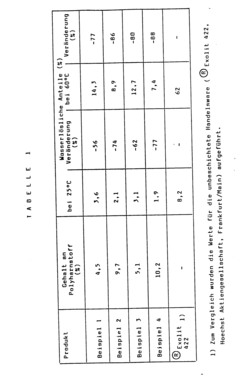
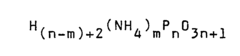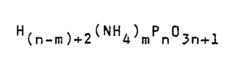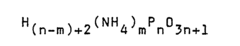Ammonium hydroxide as a flame retardant agent
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide FR Background and Objectives
Ammonium hydroxide, a compound of nitrogen and hydrogen with the chemical formula NH4OH, has gained significant attention in recent years as a potential flame retardant agent. The development of effective and environmentally friendly flame retardants has become increasingly important due to growing concerns about fire safety and the environmental impact of traditional flame retardant materials.
The use of flame retardants dates back to ancient times, with early civilizations using alum and vinegar to reduce the flammability of wood. However, the modern era of flame retardants began in the 1970s with the introduction of halogenated compounds. These materials, while effective, have faced scrutiny due to their persistence in the environment and potential health risks.
Ammonium hydroxide represents a new direction in flame retardant technology, offering a potentially safer and more sustainable alternative to traditional options. Its flame retardant properties are attributed to its ability to release ammonia gas when exposed to heat, which can dilute flammable gases and potentially inhibit the combustion process.
The primary objective of research into ammonium hydroxide as a flame retardant agent is to develop a comprehensive understanding of its effectiveness, mechanisms of action, and potential applications across various materials and industries. This includes investigating its performance in different polymer matrices, textiles, and wood products, as well as exploring optimal formulations and application methods.
Another crucial goal is to assess the environmental and health impacts of ammonium hydroxide-based flame retardants. This involves studying their toxicity, biodegradability, and long-term effects on ecosystems. Researchers aim to ensure that this new flame retardant solution addresses the shortcomings of its predecessors while maintaining or improving upon their fire-retardant efficacy.
The development of ammonium hydroxide as a flame retardant also aligns with broader industry trends towards sustainable and bio-based materials. As regulations become more stringent and consumer awareness grows, there is an increasing demand for eco-friendly flame retardant solutions that can meet both safety standards and environmental requirements.
Furthermore, researchers are exploring the potential synergistic effects of combining ammonium hydroxide with other flame retardant compounds or nanotechnologies. This approach could lead to the development of more efficient and versatile flame retardant systems, capable of addressing a wider range of fire scenarios and material applications.
The use of flame retardants dates back to ancient times, with early civilizations using alum and vinegar to reduce the flammability of wood. However, the modern era of flame retardants began in the 1970s with the introduction of halogenated compounds. These materials, while effective, have faced scrutiny due to their persistence in the environment and potential health risks.
Ammonium hydroxide represents a new direction in flame retardant technology, offering a potentially safer and more sustainable alternative to traditional options. Its flame retardant properties are attributed to its ability to release ammonia gas when exposed to heat, which can dilute flammable gases and potentially inhibit the combustion process.
The primary objective of research into ammonium hydroxide as a flame retardant agent is to develop a comprehensive understanding of its effectiveness, mechanisms of action, and potential applications across various materials and industries. This includes investigating its performance in different polymer matrices, textiles, and wood products, as well as exploring optimal formulations and application methods.
Another crucial goal is to assess the environmental and health impacts of ammonium hydroxide-based flame retardants. This involves studying their toxicity, biodegradability, and long-term effects on ecosystems. Researchers aim to ensure that this new flame retardant solution addresses the shortcomings of its predecessors while maintaining or improving upon their fire-retardant efficacy.
The development of ammonium hydroxide as a flame retardant also aligns with broader industry trends towards sustainable and bio-based materials. As regulations become more stringent and consumer awareness grows, there is an increasing demand for eco-friendly flame retardant solutions that can meet both safety standards and environmental requirements.
Furthermore, researchers are exploring the potential synergistic effects of combining ammonium hydroxide with other flame retardant compounds or nanotechnologies. This approach could lead to the development of more efficient and versatile flame retardant systems, capable of addressing a wider range of fire scenarios and material applications.
Market Analysis for Eco-friendly Flame Retardants
The market for eco-friendly flame retardants has experienced significant growth in recent years, driven by increasing environmental concerns and stringent regulations on traditional flame retardant chemicals. Ammonium hydroxide, as a potential eco-friendly flame retardant agent, is gaining attention in this evolving market landscape.
