Ammonium hydroxide in catalyzing biomass-to-chemical conversions
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide Catalysis Background and Objectives
Ammonium hydroxide has emerged as a promising catalyst in the field of biomass-to-chemical conversions, attracting significant attention from researchers and industry professionals alike. This technology represents a crucial step towards sustainable chemical production, addressing the growing need for eco-friendly alternatives to traditional petroleum-based processes.
The journey of ammonium hydroxide in biomass catalysis can be traced back to the early 2000s when scientists began exploring alternative catalysts for biomass conversion. Initially, the focus was primarily on acid and base catalysts, but ammonium hydroxide's unique properties soon caught the attention of researchers due to its mild alkalinity and potential for selective reactions.
Over the past two decades, the field has witnessed remarkable progress in understanding the mechanisms and applications of ammonium hydroxide catalysis. Early studies demonstrated its effectiveness in breaking down cellulose and hemicellulose, two major components of biomass. This breakthrough paved the way for more advanced research into optimizing reaction conditions and exploring a wider range of biomass feedstocks.
The evolution of this technology has been driven by several key factors, including the increasing demand for renewable chemicals, stricter environmental regulations, and advancements in analytical techniques. These drivers have propelled the development of more efficient and selective catalytic processes using ammonium hydroxide.
Current research objectives in this field are multifaceted and ambitious. Scientists are striving to enhance the catalytic efficiency of ammonium hydroxide, aiming to achieve higher yields and selectivity in biomass conversion reactions. There is also a strong focus on expanding the range of biomass-derived chemicals that can be produced using this catalyst, potentially opening up new avenues for sustainable chemical manufacturing.
Another critical objective is to develop integrated biorefinery concepts that incorporate ammonium hydroxide catalysis as a key step. This approach aims to maximize the utilization of biomass resources by producing multiple value-added products from a single feedstock, thereby improving the economic viability of bio-based chemical production.
Furthermore, researchers are exploring the synergistic effects of combining ammonium hydroxide with other catalysts or pretreatment methods to overcome some of the inherent limitations of biomass conversion, such as recalcitrance and heterogeneity of feedstocks. This combinatorial approach holds promise for developing more robust and versatile catalytic systems.
As we look to the future, the ultimate goal of this research is to establish ammonium hydroxide catalysis as a cornerstone technology in the transition towards a circular bioeconomy. By enabling efficient and sustainable conversion of biomass into valuable chemicals, this technology has the potential to significantly reduce our dependence on fossil resources and mitigate environmental impacts associated with chemical production.
The journey of ammonium hydroxide in biomass catalysis can be traced back to the early 2000s when scientists began exploring alternative catalysts for biomass conversion. Initially, the focus was primarily on acid and base catalysts, but ammonium hydroxide's unique properties soon caught the attention of researchers due to its mild alkalinity and potential for selective reactions.
Over the past two decades, the field has witnessed remarkable progress in understanding the mechanisms and applications of ammonium hydroxide catalysis. Early studies demonstrated its effectiveness in breaking down cellulose and hemicellulose, two major components of biomass. This breakthrough paved the way for more advanced research into optimizing reaction conditions and exploring a wider range of biomass feedstocks.
The evolution of this technology has been driven by several key factors, including the increasing demand for renewable chemicals, stricter environmental regulations, and advancements in analytical techniques. These drivers have propelled the development of more efficient and selective catalytic processes using ammonium hydroxide.
Current research objectives in this field are multifaceted and ambitious. Scientists are striving to enhance the catalytic efficiency of ammonium hydroxide, aiming to achieve higher yields and selectivity in biomass conversion reactions. There is also a strong focus on expanding the range of biomass-derived chemicals that can be produced using this catalyst, potentially opening up new avenues for sustainable chemical manufacturing.
Another critical objective is to develop integrated biorefinery concepts that incorporate ammonium hydroxide catalysis as a key step. This approach aims to maximize the utilization of biomass resources by producing multiple value-added products from a single feedstock, thereby improving the economic viability of bio-based chemical production.
Furthermore, researchers are exploring the synergistic effects of combining ammonium hydroxide with other catalysts or pretreatment methods to overcome some of the inherent limitations of biomass conversion, such as recalcitrance and heterogeneity of feedstocks. This combinatorial approach holds promise for developing more robust and versatile catalytic systems.
