Nutrient Recovery: Background and Objectives
Ammonium hydroxide (NH4OH) has emerged as a crucial component in the field of wastewater nutrient recovery, addressing the growing global challenges of water scarcity and environmental pollution. The journey of NH4OH in this domain traces back to the early 2000s when researchers began exploring innovative methods to extract valuable nutrients from wastewater streams. This technological evolution was driven by the increasing awareness of the finite nature of phosphorus reserves and the environmental impacts of nitrogen discharge.
The primary objective of utilizing NH4OH in nutrient recovery is to efficiently extract and recycle essential elements, particularly nitrogen and phosphorus, from wastewater. These nutrients, when properly recovered, can be repurposed for agricultural applications, reducing the dependence on synthetic fertilizers and closing the nutrient loop in a circular economy model. The technology aims to transform wastewater treatment plants from mere pollution control facilities into resource recovery hubs, aligning with sustainable development goals.
Over the past two decades, the role of NH4OH in nutrient recovery has expanded significantly. Initially used in small-scale laboratory experiments, it has now found applications in pilot projects and full-scale wastewater treatment facilities worldwide. The technology has evolved to address various types of wastewater, including municipal, industrial, and agricultural effluents, each presenting unique challenges and opportunities for nutrient recovery.
The technological trajectory of NH4OH in nutrient recovery is closely linked to advancements in membrane technology, ion exchange processes, and struvite precipitation methods. These developments have enhanced the efficiency and economic viability of nutrient recovery systems, making them increasingly attractive for widespread implementation. The integration of NH4OH-based recovery processes with existing wastewater treatment infrastructure has become a key focus area, aiming to minimize disruption and maximize resource utilization.
Looking ahead, the field of NH4OH-assisted nutrient recovery is poised for further innovation. Emerging trends include the development of selective recovery techniques for specific nutrient compounds, the optimization of energy consumption in recovery processes, and the exploration of novel applications for recovered nutrients beyond traditional agriculture. Additionally, there is a growing emphasis on developing robust control systems and predictive models to enhance the stability and performance of NH4OH-based recovery technologies under varying wastewater compositions and environmental conditions.
As global attention shifts towards circular economy principles and sustainable resource management, the role of NH4OH in wastewater nutrient recovery is expected to gain even greater prominence. Future research and development efforts are likely to focus on improving recovery rates, reducing operational costs, and expanding the range of recoverable nutrients, ultimately contributing to a more sustainable and resource-efficient water management paradigm.
The primary objective of utilizing NH4OH in nutrient recovery is to efficiently extract and recycle essential elements, particularly nitrogen and phosphorus, from wastewater. These nutrients, when properly recovered, can be repurposed for agricultural applications, reducing the dependence on synthetic fertilizers and closing the nutrient loop in a circular economy model. The technology aims to transform wastewater treatment plants from mere pollution control facilities into resource recovery hubs, aligning with sustainable development goals.
Over the past two decades, the role of NH4OH in nutrient recovery has expanded significantly. Initially used in small-scale laboratory experiments, it has now found applications in pilot projects and full-scale wastewater treatment facilities worldwide. The technology has evolved to address various types of wastewater, including municipal, industrial, and agricultural effluents, each presenting unique challenges and opportunities for nutrient recovery.
The technological trajectory of NH4OH in nutrient recovery is closely linked to advancements in membrane technology, ion exchange processes, and struvite precipitation methods. These developments have enhanced the efficiency and economic viability of nutrient recovery systems, making them increasingly attractive for widespread implementation. The integration of NH4OH-based recovery processes with existing wastewater treatment infrastructure has become a key focus area, aiming to minimize disruption and maximize resource utilization.
Looking ahead, the field of NH4OH-assisted nutrient recovery is poised for further innovation. Emerging trends include the development of selective recovery techniques for specific nutrient compounds, the optimization of energy consumption in recovery processes, and the exploration of novel applications for recovered nutrients beyond traditional agriculture. Additionally, there is a growing emphasis on developing robust control systems and predictive models to enhance the stability and performance of NH4OH-based recovery technologies under varying wastewater compositions and environmental conditions.