The global flame retardant market was valued at approximately $7.4 billion in 2020 and is projected to reach $10.2 billion by 2025, with a compound annual growth rate (CAGR) of 6.7%. Within this market, the eco-friendly segment is expected to grow at an even faster rate, driven by the shift towards sustainable and non-toxic alternatives.
Key factors contributing to the market growth of eco-friendly flame retardants include increasing awareness of the harmful effects of halogenated flame retardants, stricter environmental regulations, and growing demand for sustainable building materials. The construction and automotive industries are the primary end-users of flame retardants, with the electronics sector also showing significant potential for growth.
Ammonium hydroxide, as a potential flame retardant agent, offers several advantages that align with market demands. It is non-toxic, environmentally friendly, and cost-effective compared to many traditional flame retardants. These properties make it particularly attractive for applications in textiles, wood products, and polymer composites.
The Asia-Pacific region dominates the flame retardant market, accounting for over 40% of the global market share. This is primarily due to rapid industrialization, urbanization, and the presence of major manufacturing hubs in countries like China and India. North America and Europe follow, with increasing adoption of eco-friendly alternatives driven by stringent regulations.
However, the market for ammonium hydroxide as a flame retardant faces challenges. These include competition from other eco-friendly alternatives such as phosphorus-based and nitrogen-based flame retardants, as well as the need for extensive research and development to optimize its performance across various applications.
Despite these challenges, the potential for ammonium hydroxide in the eco-friendly flame retardant market remains promising. As industries continue to seek sustainable solutions, the demand for non-toxic, environmentally friendly flame retardants is expected to grow. This presents opportunities for further research and development in ammonium hydroxide-based flame retardant formulations, potentially leading to innovative products that can capture a significant share of the expanding eco-friendly flame retardant market.
The global flame retardant market was valued at approximately $7.4 billion in 2020 and is projected to reach $10.2 billion by 2025, with a compound annual growth rate (CAGR) of 6.7%. Within this market, the eco-friendly segment is expected to grow at an even faster rate, driven by the shift towards sustainable and non-toxic alternatives.
Key factors contributing to the market growth of eco-friendly flame retardants include increasing awareness of the harmful effects of halogenated flame retardants, stricter environmental regulations, and growing demand for sustainable building materials. The construction and automotive industries are the primary end-users of flame retardants, with the electronics sector also showing significant potential for growth.
Ammonium hydroxide, as a potential flame retardant agent, offers several advantages that align with market demands. It is non-toxic, environmentally friendly, and cost-effective compared to many traditional flame retardants. These properties make it particularly attractive for applications in textiles, wood products, and polymer composites.
The Asia-Pacific region dominates the flame retardant market, accounting for over 40% of the global market share. This is primarily due to rapid industrialization, urbanization, and the presence of major manufacturing hubs in countries like China and India. North America and Europe follow, with increasing adoption of eco-friendly alternatives driven by stringent regulations.
However, the market for ammonium hydroxide as a flame retardant faces challenges. These include competition from other eco-friendly alternatives such as phosphorus-based and nitrogen-based flame retardants, as well as the need for extensive research and development to optimize its performance across various applications.
Despite these challenges, the potential for ammonium hydroxide in the eco-friendly flame retardant market remains promising. As industries continue to seek sustainable solutions, the demand for non-toxic, environmentally friendly flame retardants is expected to grow. This presents opportunities for further research and development in ammonium hydroxide-based flame retardant formulations, potentially leading to innovative products that can capture a significant share of the expanding eco-friendly flame retardant market.
Current Status and Challenges in Ammonium Hydroxide FR
The current status of ammonium hydroxide as a flame retardant agent is characterized by both promising advancements and significant challenges. Ammonium hydroxide has gained attention in the field of flame retardancy due to its potential as an eco-friendly and cost-effective alternative to traditional halogenated flame retardants.
Recent research has demonstrated the efficacy of ammonium hydroxide in various polymer systems, particularly in cellulose-based materials and some synthetic polymers. Its flame retardant mechanism primarily involves the release of ammonia gas upon heating, which dilutes flammable gases and creates a protective layer on the material surface. This intumescent effect helps to slow down heat transfer and oxygen diffusion, thereby inhibiting flame propagation.
However, the widespread adoption of ammonium hydroxide as a flame retardant faces several challenges. One of the primary concerns is its volatility and potential for off-gassing, which can lead to odor issues and reduced long-term effectiveness. This volatility also poses challenges in terms of processing and incorporation into polymer matrices, as traditional melt-blending techniques may result in significant loss of the flame retardant during manufacturing.