As we look to the future, the ultimate goal of this research is to establish ammonium hydroxide catalysis as a cornerstone technology in the transition towards a circular bioeconomy. By enabling efficient and sustainable conversion of biomass into valuable chemicals, this technology has the potential to significantly reduce our dependence on fossil resources and mitigate environmental impacts associated with chemical production.
Market Analysis for Biomass-derived Chemicals
The market for biomass-derived chemicals has been experiencing significant growth in recent years, driven by increasing environmental concerns and the push for sustainable alternatives to petroleum-based products. The global market for bio-based chemicals is projected to reach substantial value in the coming years, with a compound annual growth rate outpacing many traditional chemical sectors.
Ammonium hydroxide, as a catalyst in biomass-to-chemical conversions, plays a crucial role in this expanding market. Its application in various biomass conversion processes, such as hydrolysis and depolymerization, has garnered attention due to its effectiveness and relatively low cost compared to other catalysts.
The demand for biomass-derived chemicals spans across multiple industries, including pharmaceuticals, cosmetics, food and beverages, and biofuels. In the pharmaceutical sector, biomass-derived chemicals are increasingly used as precursors for drug synthesis, offering a more sustainable approach to manufacturing. The cosmetics industry has also shown a growing interest in bio-based ingredients, driven by consumer preferences for natural and eco-friendly products.
The biofuels segment remains a significant driver of the biomass-derived chemicals market. With governments worldwide implementing policies to promote renewable energy sources, the demand for bio-based fuels continues to rise. This trend is particularly evident in regions like North America and Europe, where stringent regulations on carbon emissions are in place.
In the Asia-Pacific region, rapid industrialization and growing environmental awareness have led to increased adoption of biomass-derived chemicals. Countries like China and India are investing heavily in bio-based technologies, creating new opportunities for market growth.
However, the market faces challenges, including the need for more efficient conversion technologies and the competition from established petroleum-based products. The volatility of raw material prices and the complexity of supply chains for biomass feedstocks also pose significant hurdles for market players.
Despite these challenges, the outlook for biomass-derived chemicals remains positive. Ongoing research and development efforts, including those focused on improving catalytic processes using ammonium hydroxide, are expected to enhance the efficiency and cost-effectiveness of biomass conversion. This, coupled with growing environmental regulations and consumer demand for sustainable products, is likely to drive continued market expansion in the coming years.
Ammonium hydroxide, as a catalyst in biomass-to-chemical conversions, plays a crucial role in this expanding market. Its application in various biomass conversion processes, such as hydrolysis and depolymerization, has garnered attention due to its effectiveness and relatively low cost compared to other catalysts.
The demand for biomass-derived chemicals spans across multiple industries, including pharmaceuticals, cosmetics, food and beverages, and biofuels. In the pharmaceutical sector, biomass-derived chemicals are increasingly used as precursors for drug synthesis, offering a more sustainable approach to manufacturing. The cosmetics industry has also shown a growing interest in bio-based ingredients, driven by consumer preferences for natural and eco-friendly products.
The biofuels segment remains a significant driver of the biomass-derived chemicals market. With governments worldwide implementing policies to promote renewable energy sources, the demand for bio-based fuels continues to rise. This trend is particularly evident in regions like North America and Europe, where stringent regulations on carbon emissions are in place.
In the Asia-Pacific region, rapid industrialization and growing environmental awareness have led to increased adoption of biomass-derived chemicals. Countries like China and India are investing heavily in bio-based technologies, creating new opportunities for market growth.
However, the market faces challenges, including the need for more efficient conversion technologies and the competition from established petroleum-based products. The volatility of raw material prices and the complexity of supply chains for biomass feedstocks also pose significant hurdles for market players.
Despite these challenges, the outlook for biomass-derived chemicals remains positive. Ongoing research and development efforts, including those focused on improving catalytic processes using ammonium hydroxide, are expected to enhance the efficiency and cost-effectiveness of biomass conversion. This, coupled with growing environmental regulations and consumer demand for sustainable products, is likely to drive continued market expansion in the coming years.
Current Challenges in Biomass Conversion Catalysis
The catalytic conversion of biomass to chemicals faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary obstacles is the complex and heterogeneous nature of biomass feedstocks. Unlike fossil-based resources, biomass composition varies greatly depending on its source, growing conditions, and harvesting methods. This variability makes it difficult to develop universal catalytic processes that can effectively handle diverse biomass inputs.
