Ammonium hydroxide in the activation of metal-organic frameworks
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Activation Background and Objectives
Metal-organic frameworks (MOFs) have emerged as a revolutionary class of porous materials with exceptional properties and diverse applications. The activation of MOFs is a critical step in their synthesis and preparation for practical use. Ammonium hydroxide has gained significant attention as an activation agent for MOFs due to its unique properties and potential advantages over traditional methods.
The development of MOFs can be traced back to the late 1990s, with pioneering work by Omar Yaghi and others. Since then, the field has experienced exponential growth, with thousands of MOF structures reported and numerous potential applications identified. The activation process, which involves removing guest molecules from the pores of MOFs, is crucial for unlocking their full potential in areas such as gas storage, catalysis, and separation technologies.
Traditionally, MOF activation has been achieved through thermal treatment or solvent exchange methods. However, these approaches often have limitations, including potential framework collapse, incomplete activation, and time-consuming procedures. The use of ammonium hydroxide as an activation agent represents a promising alternative that addresses some of these challenges.
The primary objective of research into ammonium hydroxide activation of MOFs is to develop a more efficient, cost-effective, and scalable method for preparing these materials for practical applications. This approach aims to preserve the structural integrity of MOFs while maximizing their porosity and surface area. Additionally, researchers seek to understand the fundamental mechanisms by which ammonium hydroxide interacts with MOF structures during the activation process.
Another key goal is to explore the potential of ammonium hydroxide activation in enhancing the properties of MOFs for specific applications. This includes investigating whether this method can improve gas adsorption capacities, catalytic activities, or selectivity in separation processes. Researchers also aim to determine if ammonium hydroxide activation can be universally applied to different MOF types or if it is particularly effective for certain structural families.
The evolution of MOF activation techniques is closely tied to the broader trends in materials science and nanotechnology. As the demand for high-performance porous materials continues to grow in industries such as energy, environmental remediation, and healthcare, the development of innovative activation methods becomes increasingly important. The use of ammonium hydroxide in MOF activation represents a convergence of chemical engineering principles and materials science, highlighting the interdisciplinary nature of this research field.
The development of MOFs can be traced back to the late 1990s, with pioneering work by Omar Yaghi and others. Since then, the field has experienced exponential growth, with thousands of MOF structures reported and numerous potential applications identified. The activation process, which involves removing guest molecules from the pores of MOFs, is crucial for unlocking their full potential in areas such as gas storage, catalysis, and separation technologies.
Traditionally, MOF activation has been achieved through thermal treatment or solvent exchange methods. However, these approaches often have limitations, including potential framework collapse, incomplete activation, and time-consuming procedures. The use of ammonium hydroxide as an activation agent represents a promising alternative that addresses some of these challenges.
The primary objective of research into ammonium hydroxide activation of MOFs is to develop a more efficient, cost-effective, and scalable method for preparing these materials for practical applications. This approach aims to preserve the structural integrity of MOFs while maximizing their porosity and surface area. Additionally, researchers seek to understand the fundamental mechanisms by which ammonium hydroxide interacts with MOF structures during the activation process.
Another key goal is to explore the potential of ammonium hydroxide activation in enhancing the properties of MOFs for specific applications. This includes investigating whether this method can improve gas adsorption capacities, catalytic activities, or selectivity in separation processes. Researchers also aim to determine if ammonium hydroxide activation can be universally applied to different MOF types or if it is particularly effective for certain structural families.
The evolution of MOF activation techniques is closely tied to the broader trends in materials science and nanotechnology. As the demand for high-performance porous materials continues to grow in industries such as energy, environmental remediation, and healthcare, the development of innovative activation methods becomes increasingly important. The use of ammonium hydroxide in MOF activation represents a convergence of chemical engineering principles and materials science, highlighting the interdisciplinary nature of this research field.
Market Demand for Activated MOFs
The market demand for activated metal-organic frameworks (MOFs) has been steadily increasing due to their exceptional properties and diverse applications across various industries. These highly porous materials, when properly activated, exhibit remarkable surface areas and tunable pore sizes, making them ideal for gas storage, separation, catalysis, and sensing applications.