Another significant challenge is the relatively high loading levels required to achieve adequate flame retardancy, which can negatively impact the mechanical properties and processability of the host material. This issue is particularly pronounced in applications where maintaining the original material properties is crucial, such as in structural components or high-performance textiles.
The environmental impact of ammonium hydroxide, while generally considered less harmful than halogenated alternatives, still requires further investigation. Concerns about potential ammonia emissions during the product lifecycle and end-of-life disposal need to be addressed to ensure its sustainability as a flame retardant solution.
From a regulatory perspective, the use of ammonium hydroxide as a flame retardant is still evolving. While it is generally regarded as safer than many traditional flame retardants, comprehensive toxicological studies and long-term exposure assessments are needed to fully establish its safety profile and gain widespread regulatory approval across different regions and applications.
In terms of performance, ammonium hydroxide-based flame retardants often struggle to meet the stringent fire safety standards required in certain high-risk applications, such as in the aerospace or automotive industries. Improving its flame retardant efficiency and developing synergistic systems to enhance its performance without compromising other material properties remains an active area of research.
Recent research has demonstrated the efficacy of ammonium hydroxide in various polymer systems, particularly in cellulose-based materials and some synthetic polymers. Its flame retardant mechanism primarily involves the release of ammonia gas upon heating, which dilutes flammable gases and creates a protective layer on the material surface. This intumescent effect helps to slow down heat transfer and oxygen diffusion, thereby inhibiting flame propagation.
However, the widespread adoption of ammonium hydroxide as a flame retardant faces several challenges. One of the primary concerns is its volatility and potential for off-gassing, which can lead to odor issues and reduced long-term effectiveness. This volatility also poses challenges in terms of processing and incorporation into polymer matrices, as traditional melt-blending techniques may result in significant loss of the flame retardant during manufacturing.
Another significant challenge is the relatively high loading levels required to achieve adequate flame retardancy, which can negatively impact the mechanical properties and processability of the host material. This issue is particularly pronounced in applications where maintaining the original material properties is crucial, such as in structural components or high-performance textiles.
The environmental impact of ammonium hydroxide, while generally considered less harmful than halogenated alternatives, still requires further investigation. Concerns about potential ammonia emissions during the product lifecycle and end-of-life disposal need to be addressed to ensure its sustainability as a flame retardant solution.
From a regulatory perspective, the use of ammonium hydroxide as a flame retardant is still evolving. While it is generally regarded as safer than many traditional flame retardants, comprehensive toxicological studies and long-term exposure assessments are needed to fully establish its safety profile and gain widespread regulatory approval across different regions and applications.
In terms of performance, ammonium hydroxide-based flame retardants often struggle to meet the stringent fire safety standards required in certain high-risk applications, such as in the aerospace or automotive industries. Improving its flame retardant efficiency and developing synergistic systems to enhance its performance without compromising other material properties remains an active area of research.
Existing Ammonium Hydroxide FR Solutions
01 Use of ammonium hydroxide in flame retardant compositions
Ammonium hydroxide is utilized as a component in flame retardant formulations. It can act as a neutralizing agent or pH adjuster in these compositions, contributing to the overall flame retardancy of materials. The inclusion of ammonium hydroxide can enhance the effectiveness of other flame retardant additives and improve the fire resistance of various substrates.- Use of ammonium hydroxide in flame retardant compositions: Ammonium hydroxide is utilized as a component in flame retardant formulations. It can act as a neutralizing agent or pH adjuster in these compositions, contributing to the overall flame retardancy of materials. The inclusion of ammonium hydroxide can enhance the effectiveness of other flame retardant additives and improve the fire resistance of various substrates.
- Synergistic effects with other flame retardant compounds: Ammonium hydroxide can be combined with other flame retardant compounds to create synergistic effects. These combinations can lead to improved flame retardancy performance compared to individual components. The synergistic interactions may involve chemical reactions or physical processes that enhance the overall fire resistance of treated materials.
- Application in textile flame retardancy: Ammonium hydroxide is used in flame retardant treatments for textiles. It can be incorporated into textile finishing processes to impart flame resistant properties to fabrics. The treatment may involve the application of ammonium hydroxide-based solutions or its use in combination with other flame retardant chemicals to enhance the fire resistance of textile materials.