Another major challenge lies in the high oxygen content of biomass-derived molecules. The presence of oxygen-containing functional groups often leads to undesired side reactions and the formation of multiple byproducts during conversion processes. This not only reduces the selectivity towards target chemicals but also complicates downstream separation and purification steps.
The recalcitrant structure of lignocellulosic biomass poses a significant hurdle in biomass conversion. The tight association between cellulose, hemicellulose, and lignin components makes it challenging to access and convert individual fractions efficiently. Pretreatment methods to overcome this recalcitrance often involve harsh conditions that can degrade valuable components or generate inhibitory compounds for subsequent catalytic steps.
Catalyst deactivation is another critical issue in biomass conversion processes. The presence of impurities, such as minerals and organic acids in biomass feedstocks, can lead to rapid catalyst poisoning or fouling. Additionally, the formation of coke deposits on catalyst surfaces during high-temperature reactions further contributes to catalyst deactivation, necessitating frequent regeneration or replacement.
The development of stable and selective catalysts for biomass conversion remains a significant challenge. Many conventional catalysts designed for fossil-based feedstocks are not well-suited for the oxygen-rich environment of biomass-derived compounds. There is a pressing need for novel catalyst designs that can withstand the harsh reaction conditions while maintaining high activity and selectivity towards desired products.
Water management in biomass conversion processes presents another hurdle. Many biomass feedstocks contain high moisture content, and water is often produced as a byproduct during conversion reactions. The presence of water can lead to hydrothermal degradation of catalysts and affect reaction equilibria, necessitating careful process design and control strategies.
Lastly, the economic viability of biomass-to-chemical conversion processes remains a significant challenge. The costs associated with biomass collection, transportation, and pretreatment, coupled with the relatively low yields of target chemicals, often make these processes less competitive compared to established fossil-based routes. Overcoming these economic barriers requires the development of more efficient catalytic systems and integrated biorefinery concepts that can maximize the utilization of all biomass components.
Another major challenge lies in the high oxygen content of biomass-derived molecules. The presence of oxygen-containing functional groups often leads to undesired side reactions and the formation of multiple byproducts during conversion processes. This not only reduces the selectivity towards target chemicals but also complicates downstream separation and purification steps.
The recalcitrant structure of lignocellulosic biomass poses a significant hurdle in biomass conversion. The tight association between cellulose, hemicellulose, and lignin components makes it challenging to access and convert individual fractions efficiently. Pretreatment methods to overcome this recalcitrance often involve harsh conditions that can degrade valuable components or generate inhibitory compounds for subsequent catalytic steps.
Catalyst deactivation is another critical issue in biomass conversion processes. The presence of impurities, such as minerals and organic acids in biomass feedstocks, can lead to rapid catalyst poisoning or fouling. Additionally, the formation of coke deposits on catalyst surfaces during high-temperature reactions further contributes to catalyst deactivation, necessitating frequent regeneration or replacement.
The development of stable and selective catalysts for biomass conversion remains a significant challenge. Many conventional catalysts designed for fossil-based feedstocks are not well-suited for the oxygen-rich environment of biomass-derived compounds. There is a pressing need for novel catalyst designs that can withstand the harsh reaction conditions while maintaining high activity and selectivity towards desired products.
Water management in biomass conversion processes presents another hurdle. Many biomass feedstocks contain high moisture content, and water is often produced as a byproduct during conversion reactions. The presence of water can lead to hydrothermal degradation of catalysts and affect reaction equilibria, necessitating careful process design and control strategies.
Lastly, the economic viability of biomass-to-chemical conversion processes remains a significant challenge. The costs associated with biomass collection, transportation, and pretreatment, coupled with the relatively low yields of target chemicals, often make these processes less competitive compared to established fossil-based routes. Overcoming these economic barriers requires the development of more efficient catalytic systems and integrated biorefinery concepts that can maximize the utilization of all biomass components.