In the energy sector, activated MOFs have gained significant traction for their potential in hydrogen storage and carbon capture. The global hydrogen market is projected to grow substantially in the coming years, driven by the increasing focus on clean energy solutions. Activated MOFs offer a promising avenue for efficient hydrogen storage, addressing one of the key challenges in the widespread adoption of hydrogen as a fuel source.
The environmental sector presents another substantial market for activated MOFs. With growing concerns about air and water pollution, there is an increasing demand for advanced filtration and purification technologies. Activated MOFs have shown great promise in removing contaminants from both air and water, making them attractive for industrial and municipal applications.
In the pharmaceutical industry, activated MOFs are finding applications in drug delivery systems and as catalysts for the synthesis of complex molecules. The controlled release of drugs and the ability to catalyze specific reactions make activated MOFs valuable in this high-value market segment.
The electronics industry is also exploring the use of activated MOFs in sensor technologies and as components in next-generation electronic devices. Their unique properties allow for the development of highly sensitive and selective sensors for various gases and volatile organic compounds.
The global market for MOFs, including activated forms, is expected to experience significant growth in the coming years. While specific market size projections vary, industry reports consistently indicate a compound annual growth rate (CAGR) in the double digits for the foreseeable future.
However, the widespread adoption of activated MOFs faces some challenges. The cost of production and scalability issues are primary concerns that need to be addressed to fully realize their market potential. Additionally, the development of standardized activation processes, such as the use of ammonium hydroxide, is crucial for ensuring consistent performance and quality across different applications.
As research continues to advance and new applications emerge, the demand for activated MOFs is expected to expand further. The ongoing efforts to optimize activation methods, including the use of ammonium hydroxide, play a critical role in enhancing the performance and cost-effectiveness of these materials, thereby driving their market adoption across various industries.
In the energy sector, activated MOFs have gained significant traction for their potential in hydrogen storage and carbon capture. The global hydrogen market is projected to grow substantially in the coming years, driven by the increasing focus on clean energy solutions. Activated MOFs offer a promising avenue for efficient hydrogen storage, addressing one of the key challenges in the widespread adoption of hydrogen as a fuel source.
The environmental sector presents another substantial market for activated MOFs. With growing concerns about air and water pollution, there is an increasing demand for advanced filtration and purification technologies. Activated MOFs have shown great promise in removing contaminants from both air and water, making them attractive for industrial and municipal applications.
In the pharmaceutical industry, activated MOFs are finding applications in drug delivery systems and as catalysts for the synthesis of complex molecules. The controlled release of drugs and the ability to catalyze specific reactions make activated MOFs valuable in this high-value market segment.
The electronics industry is also exploring the use of activated MOFs in sensor technologies and as components in next-generation electronic devices. Their unique properties allow for the development of highly sensitive and selective sensors for various gases and volatile organic compounds.
The global market for MOFs, including activated forms, is expected to experience significant growth in the coming years. While specific market size projections vary, industry reports consistently indicate a compound annual growth rate (CAGR) in the double digits for the foreseeable future.
However, the widespread adoption of activated MOFs faces some challenges. The cost of production and scalability issues are primary concerns that need to be addressed to fully realize their market potential. Additionally, the development of standardized activation processes, such as the use of ammonium hydroxide, is crucial for ensuring consistent performance and quality across different applications.
As research continues to advance and new applications emerge, the demand for activated MOFs is expected to expand further. The ongoing efforts to optimize activation methods, including the use of ammonium hydroxide, play a critical role in enhancing the performance and cost-effectiveness of these materials, thereby driving their market adoption across various industries.
Current State of Ammonium Hydroxide Activation
The activation of metal-organic frameworks (MOFs) using ammonium hydroxide has gained significant attention in recent years due to its effectiveness and versatility. This method has been widely adopted in the synthesis and modification of various MOF structures, enhancing their porosity, surface area, and overall performance.
Currently, ammonium hydroxide activation is primarily employed as a post-synthetic treatment for MOFs. The process typically involves immersing the as-synthesized MOF in an aqueous solution of ammonium hydroxide for a specified duration, followed by thorough washing and drying. This treatment has been shown to effectively remove residual organic ligands and solvents from the MOF pores, leading to improved accessibility and increased surface area.