As global attention shifts towards circular economy principles and sustainable resource management, the role of NH4OH in wastewater nutrient recovery is expected to gain even greater prominence. Future research and development efforts are likely to focus on improving recovery rates, reducing operational costs, and expanding the range of recoverable nutrients, ultimately contributing to a more sustainable and resource-efficient water management paradigm.
Market Analysis for Nutrient Recovery Technologies
The market for nutrient recovery technologies in wastewater treatment has been experiencing significant growth in recent years, driven by increasing environmental regulations, resource scarcity, and the push towards circular economy principles. The global market for nutrient recovery from wastewater is projected to reach several billion dollars by 2025, with a compound annual growth rate exceeding 10%.
Ammonium hydroxide plays a crucial role in this market as both a target for recovery and a potential product. The demand for recovered ammonia is particularly strong in the agricultural sector, where it serves as a valuable fertilizer. Additionally, industries such as pharmaceuticals, textiles, and cleaning products utilize ammonia as a raw material, further expanding the market potential for recovered ammonium.
The market for nutrient recovery technologies can be segmented based on the type of nutrient recovered, with nitrogen (primarily in the form of ammonia) and phosphorus being the most sought-after. Geographically, North America and Europe lead the market due to stringent environmental regulations and advanced wastewater treatment infrastructure. However, rapid industrialization and urbanization in Asia-Pacific regions are expected to drive significant market growth in the coming years.
Key market drivers include the increasing focus on sustainable water management practices, rising costs of synthetic fertilizers, and growing awareness of the environmental impacts of nutrient pollution. Government initiatives and regulations aimed at reducing nutrient discharge into water bodies are also propelling market growth. For instance, the European Union's Water Framework Directive and the United States' Clean Water Act have set strict limits on nutrient levels in wastewater effluents, incentivizing the adoption of recovery technologies.
The market faces challenges such as high initial investment costs for implementing nutrient recovery systems and the need for specialized expertise in operating these technologies. However, ongoing research and development efforts are focused on improving the efficiency and cost-effectiveness of nutrient recovery processes, particularly those involving ammonium hydroxide.
Emerging trends in the market include the integration of nutrient recovery with other wastewater treatment processes, the development of decentralized recovery systems for smaller communities, and the exploration of novel applications for recovered nutrients beyond traditional fertilizers. The potential for recovering other valuable compounds alongside nutrients, such as bioplastics precursors or energy sources, is also gaining attention and could significantly expand the market scope in the future.
Ammonium hydroxide plays a crucial role in this market as both a target for recovery and a potential product. The demand for recovered ammonia is particularly strong in the agricultural sector, where it serves as a valuable fertilizer. Additionally, industries such as pharmaceuticals, textiles, and cleaning products utilize ammonia as a raw material, further expanding the market potential for recovered ammonium.
The market for nutrient recovery technologies can be segmented based on the type of nutrient recovered, with nitrogen (primarily in the form of ammonia) and phosphorus being the most sought-after. Geographically, North America and Europe lead the market due to stringent environmental regulations and advanced wastewater treatment infrastructure. However, rapid industrialization and urbanization in Asia-Pacific regions are expected to drive significant market growth in the coming years.
Key market drivers include the increasing focus on sustainable water management practices, rising costs of synthetic fertilizers, and growing awareness of the environmental impacts of nutrient pollution. Government initiatives and regulations aimed at reducing nutrient discharge into water bodies are also propelling market growth. For instance, the European Union's Water Framework Directive and the United States' Clean Water Act have set strict limits on nutrient levels in wastewater effluents, incentivizing the adoption of recovery technologies.
The market faces challenges such as high initial investment costs for implementing nutrient recovery systems and the need for specialized expertise in operating these technologies. However, ongoing research and development efforts are focused on improving the efficiency and cost-effectiveness of nutrient recovery processes, particularly those involving ammonium hydroxide.
Emerging trends in the market include the integration of nutrient recovery with other wastewater treatment processes, the development of decentralized recovery systems for smaller communities, and the exploration of novel applications for recovered nutrients beyond traditional fertilizers. The potential for recovering other valuable compounds alongside nutrients, such as bioplastics precursors or energy sources, is also gaining attention and could significantly expand the market scope in the future.
Current Challenges in Wastewater Nutrient Recovery
Wastewater nutrient recovery faces several significant challenges that hinder its widespread implementation and efficiency. One of the primary obstacles is the complex composition of wastewater, which contains a diverse array of contaminants and nutrients in varying concentrations. This heterogeneity makes it difficult to develop a one-size-fits-all solution for nutrient recovery.