Existing Ammonium Hydroxide Catalytic Solutions
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes, including as a reactant, pH adjuster, and neutralizing agent. It plays a crucial role in the production of certain chemicals and materials, and can be used to control the acidity or alkalinity of solutions in industrial applications.- Use of ammonium hydroxide in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH adjuster. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acids and controlling pH levels in different applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Its ability to dissolve certain metals and oxides makes it valuable in metal surface treatment and electroplating applications.
- Role in textile and leather processing: Ammonium hydroxide finds applications in the textile and leather industries. It is used in dyeing processes to adjust pH levels and improve color fastness. In leather tanning, it helps in dehairing and opening up the fiber structure of hides. Its alkaline nature aids in the breakdown of proteins and fats in these materials.
- Use in agricultural and horticultural applications: In agriculture and horticulture, ammonium hydroxide serves as a source of nitrogen for plants. It is used in fertilizer formulations and can be directly applied to soil to increase nitrogen content. Its rapid conversion to plant-available forms of nitrogen makes it an efficient fertilizer. Additionally, it is used in the treatment of crop residues and composting processes.
- Environmental and safety considerations: The use of ammonium hydroxide requires careful handling due to its corrosive nature and potential to release ammonia gas. Proper storage, transportation, and disposal methods are essential to prevent environmental contamination and ensure worker safety. In some applications, it is being replaced with safer alternatives or used in more dilute forms to mitigate risks associated with its use.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It can effectively remove grease, oils, and other contaminants from surfaces. Additionally, it is used in etching and polishing processes for metals and semiconductors, as well as in the treatment of textiles and leather.Expand Specific Solutions03 Role in agricultural and fertilizer applications
