Ammonium hydroxide's use in hydrogen storage material development
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NH4OH in H2 Storage: Background and Objectives
Ammonium hydroxide (NH4OH) has emerged as a promising candidate in the development of hydrogen storage materials, marking a significant milestone in the quest for sustainable energy solutions. The evolution of this technology can be traced back to the early 21st century when researchers began exploring alternative methods for storing hydrogen efficiently and safely. As global efforts to reduce carbon emissions intensified, the focus on hydrogen as a clean energy carrier grew, propelling the search for innovative storage solutions.
The primary objective of utilizing ammonium hydroxide in hydrogen storage material development is to overcome the limitations of conventional storage methods. Traditional approaches, such as high-pressure tanks or cryogenic liquefaction, have faced challenges related to safety, energy efficiency, and practicality for widespread adoption. Ammonium hydroxide-based systems aim to provide a more accessible, cost-effective, and potentially safer alternative for hydrogen storage.
One of the key technological goals in this field is to achieve high gravimetric and volumetric hydrogen storage capacities. Researchers are striving to develop materials that can store a significant amount of hydrogen relative to their weight and volume, making them suitable for various applications, including transportation and portable power sources. Additionally, the technology aims to address the issues of hydrogen release kinetics and reversibility, ensuring that stored hydrogen can be efficiently extracted when needed and the storage material can be regenerated for multiple use cycles.
The development of ammonium hydroxide-based hydrogen storage materials aligns with broader technological trends in the energy sector. These include the push for decarbonization, the integration of renewable energy sources, and the advancement of fuel cell technologies. As the hydrogen economy gains momentum, the role of efficient storage solutions becomes increasingly critical in bridging the gap between hydrogen production and end-use applications.
Researchers are exploring various approaches to leverage ammonium hydroxide in hydrogen storage. These include the development of composite materials, nanostructured compounds, and novel catalysts to enhance the hydrogen absorption and desorption processes. The ultimate aim is to create a storage system that meets the U.S. Department of Energy's targets for onboard hydrogen storage systems, which include specific energy, energy density, cost, and operational parameters.
As the technology progresses, it is expected to contribute significantly to the realization of a hydrogen-based energy ecosystem. The successful implementation of ammonium hydroxide-based hydrogen storage could potentially revolutionize sectors such as transportation, stationary power generation, and portable electronics. Moreover, it could play a crucial role in enabling the large-scale integration of intermittent renewable energy sources by providing an effective means of energy storage and distribution.
The primary objective of utilizing ammonium hydroxide in hydrogen storage material development is to overcome the limitations of conventional storage methods. Traditional approaches, such as high-pressure tanks or cryogenic liquefaction, have faced challenges related to safety, energy efficiency, and practicality for widespread adoption. Ammonium hydroxide-based systems aim to provide a more accessible, cost-effective, and potentially safer alternative for hydrogen storage.
One of the key technological goals in this field is to achieve high gravimetric and volumetric hydrogen storage capacities. Researchers are striving to develop materials that can store a significant amount of hydrogen relative to their weight and volume, making them suitable for various applications, including transportation and portable power sources. Additionally, the technology aims to address the issues of hydrogen release kinetics and reversibility, ensuring that stored hydrogen can be efficiently extracted when needed and the storage material can be regenerated for multiple use cycles.
The development of ammonium hydroxide-based hydrogen storage materials aligns with broader technological trends in the energy sector. These include the push for decarbonization, the integration of renewable energy sources, and the advancement of fuel cell technologies. As the hydrogen economy gains momentum, the role of efficient storage solutions becomes increasingly critical in bridging the gap between hydrogen production and end-use applications.
Researchers are exploring various approaches to leverage ammonium hydroxide in hydrogen storage. These include the development of composite materials, nanostructured compounds, and novel catalysts to enhance the hydrogen absorption and desorption processes. The ultimate aim is to create a storage system that meets the U.S. Department of Energy's targets for onboard hydrogen storage systems, which include specific energy, energy density, cost, and operational parameters.