The presence of competing ions and interfering compounds in wastewater often reduces the effectiveness of nutrient recovery processes. For instance, the recovery of phosphorus can be impeded by the presence of calcium, magnesium, and other metals that form insoluble precipitates. Similarly, the recovery of nitrogen in the form of ammonia can be affected by the presence of organic matter and other nitrogen-containing compounds.
Energy consumption remains a significant challenge in nutrient recovery processes. Many current technologies, such as struvite precipitation or ion exchange, require substantial energy inputs for operation, which can offset the environmental benefits of nutrient recovery. Developing energy-efficient methods that can operate at ambient temperatures and pressures is crucial for improving the sustainability of these processes.
The economic viability of nutrient recovery systems is another major hurdle. The cost of implementing and operating these systems often outweighs the immediate economic benefits, particularly when compared to traditional wastewater treatment methods. This economic barrier is further compounded by the fluctuating market prices of recovered nutrients, which can affect the long-term profitability of recovery operations.
Scale formation and fouling of equipment are persistent operational challenges in nutrient recovery systems. These issues can lead to reduced efficiency, increased maintenance requirements, and higher operational costs. Developing materials and processes that are resistant to scaling and fouling is essential for improving the reliability and longevity of nutrient recovery systems.
The regulatory landscape surrounding nutrient recovery from wastewater is often complex and varies significantly across different regions. This lack of standardization can create uncertainty for technology developers and potential adopters, slowing down innovation and implementation. Establishing clear guidelines and standards for nutrient recovery processes and products is necessary to facilitate wider adoption.
Lastly, there is a need for more efficient and selective recovery methods that can target specific nutrients while minimizing the co-recovery of contaminants. Current technologies often struggle to achieve high purity in recovered products, which can limit their marketability and potential applications. Developing more selective recovery processes, possibly through the use of novel materials or advanced separation techniques, remains an active area of research and development in the field of wastewater nutrient recovery.
The presence of competing ions and interfering compounds in wastewater often reduces the effectiveness of nutrient recovery processes. For instance, the recovery of phosphorus can be impeded by the presence of calcium, magnesium, and other metals that form insoluble precipitates. Similarly, the recovery of nitrogen in the form of ammonia can be affected by the presence of organic matter and other nitrogen-containing compounds.
Energy consumption remains a significant challenge in nutrient recovery processes. Many current technologies, such as struvite precipitation or ion exchange, require substantial energy inputs for operation, which can offset the environmental benefits of nutrient recovery. Developing energy-efficient methods that can operate at ambient temperatures and pressures is crucial for improving the sustainability of these processes.
The economic viability of nutrient recovery systems is another major hurdle. The cost of implementing and operating these systems often outweighs the immediate economic benefits, particularly when compared to traditional wastewater treatment methods. This economic barrier is further compounded by the fluctuating market prices of recovered nutrients, which can affect the long-term profitability of recovery operations.
Scale formation and fouling of equipment are persistent operational challenges in nutrient recovery systems. These issues can lead to reduced efficiency, increased maintenance requirements, and higher operational costs. Developing materials and processes that are resistant to scaling and fouling is essential for improving the reliability and longevity of nutrient recovery systems.
The regulatory landscape surrounding nutrient recovery from wastewater is often complex and varies significantly across different regions. This lack of standardization can create uncertainty for technology developers and potential adopters, slowing down innovation and implementation. Establishing clear guidelines and standards for nutrient recovery processes and products is necessary to facilitate wider adoption.
Lastly, there is a need for more efficient and selective recovery methods that can target specific nutrients while minimizing the co-recovery of contaminants. Current technologies often struggle to achieve high purity in recovered products, which can limit their marketability and potential applications. Developing more selective recovery processes, possibly through the use of novel materials or advanced separation techniques, remains an active area of research and development in the field of wastewater nutrient recovery.