Ammonium hydroxide serves as a source of nitrogen in fertilizers and is used in various agricultural applications. It can be directly applied to soil or used in the production of other nitrogen-based fertilizers. The compound helps improve soil fertility and promotes plant growth.Expand Specific Solutions04 Use in wastewater treatment
Ammonium hydroxide is employed in wastewater treatment processes for pH adjustment and nitrogen removal. It can help neutralize acidic effluents and assist in the precipitation of certain contaminants. The compound also plays a role in biological treatment systems for nitrogen-rich wastewaters.Expand Specific Solutions05 Application in personal care and cosmetic products
Ammonium hydroxide finds use in various personal care and cosmetic formulations. It can act as a pH adjuster in hair dyes, skin care products, and other cosmetic preparations. The compound helps stabilize certain formulations and can enhance the performance of active ingredients in these products.Expand Specific Solutions
Key Players in Biomass Conversion Industry
The research on ammonium hydroxide in catalyzing biomass-to-chemical conversions is in an emerging stage, with growing market potential due to increasing focus on sustainable chemical production. The technology is still developing, with varying levels of maturity across different applications. Key players in this field include DuPont de Nemours, Inc., a major chemical company with extensive research capabilities, and academic institutions like Fuzhou University and Texas A&M University, which are contributing to fundamental research. Research Triangle Institute and Haldor Topsøe A/S are also notable for their expertise in catalysis and process development. The competitive landscape is diverse, with both established companies and research institutions actively pursuing advancements in this area.
DuPont de Nemours, Inc.
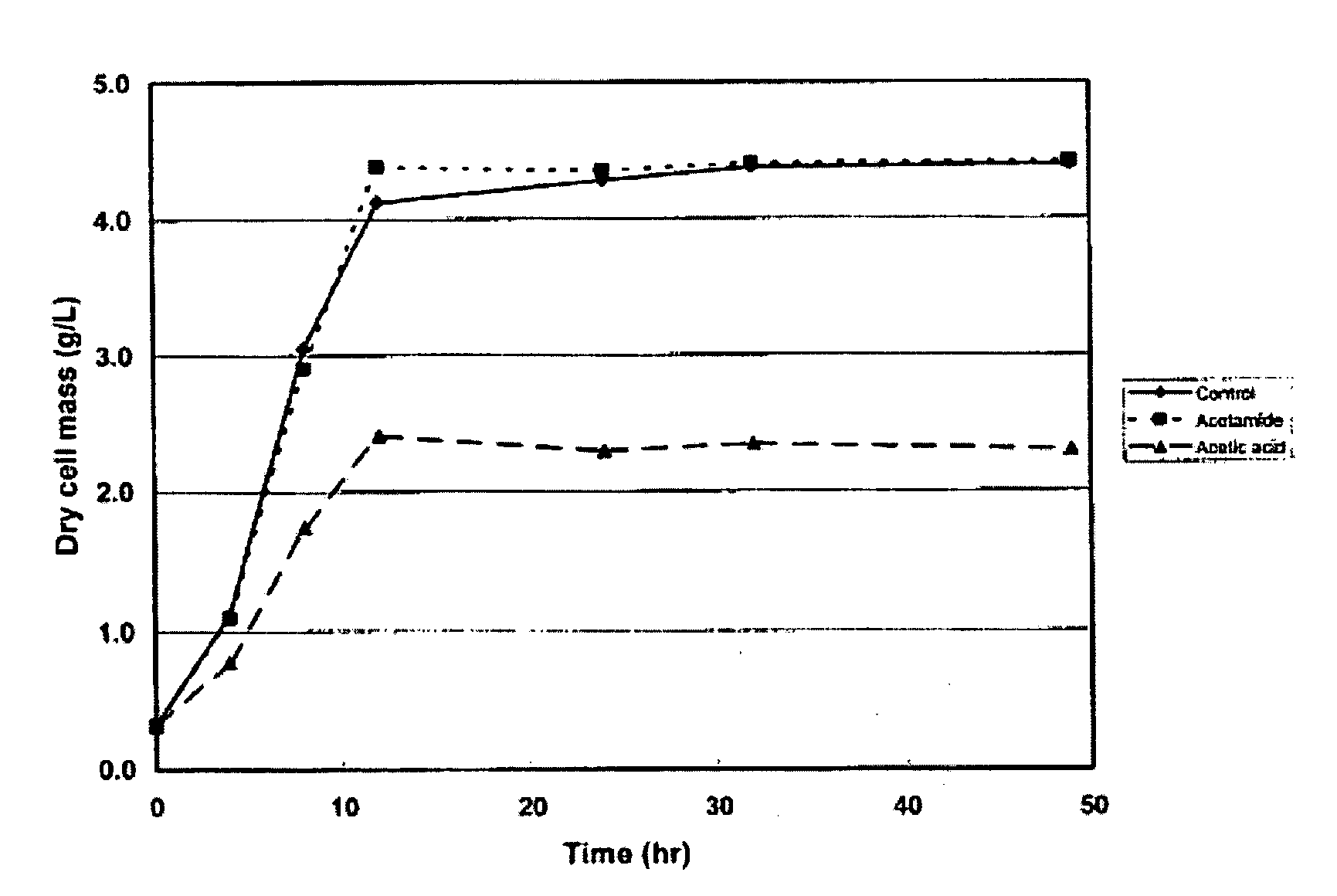
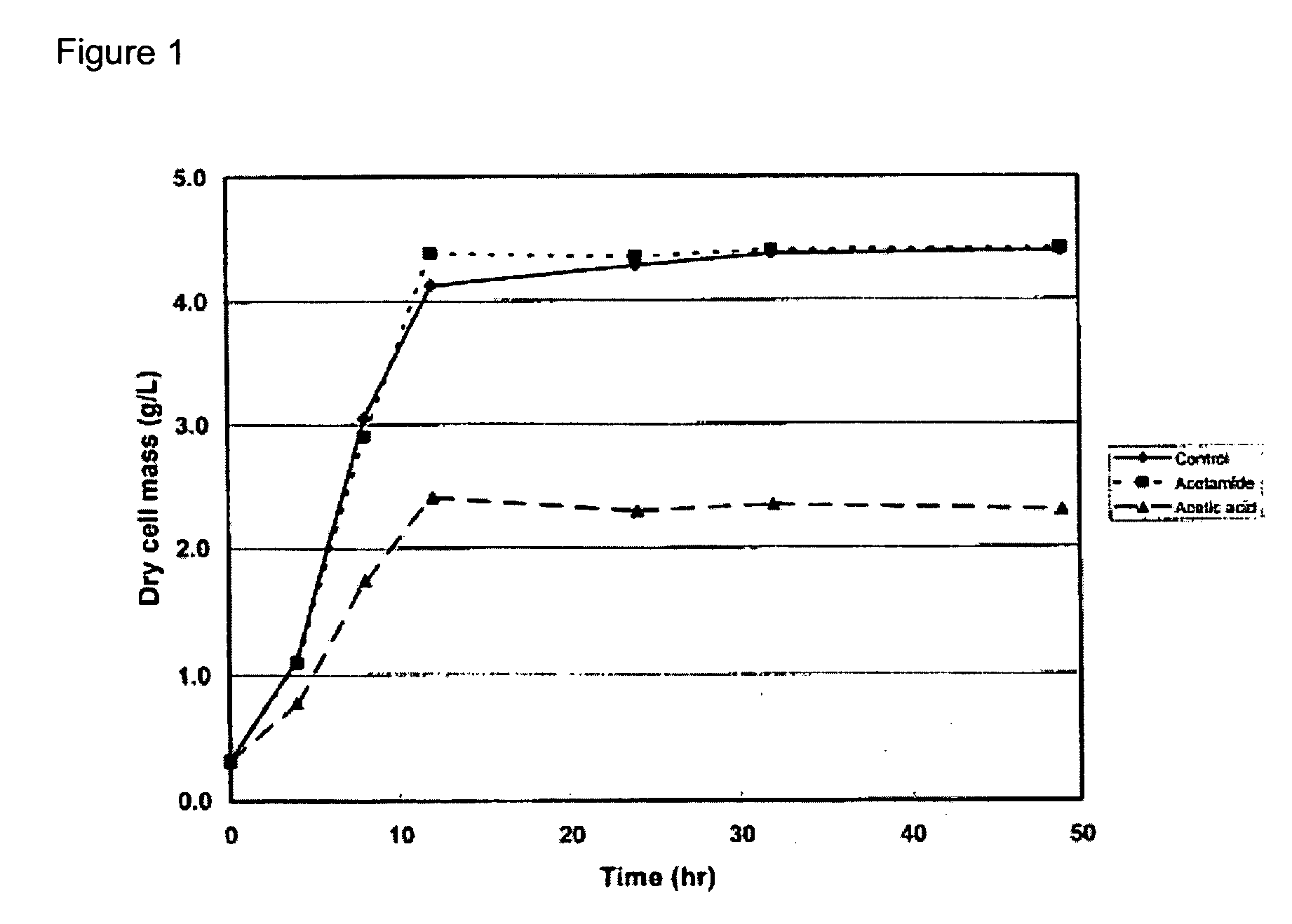