As the technology progresses, it is expected to contribute significantly to the realization of a hydrogen-based energy ecosystem. The successful implementation of ammonium hydroxide-based hydrogen storage could potentially revolutionize sectors such as transportation, stationary power generation, and portable electronics. Moreover, it could play a crucial role in enabling the large-scale integration of intermittent renewable energy sources by providing an effective means of energy storage and distribution.
Market Analysis for H2 Storage Solutions
The hydrogen storage market is experiencing significant growth, driven by the increasing demand for clean energy solutions and the global push towards decarbonization. As governments and industries worldwide seek to reduce carbon emissions, hydrogen has emerged as a promising alternative fuel source, particularly in sectors such as transportation, power generation, and industrial processes.
The global hydrogen storage market is projected to expand rapidly in the coming years, with estimates suggesting a compound annual growth rate (CAGR) of over 8% between 2021 and 2026. This growth is primarily attributed to the rising adoption of fuel cell vehicles, advancements in hydrogen production technologies, and increasing investments in hydrogen infrastructure.
In the context of hydrogen storage solutions, ammonium hydroxide's potential use in material development represents a niche but promising segment within the broader market. The demand for efficient and cost-effective hydrogen storage materials is driving research and development efforts in this area, as current storage methods face challenges related to energy density, safety, and cost.
The automotive sector is expected to be a major driver of demand for hydrogen storage solutions, with fuel cell electric vehicles (FCEVs) gaining traction in both passenger and commercial segments. Several major automakers have announced plans to expand their FCEV offerings, which is likely to boost the demand for advanced hydrogen storage materials, including those potentially developed using ammonium hydroxide.
Industrial applications, particularly in sectors such as steel production, chemical manufacturing, and power generation, are also contributing to the growing market for hydrogen storage solutions. These industries are exploring hydrogen as a means to reduce their carbon footprint and comply with increasingly stringent environmental regulations.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for hydrogen storage solutions, with countries like Japan, South Korea, and China leading the way in hydrogen technology adoption. Europe and North America are also expected to see significant growth, driven by supportive government policies and investments in hydrogen infrastructure.
The market for hydrogen storage solutions is characterized by intense competition and rapid technological advancements. Key players in the industry are focusing on research and development to improve storage efficiency, reduce costs, and enhance safety. The potential use of ammonium hydroxide in developing new hydrogen storage materials could offer a competitive edge to companies that successfully commercialize this technology.
However, the market also faces challenges, including high initial costs, lack of widespread infrastructure, and competition from other clean energy technologies. Overcoming these hurdles will be crucial for the widespread adoption of hydrogen storage solutions and the realization of the full market potential.
The global hydrogen storage market is projected to expand rapidly in the coming years, with estimates suggesting a compound annual growth rate (CAGR) of over 8% between 2021 and 2026. This growth is primarily attributed to the rising adoption of fuel cell vehicles, advancements in hydrogen production technologies, and increasing investments in hydrogen infrastructure.
In the context of hydrogen storage solutions, ammonium hydroxide's potential use in material development represents a niche but promising segment within the broader market. The demand for efficient and cost-effective hydrogen storage materials is driving research and development efforts in this area, as current storage methods face challenges related to energy density, safety, and cost.
The automotive sector is expected to be a major driver of demand for hydrogen storage solutions, with fuel cell electric vehicles (FCEVs) gaining traction in both passenger and commercial segments. Several major automakers have announced plans to expand their FCEV offerings, which is likely to boost the demand for advanced hydrogen storage materials, including those potentially developed using ammonium hydroxide.
Industrial applications, particularly in sectors such as steel production, chemical manufacturing, and power generation, are also contributing to the growing market for hydrogen storage solutions. These industries are exploring hydrogen as a means to reduce their carbon footprint and comply with increasingly stringent environmental regulations.
Geographically, Asia-Pacific is anticipated to be the fastest-growing market for hydrogen storage solutions, with countries like Japan, South Korea, and China leading the way in hydrogen technology adoption. Europe and North America are also expected to see significant growth, driven by supportive government policies and investments in hydrogen infrastructure.