- Role in intumescent flame retardant systems: Ammonium hydroxide can play a role in intumescent flame retardant systems. These systems form a protective char layer when exposed to heat, providing insulation and reducing heat transfer. Ammonium hydroxide may contribute to the expansion or foaming process of the intumescent coating, enhancing its effectiveness in protecting the underlying material from fire.
- Use in flame retardant polymer compositions: Ammonium hydroxide is incorporated into flame retardant polymer compositions. It can be used as a reactive component or as part of a flame retardant additive system in various polymer formulations. The presence of ammonium hydroxide can contribute to the overall flame retardancy of the polymer, improving its resistance to ignition and flame spread.
02 Synergistic effects with other flame retardant compounds
Ammonium hydroxide can be combined with other flame retardant compounds to create synergistic effects. These combinations can lead to improved flame retardancy performance compared to individual components. The synergistic interactions may involve chemical reactions or physical processes that enhance the overall fire resistance of treated materials.Expand Specific Solutions03 Application in textile flame retardancy
Ammonium hydroxide is used in flame retardant treatments for textiles. It can be incorporated into textile finishing processes to impart flame resistant properties to fabrics. The treatment may involve the application of ammonium hydroxide-based solutions or its use in combination with other flame retardant chemicals to enhance the fire resistance of textile materials.Expand Specific Solutions04 Role in intumescent flame retardant systems
Ammonium hydroxide can play a role in intumescent flame retardant systems. These systems form a protective char layer when exposed to heat, providing insulation and limiting the spread of fire. Ammonium hydroxide may contribute to the expansion and char formation processes, enhancing the effectiveness of intumescent flame retardant coatings or additives.Expand Specific Solutions05 Use in flame retardant polymer compositions
Ammonium hydroxide is incorporated into flame retardant polymer compositions. It can be used as a reactive component or as part of a flame retardant additive system in various polymer formulations. The presence of ammonium hydroxide in these compositions can contribute to improved flame resistance, reduced smoke generation, and enhanced overall fire performance of the polymer materials.Expand Specific Solutions
Key Players in Flame Retardant Industry
The research on ammonium hydroxide as a flame retardant agent is in a developing stage, with growing market potential due to increasing fire safety regulations across industries. The global flame retardant market is expanding, driven by stringent safety standards and rising awareness. While the technology is not fully mature, several key players are actively involved in its development. Companies like Hoechst AG, Shin-Etsu Chemical Co., Ltd., and BK Giulini GmbH are leveraging their expertise in chemical manufacturing to advance ammonium hydroxide-based flame retardants. Academic institutions such as Harbin Engineering University and Shandong University are contributing to research efforts, indicating a collaborative approach between industry and academia to refine this technology.
Harbin Engineering University
Technical Solution: Researchers at Harbin Engineering University have made significant strides in the application of ammonium hydroxide as a flame retardant agent for wood-based materials. Their approach involves a two-step treatment process: first, impregnating wood with ammonium hydroxide solution, followed by a heat treatment to form stable nitrogen compounds within the wood structure[7]. This method has shown to significantly improve the fire resistance of treated wood, with char formation increasing by up to 30% compared to untreated samples[9]. The university's research also explores the combination of ammonium hydroxide with other inorganic compounds to create multi-functional flame retardant systems that offer both fire resistance and improved mechanical properties[11].
Strengths: Effective for wood-based materials, dual functionality (flame retardancy and mechanical improvement), environmentally friendly approach. Weaknesses: Limited to wood and cellulosic materials, potential for color changes in treated materials.
Chemische Fabrik Budenheim KG
Technical Solution: Chemische Fabrik Budenheim KG has developed a novel ammonium hydroxide-based flame retardant system for polymers. Their approach involves incorporating ammonium hydroxide into a phosphorus-containing compound, creating a synergistic effect that enhances flame retardancy[1]. The company's research has shown that this combination can achieve a UL94 V-0 rating in various polymer systems at lower loadings compared to traditional flame retardants[3]. They have also focused on improving the stability of ammonium hydroxide in polymer matrices, addressing the volatility issue often associated with this compound[5].
Strengths: Synergistic effect with phosphorus compounds, lower loading requirements, improved stability in polymers. Weaknesses: Potential for ammonia off-gassing, may affect polymer processing due to alkaline nature.
Core Innovations in Ammonium Hydroxide FR
Flameproofing agent
PatentInactiveJP2017105870A
Innovation
- A flame retardant formulation comprising a flame retardant compound, metal hydroxide, and an anti-deliquescence agent, including bicarbonate and magnesium sulfate, which prevents deliquescence and maintains flame retardancy and adhesion.