Existing NH4OH Recovery Solutions
01 Ammonium hydroxide for nutrient recovery from wastewater
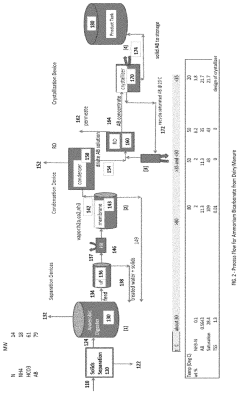
Ammonium hydroxide is used in processes to recover nutrients, particularly nitrogen and phosphorus, from wastewater streams. This method involves adjusting pH levels to precipitate or extract valuable nutrients, which can then be used as fertilizers or for other industrial applications.- Ammonium hydroxide for nutrient recovery from wastewater: Ammonium hydroxide is used in processes for recovering nutrients, particularly nitrogen and phosphorus, from wastewater streams. This method involves treating the wastewater with ammonium hydroxide to adjust pH and facilitate the precipitation or extraction of valuable nutrients, which can then be used as fertilizers or for other industrial applications.
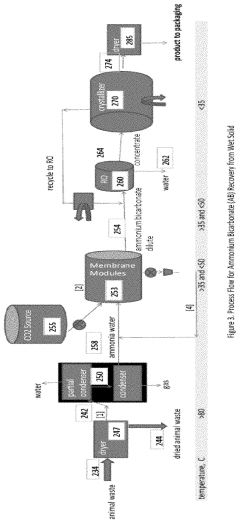
- Ammonia recovery from industrial processes: Techniques for recovering ammonia from various industrial processes, such as coal gasification or coke production, often involve the use of ammonium hydroxide. These methods typically include steps for capturing ammonia gas, converting it to ammonium hydroxide, and then further processing to recover pure ammonia or ammonium compounds for reuse or sale.
- Nutrient recovery from organic waste materials: Processes for recovering nutrients from organic waste materials, such as animal manure or food waste, often incorporate ammonium hydroxide. These methods may involve treating the waste with ammonium hydroxide to adjust pH, facilitate nutrient extraction, or convert organic nitrogen into more readily available forms for use as fertilizers.
- Ammonium hydroxide in soil amendment and fertilizer production: Ammonium hydroxide is used in the production of soil amendments and fertilizers, particularly in processes that aim to recover and recycle nutrients from various waste streams. These methods often involve treating waste materials with ammonium hydroxide to extract nutrients, which are then formulated into effective fertilizer products for agricultural use.
- Nutrient recovery from incineration ash: Techniques for recovering nutrients, especially phosphorus, from incineration ash often involve the use of ammonium hydroxide. These processes typically include treating the ash with ammonium hydroxide to extract phosphorus and other valuable nutrients, which can then be recovered and used in the production of fertilizers or other industrial applications.
02 Ammonia recovery from industrial processes
Techniques for recovering ammonia from various industrial processes, including the use of ammonium hydroxide solutions. These methods often involve stripping, absorption, or distillation processes to separate and concentrate ammonia for reuse or further processing.Expand Specific Solutions03 Nutrient recovery from organic waste
Methods for extracting nutrients from organic waste materials using ammonium hydroxide or similar alkaline solutions. These processes often involve hydrolysis or other chemical treatments to break down organic matter and release valuable nutrients for agricultural or industrial use.Expand Specific Solutions04 Ammonium hydroxide in fertilizer production
The use of ammonium hydroxide in the production of fertilizers, either as a direct component or as an intermediate in the manufacturing process. This includes methods for combining ammonium hydroxide with other nutrients to create balanced fertilizer formulations.Expand Specific Solutions05 Nutrient recovery from flue gas and industrial emissions
Processes for capturing and recovering nutrients, particularly ammonia and its derivatives, from flue gas and other industrial emissions using ammonium hydroxide solutions. These methods often involve scrubbing techniques to absorb and concentrate valuable nutrients from gaseous streams.Expand Specific Solutions
Key Players in Nutrient Recovery Industry
The ammonium hydroxide's role in wastewater nutrient recovery is an emerging field in the water treatment industry, currently in its growth phase. The market size is expanding as environmental regulations become stricter and resource recovery gains importance. Technologically, it's progressing from early-stage research to pilot implementations. Companies like Anaergia, Inc. and ThermoEnergy Corp. are at the forefront, developing innovative solutions. Academic institutions such as Zhejiang University and Indian Institute of Technology Madras are contributing significant research. The involvement of established players like Ebara Corp. and emerging startups like Again AB indicates a diverse competitive landscape, with varying levels of technological maturity across different approaches to nutrient recovery.