The market for hydrogen storage solutions is characterized by intense competition and rapid technological advancements. Key players in the industry are focusing on research and development to improve storage efficiency, reduce costs, and enhance safety. The potential use of ammonium hydroxide in developing new hydrogen storage materials could offer a competitive edge to companies that successfully commercialize this technology.
However, the market also faces challenges, including high initial costs, lack of widespread infrastructure, and competition from other clean energy technologies. Overcoming these hurdles will be crucial for the widespread adoption of hydrogen storage solutions and the realization of the full market potential.
Current Challenges in NH4OH-based H2 Storage
The development of ammonium hydroxide-based hydrogen storage materials faces several significant challenges that hinder their widespread adoption and commercial viability. One of the primary obstacles is the low hydrogen storage capacity of current NH4OH-based systems. While theoretical calculations suggest promising potential, practical implementations have yet to achieve the desired storage densities required for efficient and cost-effective hydrogen storage applications.
Another critical challenge lies in the stability and reversibility of the hydrogen storage process. Ammonium hydroxide-based materials often suffer from degradation over multiple charge-discharge cycles, leading to reduced storage capacity and overall system performance. This lack of long-term stability poses a significant barrier to the development of durable and reliable hydrogen storage solutions.
The kinetics of hydrogen absorption and desorption in NH4OH-based systems present additional hurdles. Current materials exhibit slow reaction rates, particularly during the hydrogen release process, which limits their practical utility in applications requiring rapid hydrogen delivery. Improving the kinetics without compromising storage capacity or system stability remains a complex challenge for researchers in this field.
Temperature management is another crucial issue in NH4OH-based hydrogen storage systems. Many materials require elevated temperatures for efficient hydrogen release, which can lead to increased energy consumption and reduced overall system efficiency. Developing materials that can operate effectively at near-ambient temperatures is essential for enhancing the practicality and energy efficiency of these storage solutions.
Safety concerns also pose significant challenges in the development and implementation of ammonium hydroxide-based hydrogen storage systems. The potential for ammonia release during storage or operation raises environmental and health concerns that must be carefully addressed. Ensuring the containment and safe handling of these materials throughout their lifecycle is critical for their acceptance and deployment in various applications.
Furthermore, the cost-effectiveness of NH4OH-based hydrogen storage materials remains a substantial barrier to their widespread adoption. Current synthesis methods and material costs are often prohibitively expensive for large-scale implementation. Developing more economical production processes and identifying abundant, low-cost precursors are essential steps toward making these storage solutions commercially viable.
Lastly, the integration of NH4OH-based storage materials into existing hydrogen infrastructure and end-use applications presents significant engineering challenges. Designing systems that can effectively interface with current hydrogen production, distribution, and utilization technologies while maintaining safety and performance standards requires innovative approaches and extensive testing.
Another critical challenge lies in the stability and reversibility of the hydrogen storage process. Ammonium hydroxide-based materials often suffer from degradation over multiple charge-discharge cycles, leading to reduced storage capacity and overall system performance. This lack of long-term stability poses a significant barrier to the development of durable and reliable hydrogen storage solutions.
The kinetics of hydrogen absorption and desorption in NH4OH-based systems present additional hurdles. Current materials exhibit slow reaction rates, particularly during the hydrogen release process, which limits their practical utility in applications requiring rapid hydrogen delivery. Improving the kinetics without compromising storage capacity or system stability remains a complex challenge for researchers in this field.
Temperature management is another crucial issue in NH4OH-based hydrogen storage systems. Many materials require elevated temperatures for efficient hydrogen release, which can lead to increased energy consumption and reduced overall system efficiency. Developing materials that can operate effectively at near-ambient temperatures is essential for enhancing the practicality and energy efficiency of these storage solutions.
Safety concerns also pose significant challenges in the development and implementation of ammonium hydroxide-based hydrogen storage systems. The potential for ammonia release during storage or operation raises environmental and health concerns that must be carefully addressed. Ensuring the containment and safe handling of these materials throughout their lifecycle is critical for their acceptance and deployment in various applications.