Hydrolysis-stable flame-retardant agent based on ammonium polyphosphate
PatentInactiveEP0180790A1
Innovation
- A hydrolysis-stable, microencapsulated flame retardant is created using free-flowing, powdered ammonium polyphosphate coated with a polyurea reaction product of polyisocyanate and water, reducing water solubility without releasing formaldehyde.
Environmental Impact Assessment
The environmental impact assessment of ammonium hydroxide as a flame retardant agent is a critical aspect of its research and potential application. Ammonium hydroxide, while effective in fire suppression, presents several environmental considerations that must be carefully evaluated.
One of the primary concerns is the potential for ammonia release into the atmosphere. When ammonium hydroxide is used as a flame retardant, there is a risk of ammonia gas being emitted during fire events or through gradual evaporation. Ammonia is a known air pollutant that can contribute to the formation of particulate matter and have adverse effects on air quality. Additionally, ammonia can react with other atmospheric compounds to form secondary pollutants, potentially exacerbating smog and acid rain issues in affected areas.
Water contamination is another significant environmental consideration. Ammonium hydroxide is highly soluble in water, and its runoff from treated materials or firefighting activities can lead to increased nitrogen levels in aquatic ecosystems. This nutrient enrichment may result in eutrophication, causing algal blooms and disrupting the balance of aquatic life. Furthermore, elevated ammonia concentrations in water bodies can be toxic to fish and other aquatic organisms, potentially impacting biodiversity and ecosystem health.
Soil impacts must also be taken into account. The application of ammonium hydroxide-based flame retardants can alter soil pH and nitrogen content. While this may have beneficial effects in some agricultural contexts, it can also lead to soil degradation and changes in microbial communities in natural environments. The long-term effects on soil fertility and structure require thorough investigation to ensure sustainable use of this flame retardant agent.
The production and disposal of ammonium hydroxide-based flame retardants also warrant consideration in the environmental impact assessment. Manufacturing processes may contribute to industrial emissions and resource consumption. End-of-life management of treated materials poses challenges, as improper disposal could lead to leaching of ammonium compounds into the environment.
To mitigate these potential environmental impacts, research should focus on developing application methods that minimize ammonia release and optimize the retention of the flame retardant within treated materials. Additionally, exploring biodegradable alternatives or encapsulation technologies could help reduce the long-term environmental persistence of ammonium hydroxide-based flame retardants.
Comprehensive life cycle assessments and ecological risk evaluations are essential to fully understand and quantify the environmental implications of using ammonium hydroxide as a flame retardant agent. These studies should compare the environmental footprint of ammonium hydroxide with other flame retardant options to inform decision-making and guide the development of more sustainable fire safety solutions.
One of the primary concerns is the potential for ammonia release into the atmosphere. When ammonium hydroxide is used as a flame retardant, there is a risk of ammonia gas being emitted during fire events or through gradual evaporation. Ammonia is a known air pollutant that can contribute to the formation of particulate matter and have adverse effects on air quality. Additionally, ammonia can react with other atmospheric compounds to form secondary pollutants, potentially exacerbating smog and acid rain issues in affected areas.
Water contamination is another significant environmental consideration. Ammonium hydroxide is highly soluble in water, and its runoff from treated materials or firefighting activities can lead to increased nitrogen levels in aquatic ecosystems. This nutrient enrichment may result in eutrophication, causing algal blooms and disrupting the balance of aquatic life. Furthermore, elevated ammonia concentrations in water bodies can be toxic to fish and other aquatic organisms, potentially impacting biodiversity and ecosystem health.
Soil impacts must also be taken into account. The application of ammonium hydroxide-based flame retardants can alter soil pH and nitrogen content. While this may have beneficial effects in some agricultural contexts, it can also lead to soil degradation and changes in microbial communities in natural environments. The long-term effects on soil fertility and structure require thorough investigation to ensure sustainable use of this flame retardant agent.
The production and disposal of ammonium hydroxide-based flame retardants also warrant consideration in the environmental impact assessment. Manufacturing processes may contribute to industrial emissions and resource consumption. End-of-life management of treated materials poses challenges, as improper disposal could lead to leaching of ammonium compounds into the environment.
To mitigate these potential environmental impacts, research should focus on developing application methods that minimize ammonia release and optimize the retention of the flame retardant within treated materials. Additionally, exploring biodegradable alternatives or encapsulation technologies could help reduce the long-term environmental persistence of ammonium hydroxide-based flame retardants.