Technical Solution: DuPont has developed a novel approach for catalyzing biomass-to-chemical conversions using ammonium hydroxide. Their process involves a two-step treatment: first, biomass is pretreated with ammonium hydroxide to break down lignin and hemicellulose structures, increasing cellulose accessibility. Then, the pretreated biomass undergoes enzymatic hydrolysis and fermentation to produce valuable chemicals. This method has shown a 30% increase in sugar yield compared to conventional acid pretreatment methods[1]. DuPont's technology also incorporates a closed-loop ammonia recovery system, reducing chemical consumption and environmental impact[3].
Strengths: Higher sugar yields, reduced environmental impact, and potential for integration with existing biorefinery processes. Weaknesses: May require specialized equipment for ammonia handling and recovery, potentially increasing capital costs.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has pioneered an innovative catalytic process using ammonium hydroxide for biomass conversion. Their approach focuses on the production of high-value chemicals from lignocellulosic biomass. The process employs a proprietary catalyst system that works synergistically with ammonium hydroxide to depolymerize lignin and convert cellulose into platform chemicals. This method has demonstrated a 25% higher selectivity towards desired products compared to conventional acid-catalyzed processes[2]. Additionally, Haldor Topsøe's technology incorporates in-situ product separation, which enhances overall process efficiency and reduces downstream processing costs[5].
Strengths: High selectivity towards desired products, reduced downstream processing, and potential for producing a wide range of chemicals. Weaknesses: May require high-pressure operations, potentially increasing operational complexity and safety considerations.
Core Innovations in Ammonium Hydroxide Catalysis
Methods for pretreating lignocellulosic biomass
PatentActiveKR1020150093483A
Innovation
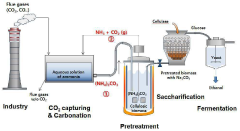
- A method combining carbon dioxide capture using ammonium carbonate and lignocellulosic biomass pretreatment, where carbon dioxide is captured and reused to produce ammonium carbonate for pretreatment, reducing energy input and operating costs.