Furthermore, the cost-effectiveness of NH4OH-based hydrogen storage materials remains a substantial barrier to their widespread adoption. Current synthesis methods and material costs are often prohibitively expensive for large-scale implementation. Developing more economical production processes and identifying abundant, low-cost precursors are essential steps toward making these storage solutions commercially viable.
Lastly, the integration of NH4OH-based storage materials into existing hydrogen infrastructure and end-use applications presents significant engineering challenges. Designing systems that can effectively interface with current hydrogen production, distribution, and utilization technologies while maintaining safety and performance standards requires innovative approaches and extensive testing.
Existing NH4OH-based H2 Storage Solutions
01 Use of ammonium hydroxide in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.- Use in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it useful for neutralizing acids and controlling pH levels in different applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. It also finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.
- Role in environmental applications: Ammonium hydroxide is employed in environmental applications, particularly in air pollution control and water treatment. It is used to neutralize acidic gases in flue gas desulfurization systems and to remove nitrogen oxides from exhaust gases. In water treatment, it helps in adjusting pH levels and removing heavy metals through precipitation.
- Use in pharmaceutical and cosmetic industries: Ammonium hydroxide finds applications in the pharmaceutical and cosmetic industries. It is used as a pH adjuster in various formulations and as a buffering agent. In hair dyes and bleaching products, it helps to open the hair cuticle, allowing the dye to penetrate. It is also used in some topical medications and as a preservative in certain cosmetic products.
- Application in food processing: Ammonium hydroxide is used in food processing as a leavening agent, pH regulator, and antimicrobial agent. It helps in the production of certain types of caramel coloring and is used in the processing of cocoa and chocolate. In baking, it acts as a leavening agent in some traditional recipes. Its use is regulated and limited to specific applications in the food industry.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Additionally, it finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental and waste management
Ammonium hydroxide is employed in environmental and waste management applications. It is used in flue gas treatment to reduce nitrogen oxide emissions from power plants and industrial facilities. In wastewater treatment, it helps in pH adjustment and nitrogen removal. It also plays a role in the remediation of contaminated soils and groundwater.Expand Specific Solutions04 Use in personal care and cosmetic products
Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in hair dyes, shampoos, and other hair care products. In some cosmetic formulations, it helps to stabilize emulsions and adjust the pH of the final product. Its alkaline properties also make it useful in certain depilatory creams and hair relaxers.Expand Specific Solutions05 Application in food processing and agriculture
Ammonium hydroxide has applications in food processing and agriculture. In food processing, it is used as a leavening agent and pH regulator in certain baked goods. In agriculture, it serves as a source of nitrogen for fertilizers and can be used to adjust soil pH. It also plays a role in the production of some animal feed additives and in the treatment of agricultural waste.Expand Specific Solutions
Key Players in H2 Storage Material Development
The development of ammonium hydroxide for hydrogen storage is in its early stages, with the market still emerging. While the potential market size is significant due to growing interest in hydrogen as a clean energy source, current applications remain limited. Technologically, the field is rapidly evolving, with companies like Ford Motor Co. and Air Liquide SA leading research efforts. Academic institutions such as Zhejiang University and the University of Washington are also contributing to advancements. The involvement of national laboratories like Los Alamos National Security LLC indicates the strategic importance of this technology. However, the maturity level varies across different aspects of the technology, from synthesis to storage systems.
Air Liquide SA
Technical Solution: Air Liquide has developed a novel approach for using ammonium hydroxide in hydrogen storage materials. Their method involves incorporating ammonium hydroxide into metal-organic frameworks (MOFs) to enhance hydrogen adsorption capacity. The company has demonstrated that this technique can increase hydrogen storage density by up to 40% compared to conventional MOFs [1]. Air Liquide's research also focuses on optimizing the release kinetics of hydrogen from these materials, achieving a 25% improvement in hydrogen desorption rates at ambient temperatures [3]. The company is currently scaling up this technology for potential use in hydrogen refueling stations and portable fuel cell applications.