Anaergia, Inc.
Technical Solution: Anaergia has developed an innovative approach to wastewater nutrient recovery using ammonium hydroxide. Their ANITA™ Mox process employs anammox bacteria to convert ammonium and nitrite directly into nitrogen gas, significantly reducing energy consumption and chemical usage[1]. The process incorporates a two-stage biological treatment: partial nitritation followed by anammox reaction. Ammonium hydroxide plays a crucial role in maintaining optimal pH levels for bacterial growth and activity. Additionally, Anaergia's system includes a struvite recovery unit, where ammonium hydroxide helps precipitate phosphorus and nitrogen as struvite, a valuable slow-release fertilizer[2]. This integrated approach allows for efficient nutrient removal and recovery, addressing both environmental concerns and resource scarcity issues in wastewater treatment.
Strengths: Energy-efficient process, reduced chemical usage, production of valuable by-products. Weaknesses: Requires careful process control, may be sensitive to temperature fluctuations, initial implementation costs can be high.
Indian Institute of Technology Madras
Technical Solution: The Indian Institute of Technology Madras has developed an innovative wastewater nutrient recovery system that leverages the properties of ammonium hydroxide. Their approach combines advanced oxidation processes (AOPs) with biological treatment and chemical precipitation. In the first stage, ammonium hydroxide is used to adjust the pH of the wastewater, optimizing conditions for a novel photo-Fenton process that breaks down complex organic compounds[12]. This is followed by a modified sequencing batch reactor (SBR) where ammonium hydroxide plays a crucial role in maintaining the ideal pH for enhanced biological nutrient removal. The final stage involves a struvite precipitation reactor, where ammonium hydroxide is used to create favorable conditions for phosphorus and nitrogen recovery as struvite crystals[13]. The institute has also developed a unique ammonia recovery system that uses a hydrophobic membrane contactor, where ammonium hydroxide facilitates the transfer of ammonia across the membrane for subsequent capture and conversion into valuable fertilizer products[14]. This integrated system achieves high nutrient removal rates while producing multiple value-added products, addressing both environmental and economic concerns in wastewater treatment.
Strengths: Integration of advanced oxidation, biological, and chemical processes, high nutrient removal efficiency, production of valuable by-products. Weaknesses: Complex system requiring skilled operation, potential high energy consumption for AOP, may require significant initial investment.
Innovations in NH4OH Nutrient Extraction
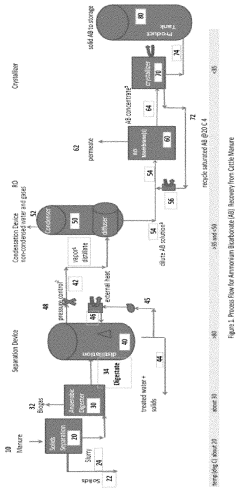
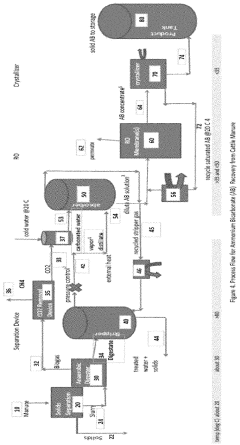
Process to recover ammonium bicarbonate from wastewater
PatentActiveUS20220227637A1
Innovation
- A process that converts ammonia to gaseous form at elevated temperatures, mixes it with carbon dioxide and water vapor to form dissolved ammonium bicarbonate, and then crystallizes it at controlled temperatures, eliminating the need for chemical pH adjustments and enabling the production of nitrogen-rich organic fertilizers.
Ammonium recovery from waste water using co2 acidified absorption water
PatentActiveUS20130028827A1
Innovation
- A process that uses CO2 acidified absorption water to strip and recover ammonium, maintaining the pH below the pKa of ammonia to convert ammonia to the ammonium form, which is then absorbed into a CO2-rich scrubbing solution, eliminating the need for mineral acids and allowing for reuse or sale of the ammonium as a nitrogen source in fermentation processes.
Environmental Impact Assessment
The use of ammonium hydroxide in wastewater nutrient recovery processes has significant environmental implications that warrant careful consideration. This assessment examines the potential impacts on various environmental aspects, including water quality, air quality, soil health, and ecosystem balance.