One of the key advantages of ammonium hydroxide activation is its ability to preserve the crystalline structure of MOFs while simultaneously enhancing their textural properties. Studies have demonstrated that this method can increase the Brunauer-Emmett-Teller (BET) surface area of MOFs by up to 30-50%, depending on the specific MOF composition and activation conditions.
The mechanism of ammonium hydroxide activation is believed to involve the partial deprotonation of organic linkers and the formation of ammonium cations within the MOF structure. This process can lead to the creation of additional mesopores and the expansion of existing micropores, resulting in improved gas adsorption and catalytic properties.
Recent research has focused on optimizing the activation conditions, including concentration of ammonium hydroxide, treatment duration, and temperature. It has been found that these parameters significantly influence the extent of activation and the resulting MOF properties. For instance, higher concentrations of ammonium hydroxide and longer treatment times generally lead to more pronounced activation effects, but excessive exposure can potentially cause structural degradation.
The applicability of ammonium hydroxide activation has been demonstrated across a wide range of MOF types, including zirconium-based MOFs (e.g., UiO-66), copper-based MOFs (e.g., HKUST-1), and zinc-based MOFs (e.g., ZIF-8). However, the effectiveness of this method can vary depending on the chemical stability and composition of the MOF, necessitating careful optimization for each specific system.
In addition to its use as a standalone activation method, ammonium hydroxide treatment has also been explored in combination with other activation techniques, such as solvent exchange or thermal treatment. These hybrid approaches have shown promise in further enhancing MOF properties and expanding the range of applications for activated MOFs.
Currently, ammonium hydroxide activation is primarily employed as a post-synthetic treatment for MOFs. The process typically involves immersing the as-synthesized MOF in an aqueous solution of ammonium hydroxide for a specified duration, followed by thorough washing and drying. This treatment has been shown to effectively remove residual organic ligands and solvents from the MOF pores, leading to improved accessibility and increased surface area.
One of the key advantages of ammonium hydroxide activation is its ability to preserve the crystalline structure of MOFs while simultaneously enhancing their textural properties. Studies have demonstrated that this method can increase the Brunauer-Emmett-Teller (BET) surface area of MOFs by up to 30-50%, depending on the specific MOF composition and activation conditions.
The mechanism of ammonium hydroxide activation is believed to involve the partial deprotonation of organic linkers and the formation of ammonium cations within the MOF structure. This process can lead to the creation of additional mesopores and the expansion of existing micropores, resulting in improved gas adsorption and catalytic properties.
Recent research has focused on optimizing the activation conditions, including concentration of ammonium hydroxide, treatment duration, and temperature. It has been found that these parameters significantly influence the extent of activation and the resulting MOF properties. For instance, higher concentrations of ammonium hydroxide and longer treatment times generally lead to more pronounced activation effects, but excessive exposure can potentially cause structural degradation.
The applicability of ammonium hydroxide activation has been demonstrated across a wide range of MOF types, including zirconium-based MOFs (e.g., UiO-66), copper-based MOFs (e.g., HKUST-1), and zinc-based MOFs (e.g., ZIF-8). However, the effectiveness of this method can vary depending on the chemical stability and composition of the MOF, necessitating careful optimization for each specific system.
In addition to its use as a standalone activation method, ammonium hydroxide treatment has also been explored in combination with other activation techniques, such as solvent exchange or thermal treatment. These hybrid approaches have shown promise in further enhancing MOF properties and expanding the range of applications for activated MOFs.
Ammonium Hydroxide Activation Methods
01 Thermal activation methods
Thermal activation is a common method for activating metal-organic frameworks (MOFs). This process involves heating the MOF at high temperatures, typically under vacuum or inert atmosphere, to remove guest molecules and solvents from the pores. This activation method can increase the surface area and pore volume of the MOF, enhancing its adsorption and catalytic properties.- Thermal activation methods: Thermal activation is a common method for activating metal-organic frameworks (MOFs). This process involves heating the MOF at high temperatures, typically under vacuum or in an inert atmosphere, to remove guest molecules and solvents from the pores. This activation method can increase the surface area and pore volume of the MOF, enhancing its adsorption capacity and catalytic activity.