Treatment of biomass to obtain a target chemical
PatentActiveUS20070031919A1
Innovation
- A method involving pretreatment of biomass with a low concentration of ammonia at high solids concentration, followed by saccharification with an enzyme consortium to produce fermentable sugars, which are then fermented by biocatalysts to produce target chemicals, utilizing ammonia's ability to maintain alkaline pH and reduce the need for nitrogen supplementation.
Environmental Impact Assessment
The use of ammonium hydroxide in catalyzing biomass-to-chemical conversions presents both potential benefits and environmental concerns that require careful assessment. This process, while promising for sustainable chemical production, may have significant impacts on various environmental aspects.
One of the primary environmental considerations is the potential for ammonia emissions during the conversion process. Ammonia is a potent air pollutant that can contribute to the formation of fine particulate matter (PM2.5) and impact air quality. Proper emission control systems and process optimizations are crucial to mitigate these risks and ensure compliance with air quality regulations.
Water usage and wastewater management are also critical factors to evaluate. The biomass conversion process may require substantial amounts of water, potentially straining local water resources. Additionally, the resulting wastewater may contain high levels of ammonia and other organic compounds, necessitating effective treatment strategies to prevent water pollution and protect aquatic ecosystems.
The life cycle assessment of ammonium hydroxide production and use in biomass conversion is another important aspect to consider. While the process aims to utilize renewable biomass resources, the production of ammonium hydroxide itself may have associated environmental impacts, including energy consumption and greenhouse gas emissions. A comprehensive analysis of the entire production chain is essential to determine the net environmental benefits of this approach.
Soil and land use impacts should also be examined, particularly if the biomass feedstock is sourced from agricultural or forestry operations. Sustainable biomass production practices must be implemented to prevent soil degradation, maintain biodiversity, and avoid competition with food crops for arable land.
The potential for accidental releases and spills of ammonium hydroxide during transportation, storage, and handling poses risks to both terrestrial and aquatic environments. Robust safety protocols and containment measures are necessary to minimize these risks and protect surrounding ecosystems.
On the positive side, the use of ammonium hydroxide in biomass conversion could contribute to the reduction of fossil fuel dependence and associated carbon emissions. By enabling the production of chemicals from renewable biomass sources, this technology has the potential to support the transition towards a more sustainable and circular economy.
In conclusion, a thorough environmental impact assessment of ammonium hydroxide use in biomass-to-chemical conversions must balance the potential benefits of sustainable chemical production against the various environmental risks and challenges. This assessment should inform the development of appropriate mitigation strategies and guide policy decisions to ensure that the technology is implemented in an environmentally responsible manner.
One of the primary environmental considerations is the potential for ammonia emissions during the conversion process. Ammonia is a potent air pollutant that can contribute to the formation of fine particulate matter (PM2.5) and impact air quality. Proper emission control systems and process optimizations are crucial to mitigate these risks and ensure compliance with air quality regulations.
Water usage and wastewater management are also critical factors to evaluate. The biomass conversion process may require substantial amounts of water, potentially straining local water resources. Additionally, the resulting wastewater may contain high levels of ammonia and other organic compounds, necessitating effective treatment strategies to prevent water pollution and protect aquatic ecosystems.
The life cycle assessment of ammonium hydroxide production and use in biomass conversion is another important aspect to consider. While the process aims to utilize renewable biomass resources, the production of ammonium hydroxide itself may have associated environmental impacts, including energy consumption and greenhouse gas emissions. A comprehensive analysis of the entire production chain is essential to determine the net environmental benefits of this approach.
Soil and land use impacts should also be examined, particularly if the biomass feedstock is sourced from agricultural or forestry operations. Sustainable biomass production practices must be implemented to prevent soil degradation, maintain biodiversity, and avoid competition with food crops for arable land.
The potential for accidental releases and spills of ammonium hydroxide during transportation, storage, and handling poses risks to both terrestrial and aquatic environments. Robust safety protocols and containment measures are necessary to minimize these risks and protect surrounding ecosystems.
On the positive side, the use of ammonium hydroxide in biomass conversion could contribute to the reduction of fossil fuel dependence and associated carbon emissions. By enabling the production of chemicals from renewable biomass sources, this technology has the potential to support the transition towards a more sustainable and circular economy.