In terms of water quality, the application of ammonium hydroxide can lead to both positive and negative outcomes. On the positive side, it aids in the efficient removal of nutrients, particularly nitrogen and phosphorus, from wastewater. This reduction in nutrient load helps prevent eutrophication in receiving water bodies, thereby protecting aquatic ecosystems from algal blooms and oxygen depletion. However, if not properly managed, excess ammonium hydroxide can increase the pH of treated water, potentially affecting the aquatic life in discharge areas.
Air quality impacts are primarily associated with the potential release of ammonia gas during the treatment process. Proper handling and containment measures are crucial to minimize atmospheric emissions. Ammonia, if released in significant quantities, can contribute to the formation of particulate matter and impact local air quality. Additionally, it may lead to the deposition of nitrogen in nearby ecosystems, potentially altering soil chemistry and vegetation patterns.
Soil health can be indirectly affected by the use of ammonium hydroxide in nutrient recovery. The recovered nutrients, when used as fertilizers, can improve soil fertility and reduce the need for synthetic fertilizers. This can lead to more sustainable agricultural practices and reduced environmental impacts associated with fertilizer production. However, care must be taken to ensure that the recovered nutrients are free from contaminants that could accumulate in soil over time.
The broader ecosystem impacts of ammonium hydroxide use in nutrient recovery are complex and interconnected. While the reduction of nutrient pollution in water bodies is generally beneficial for aquatic ecosystems, the potential for pH changes and ammonia emissions necessitates careful monitoring and management. The recovered nutrients can support more sustainable agricultural practices, potentially reducing the overall environmental footprint of food production.
Long-term environmental effects should also be considered, including the potential for bioaccumulation of any trace contaminants in the recovered nutrients. Regular monitoring and assessment of soil, water, and air quality in areas where recovered nutrients are applied is essential to ensure long-term environmental sustainability.
In conclusion, while ammonium hydroxide plays a crucial role in wastewater nutrient recovery, its environmental impacts are multifaceted. Proper management, stringent safety protocols, and ongoing environmental monitoring are essential to maximize the benefits of nutrient recovery while minimizing potential negative impacts on the environment.
In terms of water quality, the application of ammonium hydroxide can lead to both positive and negative outcomes. On the positive side, it aids in the efficient removal of nutrients, particularly nitrogen and phosphorus, from wastewater. This reduction in nutrient load helps prevent eutrophication in receiving water bodies, thereby protecting aquatic ecosystems from algal blooms and oxygen depletion. However, if not properly managed, excess ammonium hydroxide can increase the pH of treated water, potentially affecting the aquatic life in discharge areas.
Air quality impacts are primarily associated with the potential release of ammonia gas during the treatment process. Proper handling and containment measures are crucial to minimize atmospheric emissions. Ammonia, if released in significant quantities, can contribute to the formation of particulate matter and impact local air quality. Additionally, it may lead to the deposition of nitrogen in nearby ecosystems, potentially altering soil chemistry and vegetation patterns.
Soil health can be indirectly affected by the use of ammonium hydroxide in nutrient recovery. The recovered nutrients, when used as fertilizers, can improve soil fertility and reduce the need for synthetic fertilizers. This can lead to more sustainable agricultural practices and reduced environmental impacts associated with fertilizer production. However, care must be taken to ensure that the recovered nutrients are free from contaminants that could accumulate in soil over time.
The broader ecosystem impacts of ammonium hydroxide use in nutrient recovery are complex and interconnected. While the reduction of nutrient pollution in water bodies is generally beneficial for aquatic ecosystems, the potential for pH changes and ammonia emissions necessitates careful monitoring and management. The recovered nutrients can support more sustainable agricultural practices, potentially reducing the overall environmental footprint of food production.
Long-term environmental effects should also be considered, including the potential for bioaccumulation of any trace contaminants in the recovered nutrients. Regular monitoring and assessment of soil, water, and air quality in areas where recovered nutrients are applied is essential to ensure long-term environmental sustainability.
In conclusion, while ammonium hydroxide plays a crucial role in wastewater nutrient recovery, its environmental impacts are multifaceted. Proper management, stringent safety protocols, and ongoing environmental monitoring are essential to maximize the benefits of nutrient recovery while minimizing potential negative impacts on the environment.