Comprehensive life cycle assessments and ecological risk evaluations are essential to fully understand and quantify the environmental implications of using ammonium hydroxide as a flame retardant agent. These studies should compare the environmental footprint of ammonium hydroxide with other flame retardant options to inform decision-making and guide the development of more sustainable fire safety solutions.
Regulatory Framework for Flame Retardants
The regulatory framework for flame retardants has become increasingly stringent in recent years, reflecting growing concerns about the environmental and health impacts of certain chemical compounds. In the context of ammonium hydroxide as a flame retardant agent, it is essential to understand the current regulatory landscape and its implications for research and development in this field.
At the international level, the Stockholm Convention on Persistent Organic Pollutants (POPs) has played a significant role in shaping flame retardant regulations. This treaty, which came into force in 2004, aims to eliminate or restrict the production and use of POPs, including some brominated flame retardants. While ammonium hydroxide is not directly targeted by this convention, the increased scrutiny on flame retardants has led to a shift towards more environmentally friendly alternatives.
In the United States, the Environmental Protection Agency (EPA) regulates flame retardants under the Toxic Substances Control Act (TSCA). The EPA has the authority to evaluate and restrict the use of flame retardants that pose unreasonable risks to human health or the environment. Additionally, individual states have implemented their own regulations, with California's Proposition 65 and Technical Bulletin 117-2013 being notable examples that have influenced flame retardant use nationwide.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to register chemical substances and provide safety information. The EU has also adopted specific regulations on flame retardants in electrical and electronic equipment through the Restriction of Hazardous Substances (RoHS) Directive.
In Asia, countries like Japan and China have established their own regulatory frameworks for flame retardants. Japan's Chemical Substances Control Law (CSCL) and China's Measures for Environmental Administration of New Chemical Substances both aim to assess and manage the risks associated with chemical substances, including flame retardants.
These regulatory frameworks have significant implications for the research and development of ammonium hydroxide as a flame retardant agent. Researchers and manufacturers must consider compliance with these regulations throughout the product development lifecycle, from initial testing to market introduction. This includes conducting thorough safety assessments, evaluating potential environmental impacts, and ensuring that the use of ammonium hydroxide meets the increasingly stringent standards set by various regulatory bodies.
Furthermore, the evolving nature of flame retardant regulations necessitates ongoing monitoring and adaptation in research strategies. As new scientific evidence emerges and public awareness of environmental and health issues grows, regulatory requirements are likely to become more comprehensive and demanding. This dynamic regulatory landscape underscores the importance of developing flame retardants that not only meet current standards but also anticipate future regulatory trends.
At the international level, the Stockholm Convention on Persistent Organic Pollutants (POPs) has played a significant role in shaping flame retardant regulations. This treaty, which came into force in 2004, aims to eliminate or restrict the production and use of POPs, including some brominated flame retardants. While ammonium hydroxide is not directly targeted by this convention, the increased scrutiny on flame retardants has led to a shift towards more environmentally friendly alternatives.
In the United States, the Environmental Protection Agency (EPA) regulates flame retardants under the Toxic Substances Control Act (TSCA). The EPA has the authority to evaluate and restrict the use of flame retardants that pose unreasonable risks to human health or the environment. Additionally, individual states have implemented their own regulations, with California's Proposition 65 and Technical Bulletin 117-2013 being notable examples that have influenced flame retardant use nationwide.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to register chemical substances and provide safety information. The EU has also adopted specific regulations on flame retardants in electrical and electronic equipment through the Restriction of Hazardous Substances (RoHS) Directive.
In Asia, countries like Japan and China have established their own regulatory frameworks for flame retardants. Japan's Chemical Substances Control Law (CSCL) and China's Measures for Environmental Administration of New Chemical Substances both aim to assess and manage the risks associated with chemical substances, including flame retardants.
These regulatory frameworks have significant implications for the research and development of ammonium hydroxide as a flame retardant agent. Researchers and manufacturers must consider compliance with these regulations throughout the product development lifecycle, from initial testing to market introduction. This includes conducting thorough safety assessments, evaluating potential environmental impacts, and ensuring that the use of ammonium hydroxide meets the increasingly stringent standards set by various regulatory bodies.
Furthermore, the evolving nature of flame retardant regulations necessitates ongoing monitoring and adaptation in research strategies. As new scientific evidence emerges and public awareness of environmental and health issues grows, regulatory requirements are likely to become more comprehensive and demanding. This dynamic regulatory landscape underscores the importance of developing flame retardants that not only meet current standards but also anticipate future regulatory trends.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!