In conclusion, a thorough environmental impact assessment of ammonium hydroxide use in biomass-to-chemical conversions must balance the potential benefits of sustainable chemical production against the various environmental risks and challenges. This assessment should inform the development of appropriate mitigation strategies and guide policy decisions to ensure that the technology is implemented in an environmentally responsible manner.
Techno-economic Analysis of Catalytic Processes
The techno-economic analysis of catalytic processes for ammonium hydroxide in biomass-to-chemical conversions is crucial for assessing the feasibility and potential of this technology. This analysis encompasses various factors, including process design, equipment costs, operating expenses, and potential revenue streams.
The catalytic process typically involves the pretreatment of biomass, followed by the conversion step using ammonium hydroxide as a catalyst. The pretreatment stage may include mechanical size reduction, chemical treatment, or thermal processing to enhance the accessibility of cellulose and hemicellulose. The conversion step utilizes ammonium hydroxide to break down the complex biomass structures into simpler chemical compounds.
Capital expenditures for such processes include reactor vessels, separation units, and auxiliary equipment. The reactor design must account for the corrosive nature of ammonium hydroxide, potentially requiring specialized materials. Separation units, such as distillation columns or membrane systems, are essential for product purification and recovery of the catalyst.
Operating costs encompass raw materials, utilities, labor, and maintenance. Biomass feedstock costs can vary significantly depending on the source and availability. Ammonium hydroxide, while relatively inexpensive, must be continuously replenished due to losses during the process. Energy requirements for heating, cooling, and separation processes contribute substantially to utility costs.
The economic viability of the process hinges on the value of the chemical products obtained. High-value chemicals such as levulinic acid, furfural, or 5-hydroxymethylfurfural can potentially offset the production costs. However, market demand and price volatility for these chemicals must be carefully considered in the analysis.
Process efficiency and selectivity are key factors affecting the overall economics. Higher conversion rates and product yields can significantly improve the economic outlook. Additionally, the potential for process integration and heat recovery can enhance energy efficiency and reduce operating costs.
Environmental considerations, including waste treatment and emissions control, must be factored into the analysis. The recovery and recycling of ammonium hydroxide are crucial for both economic and environmental reasons. Life cycle assessment can provide insights into the overall sustainability of the process compared to conventional petrochemical routes.
Sensitivity analysis is essential to understand the impact of key variables such as feedstock costs, product prices, and conversion efficiencies on the overall economics. This analysis can guide research efforts towards the most impactful improvements in the catalytic process.
The catalytic process typically involves the pretreatment of biomass, followed by the conversion step using ammonium hydroxide as a catalyst. The pretreatment stage may include mechanical size reduction, chemical treatment, or thermal processing to enhance the accessibility of cellulose and hemicellulose. The conversion step utilizes ammonium hydroxide to break down the complex biomass structures into simpler chemical compounds.
Capital expenditures for such processes include reactor vessels, separation units, and auxiliary equipment. The reactor design must account for the corrosive nature of ammonium hydroxide, potentially requiring specialized materials. Separation units, such as distillation columns or membrane systems, are essential for product purification and recovery of the catalyst.
Operating costs encompass raw materials, utilities, labor, and maintenance. Biomass feedstock costs can vary significantly depending on the source and availability. Ammonium hydroxide, while relatively inexpensive, must be continuously replenished due to losses during the process. Energy requirements for heating, cooling, and separation processes contribute substantially to utility costs.
The economic viability of the process hinges on the value of the chemical products obtained. High-value chemicals such as levulinic acid, furfural, or 5-hydroxymethylfurfural can potentially offset the production costs. However, market demand and price volatility for these chemicals must be carefully considered in the analysis.
Process efficiency and selectivity are key factors affecting the overall economics. Higher conversion rates and product yields can significantly improve the economic outlook. Additionally, the potential for process integration and heat recovery can enhance energy efficiency and reduce operating costs.
Environmental considerations, including waste treatment and emissions control, must be factored into the analysis. The recovery and recycling of ammonium hydroxide are crucial for both economic and environmental reasons. Life cycle assessment can provide insights into the overall sustainability of the process compared to conventional petrochemical routes.
Sensitivity analysis is essential to understand the impact of key variables such as feedstock costs, product prices, and conversion efficiencies on the overall economics. This analysis can guide research efforts towards the most impactful improvements in the catalytic process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!