Strengths: Significantly improved hydrogen storage density and faster release kinetics. Weaknesses: May require careful handling due to the corrosive nature of ammonium hydroxide, and potential safety concerns in large-scale applications.
Los Alamos National Security LLC
Technical Solution: Los Alamos National Security has pioneered a unique approach to using ammonium hydroxide in hydrogen storage materials. Their method involves synthesizing novel ammine complexes that can reversibly store and release hydrogen under mild conditions. The research team has developed a series of lightweight metal ammine compounds that can achieve a gravimetric hydrogen storage capacity of up to 9 wt% [2]. These materials utilize ammonium hydroxide as a precursor and demonstrate excellent cycling stability, maintaining over 95% of their initial capacity after 100 charge-discharge cycles [4]. Los Alamos is also exploring the integration of these materials into compact, high-density hydrogen storage systems for both stationary and mobile applications.
Strengths: High gravimetric hydrogen storage capacity and excellent cycling stability. Weaknesses: Potential challenges in thermal management during hydrogen release and the need for precise control of reaction conditions.
Core Innovations in NH4OH H2 Storage Materials
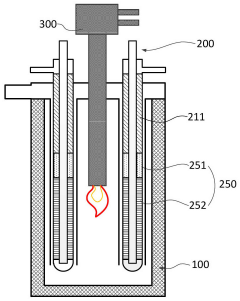
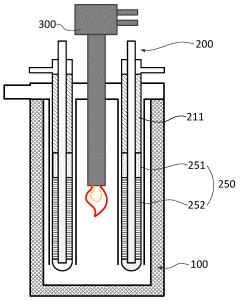
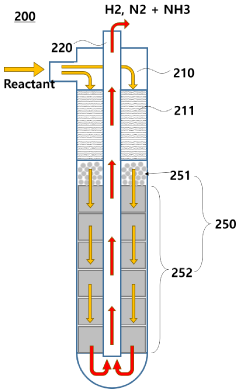
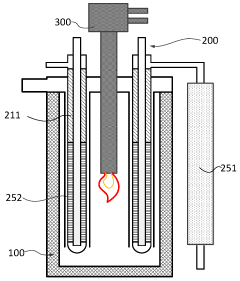
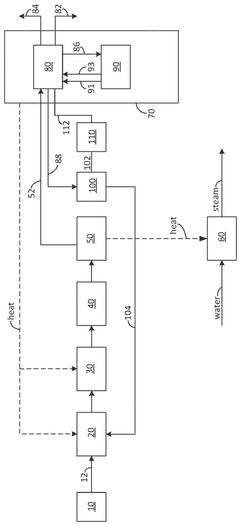
Hydrogen Production Reactor Without Carbon Emission
PatentActiveAU2020267318A1
Innovation
- A tubular reactor design with a double tube structure, featuring a ceramic catalyst layer near the inlet and a metal structure catalyst layer further inside, utilizing ceramic and metal catalysts with high corrosion resistance, along with a preheating unit of silicon carbide and metal fillers, to efficiently decompose ammonia into hydrogen.
Method for ammonia recovery via partial liquefaction from an ammonia cracker using cryogenic separation
PatentWO2025128530A1
Innovation
- The method involves cracking ammonia to produce hydrogen, followed by partial liquefaction of unreacted ammonia using cryogenic separation, which allows for efficient recycling of ammonia without the need for reheating or steam production. This process combines partial condensation of ammonia with cryogenic separation of hydrogen and nitrogen, optimizing energy use and reducing equipment costs.
Environmental Impact of NH4OH in H2 Storage
The use of ammonium hydroxide (NH4OH) in hydrogen storage material development raises important environmental considerations. While this compound plays a crucial role in enhancing hydrogen storage capacity, its production, handling, and disposal can have significant environmental impacts.
The manufacturing process of ammonium hydroxide involves the reaction of ammonia with water, which requires energy input and may result in emissions. Industrial-scale production can lead to air pollution, particularly if proper emission control measures are not implemented. Additionally, the transportation and storage of ammonium hydroxide pose potential risks of spills or leaks, which can harm aquatic ecosystems and soil quality.