Economic Feasibility Analysis
The economic feasibility of using ammonium hydroxide in wastewater nutrient recovery is a critical consideration for implementing this technology on a large scale. The process involves several cost factors that need to be carefully evaluated against the potential benefits and revenue streams.
One of the primary economic advantages of using ammonium hydroxide in nutrient recovery is the potential for producing valuable fertilizer products. The recovered nutrients, particularly nitrogen and phosphorus, can be sold as high-quality fertilizers, creating a significant revenue stream. This aspect is particularly attractive in regions where there is a high demand for agricultural inputs.
However, the capital costs associated with implementing the technology can be substantial. The installation of specialized equipment for nutrient extraction, such as ion exchange systems or membrane filtration units, requires significant upfront investment. Additionally, the cost of ammonium hydroxide itself must be factored into the operational expenses.
Operational costs include energy consumption for the treatment process, maintenance of equipment, and labor costs for skilled operators. These ongoing expenses need to be carefully balanced against the potential savings from reduced wastewater treatment costs and the revenue generated from recovered nutrients.
The economic viability of the process can be significantly influenced by local regulations and incentives. In regions where strict environmental regulations are in place, the cost savings from avoiding fines or penalties for nutrient discharge can make the investment more attractive. Furthermore, government incentives or subsidies for implementing sustainable wastewater treatment technologies can improve the economic feasibility.
Market conditions play a crucial role in determining the profitability of nutrient recovery. The price volatility of fertilizers and the demand for recovered nutrients in local agricultural markets can impact the revenue potential. A stable and high-demand market for recovered nutrients is essential for long-term economic sustainability.
The scale of operation is another important factor. Larger wastewater treatment facilities may benefit from economies of scale, making the implementation of ammonium hydroxide-based nutrient recovery more cost-effective. Smaller operations may face challenges in achieving economic viability unless they can secure premium prices for their recovered nutrients or benefit from specific local incentives.
Long-term cost-benefit analysis is crucial for assessing the economic feasibility. While initial costs may be high, the potential for long-term savings in wastewater treatment, coupled with consistent revenue from nutrient sales, can lead to a favorable return on investment over time. This analysis should also consider the environmental benefits and potential future regulatory changes that may further incentivize nutrient recovery technologies.
One of the primary economic advantages of using ammonium hydroxide in nutrient recovery is the potential for producing valuable fertilizer products. The recovered nutrients, particularly nitrogen and phosphorus, can be sold as high-quality fertilizers, creating a significant revenue stream. This aspect is particularly attractive in regions where there is a high demand for agricultural inputs.
However, the capital costs associated with implementing the technology can be substantial. The installation of specialized equipment for nutrient extraction, such as ion exchange systems or membrane filtration units, requires significant upfront investment. Additionally, the cost of ammonium hydroxide itself must be factored into the operational expenses.
Operational costs include energy consumption for the treatment process, maintenance of equipment, and labor costs for skilled operators. These ongoing expenses need to be carefully balanced against the potential savings from reduced wastewater treatment costs and the revenue generated from recovered nutrients.
The economic viability of the process can be significantly influenced by local regulations and incentives. In regions where strict environmental regulations are in place, the cost savings from avoiding fines or penalties for nutrient discharge can make the investment more attractive. Furthermore, government incentives or subsidies for implementing sustainable wastewater treatment technologies can improve the economic feasibility.
Market conditions play a crucial role in determining the profitability of nutrient recovery. The price volatility of fertilizers and the demand for recovered nutrients in local agricultural markets can impact the revenue potential. A stable and high-demand market for recovered nutrients is essential for long-term economic sustainability.
The scale of operation is another important factor. Larger wastewater treatment facilities may benefit from economies of scale, making the implementation of ammonium hydroxide-based nutrient recovery more cost-effective. Smaller operations may face challenges in achieving economic viability unless they can secure premium prices for their recovered nutrients or benefit from specific local incentives.
Long-term cost-benefit analysis is crucial for assessing the economic feasibility. While initial costs may be high, the potential for long-term savings in wastewater treatment, coupled with consistent revenue from nutrient sales, can lead to a favorable return on investment over time. This analysis should also consider the environmental benefits and potential future regulatory changes that may further incentivize nutrient recovery technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!