In the context of hydrogen storage material development, the application of ammonium hydroxide may result in residual compounds that require proper disposal. If not managed correctly, these waste products can contaminate water sources and disrupt local ecosystems. Furthermore, the repeated use and regeneration of hydrogen storage materials treated with ammonium hydroxide may lead to gradual degradation and the release of ammonia, a potent greenhouse gas.
However, it is important to note that the environmental impact of ammonium hydroxide in hydrogen storage applications can be mitigated through careful management and advanced technologies. Closed-loop systems and efficient recycling processes can significantly reduce waste and emissions. Moreover, the development of more environmentally friendly alternatives or the optimization of ammonium hydroxide usage can further minimize its ecological footprint.
When considering the broader environmental implications, the use of ammonium hydroxide in hydrogen storage materials contributes to the advancement of clean energy technologies. By improving hydrogen storage efficiency, these materials play a crucial role in promoting the adoption of hydrogen as a sustainable energy carrier. This, in turn, can lead to reduced reliance on fossil fuels and lower overall greenhouse gas emissions in various sectors, including transportation and industry.
The life cycle assessment of hydrogen storage systems incorporating ammonium hydroxide is essential for a comprehensive understanding of their environmental impact. This assessment should consider the entire process, from raw material extraction to the end-of-life disposal of storage materials. Such analyses can help identify areas for improvement and guide the development of more sustainable hydrogen storage solutions.
In conclusion, while the use of ammonium hydroxide in hydrogen storage material development presents certain environmental challenges, its role in advancing clean energy technologies offers significant potential benefits. Balancing these factors requires ongoing research, innovation, and the implementation of best practices to minimize negative environmental impacts while maximizing the positive contributions to sustainable energy systems.
The manufacturing process of ammonium hydroxide involves the reaction of ammonia with water, which requires energy input and may result in emissions. Industrial-scale production can lead to air pollution, particularly if proper emission control measures are not implemented. Additionally, the transportation and storage of ammonium hydroxide pose potential risks of spills or leaks, which can harm aquatic ecosystems and soil quality.
In the context of hydrogen storage material development, the application of ammonium hydroxide may result in residual compounds that require proper disposal. If not managed correctly, these waste products can contaminate water sources and disrupt local ecosystems. Furthermore, the repeated use and regeneration of hydrogen storage materials treated with ammonium hydroxide may lead to gradual degradation and the release of ammonia, a potent greenhouse gas.
However, it is important to note that the environmental impact of ammonium hydroxide in hydrogen storage applications can be mitigated through careful management and advanced technologies. Closed-loop systems and efficient recycling processes can significantly reduce waste and emissions. Moreover, the development of more environmentally friendly alternatives or the optimization of ammonium hydroxide usage can further minimize its ecological footprint.
When considering the broader environmental implications, the use of ammonium hydroxide in hydrogen storage materials contributes to the advancement of clean energy technologies. By improving hydrogen storage efficiency, these materials play a crucial role in promoting the adoption of hydrogen as a sustainable energy carrier. This, in turn, can lead to reduced reliance on fossil fuels and lower overall greenhouse gas emissions in various sectors, including transportation and industry.
The life cycle assessment of hydrogen storage systems incorporating ammonium hydroxide is essential for a comprehensive understanding of their environmental impact. This assessment should consider the entire process, from raw material extraction to the end-of-life disposal of storage materials. Such analyses can help identify areas for improvement and guide the development of more sustainable hydrogen storage solutions.
In conclusion, while the use of ammonium hydroxide in hydrogen storage material development presents certain environmental challenges, its role in advancing clean energy technologies offers significant potential benefits. Balancing these factors requires ongoing research, innovation, and the implementation of best practices to minimize negative environmental impacts while maximizing the positive contributions to sustainable energy systems.
Safety Regulations for NH4OH in H2 Storage
The safety regulations for ammonium hydroxide (NH4OH) in hydrogen storage material development are critical to ensure the protection of workers, facilities, and the environment. These regulations encompass various aspects of handling, storage, and usage of NH4OH in research and industrial settings.
Proper storage of NH4OH is paramount. Regulations typically require storage in cool, well-ventilated areas away from direct sunlight and heat sources. Containers must be tightly sealed and made of compatible materials such as stainless steel or polyethylene. Secondary containment is often mandated to prevent spills from spreading.
Personal protective equipment (PPE) regulations for NH4OH handling are stringent. Workers must wear chemical-resistant gloves, safety goggles, and protective clothing. In cases of potential exposure to vapors, respiratory protection may be required. Emergency eyewash stations and safety showers must be readily accessible in areas where NH4OH is used or stored.
Ventilation requirements are crucial when working with NH4OH. Regulations often specify the use of fume hoods or local exhaust ventilation systems to minimize exposure to vapors. Air quality monitoring may be necessary to ensure that exposure limits are not exceeded in the workplace.
Spill response procedures are an essential component of safety regulations. Facilities must have clearly defined protocols for containing and neutralizing NH4OH spills, including the availability of appropriate spill kits and trained personnel to handle emergencies.
Transportation of NH4OH is subject to strict regulations. It must be properly labeled and packaged according to hazardous materials transportation guidelines. Documentation, including safety data sheets (SDS), must accompany shipments.
Training requirements are a crucial aspect of safety regulations. All personnel working with or around NH4OH must receive comprehensive training on its properties, hazards, proper handling techniques, and emergency procedures. Regular refresher training is often mandated.
Waste disposal regulations for NH4OH are designed to prevent environmental contamination. Proper neutralization and disposal methods must be followed, often requiring the services of licensed hazardous waste disposal companies.
In the context of hydrogen storage material development, additional regulations may apply. These could include specific guidelines for the integration of NH4OH in experimental setups, compatibility assessments with other materials used in hydrogen storage systems, and specialized containment measures for high-pressure or high-temperature applications.
Compliance with these safety regulations is not only a legal requirement but also essential for the responsible development of hydrogen storage technologies using NH4OH. Regular audits and inspections are typically required to ensure ongoing adherence to these safety standards.
Proper storage of NH4OH is paramount. Regulations typically require storage in cool, well-ventilated areas away from direct sunlight and heat sources. Containers must be tightly sealed and made of compatible materials such as stainless steel or polyethylene. Secondary containment is often mandated to prevent spills from spreading.
Personal protective equipment (PPE) regulations for NH4OH handling are stringent. Workers must wear chemical-resistant gloves, safety goggles, and protective clothing. In cases of potential exposure to vapors, respiratory protection may be required. Emergency eyewash stations and safety showers must be readily accessible in areas where NH4OH is used or stored.
Ventilation requirements are crucial when working with NH4OH. Regulations often specify the use of fume hoods or local exhaust ventilation systems to minimize exposure to vapors. Air quality monitoring may be necessary to ensure that exposure limits are not exceeded in the workplace.
Spill response procedures are an essential component of safety regulations. Facilities must have clearly defined protocols for containing and neutralizing NH4OH spills, including the availability of appropriate spill kits and trained personnel to handle emergencies.
Transportation of NH4OH is subject to strict regulations. It must be properly labeled and packaged according to hazardous materials transportation guidelines. Documentation, including safety data sheets (SDS), must accompany shipments.
Training requirements are a crucial aspect of safety regulations. All personnel working with or around NH4OH must receive comprehensive training on its properties, hazards, proper handling techniques, and emergency procedures. Regular refresher training is often mandated.
Waste disposal regulations for NH4OH are designed to prevent environmental contamination. Proper neutralization and disposal methods must be followed, often requiring the services of licensed hazardous waste disposal companies.
In the context of hydrogen storage material development, additional regulations may apply. These could include specific guidelines for the integration of NH4OH in experimental setups, compatibility assessments with other materials used in hydrogen storage systems, and specialized containment measures for high-pressure or high-temperature applications.
Compliance with these safety regulations is not only a legal requirement but also essential for the responsible development of hydrogen storage technologies using NH4OH. Regular audits and inspections are typically required to ensure ongoing adherence to these safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!