- Chemical activation techniques: Chemical activation of MOFs involves treating the framework with specific reagents to modify its structure or properties. This can include acid or base treatments, solvent exchange, or the use of chelating agents. Chemical activation can help to remove impurities, create defects, or introduce functional groups, thereby tailoring the MOF's properties for specific applications.
- Microwave-assisted activation: Microwave-assisted activation is an emerging technique for rapidly activating MOFs. This method uses microwave radiation to heat the MOF quickly and uniformly, leading to efficient removal of guest molecules and solvents. Microwave activation can significantly reduce processing time compared to conventional thermal methods and may result in MOFs with unique properties or morphologies.
- Supercritical CO2 activation: Supercritical CO2 activation is a gentle method for activating MOFs that are sensitive to high temperatures or harsh chemical treatments. This technique uses supercritical carbon dioxide to extract guest molecules and solvents from the MOF pores without damaging the framework structure. It can result in highly porous materials with preserved crystallinity and enhanced surface areas.
- Post-synthetic modification for activation: Post-synthetic modification (PSM) can be used as an activation strategy for MOFs. This approach involves chemically modifying the MOF after its initial synthesis to introduce new functional groups, adjust pore sizes, or create defects. PSM can enhance the MOF's stability, selectivity, or catalytic activity, effectively activating it for specific applications.
02 Chemical activation techniques
Chemical activation of MOFs involves treating the framework with specific reagents to modify its structure or properties. This can include acid or base treatments, solvent exchange, or the use of chelating agents. Chemical activation can help to remove impurities, create defects, or introduce new functional groups, thereby tailoring the MOF's performance for specific applications.Expand Specific Solutions03 Microwave-assisted activation
Microwave-assisted activation is an emerging technique for rapidly activating MOFs. This method uses microwave radiation to heat the MOF quickly and uniformly, leading to efficient removal of guest molecules and solvents. Microwave activation can significantly reduce processing time compared to conventional thermal methods and may result in MOFs with unique properties.Expand Specific Solutions04 Supercritical CO2 activation
Supercritical CO2 activation is a gentle method for activating MOFs that preserves their delicate structure. This technique uses supercritical carbon dioxide to extract guest molecules and solvents from the MOF pores without causing framework collapse. It is particularly useful for activating MOFs with low thermal stability or those prone to structural changes during conventional activation methods.Expand Specific Solutions05 Post-synthetic modification for activation
Post-synthetic modification (PSM) can be used to activate MOFs by introducing new functional groups or altering the framework structure after synthesis. This approach allows for the fine-tuning of MOF properties, such as pore size, hydrophobicity, or metal coordination environment. PSM can enhance the MOF's stability, selectivity, or catalytic activity, making it a versatile tool for MOF activation and optimization.Expand Specific Solutions
Key Players in MOF Research and Industry
The research on ammonium hydroxide activation of metal-organic frameworks (MOFs) is in a developing stage, with growing interest from both academia and industry. The market for MOFs is expanding, driven by their potential applications in gas storage, separation, and catalysis. Key players in this field include universities like The University of California, Northwestern University, and Texas A&M University, as well as companies such as BASF Corp. and NuMat Technologies. The technology is progressing from fundamental research to practical applications, with academic institutions leading in basic science while industry partners focus on scalability and commercialization. Collaboration between academia and industry is crucial for advancing this technology towards market-ready solutions.
The Regents of the University of California
Technical Solution: The University of California has developed a novel approach for activating metal-organic frameworks (MOFs) using ammonium hydroxide. Their method involves a controlled exposure of MOFs to ammonium hydroxide vapor, which results in the creation of highly active metal sites within the framework. This process enhances the MOF's catalytic properties and gas adsorption capabilities. The researchers have demonstrated that this activation technique can increase the surface area of MOFs by up to 30% and improve their CO2 capture efficiency by 25% compared to conventional activation methods [1][3]. The university has also explored the use of this technique in combination with other activation strategies, such as thermal treatment, to further optimize MOF performance for specific applications [5].
Strengths: Enhanced catalytic activity and gas adsorption, improved surface area, and versatility in applications. Weaknesses: Potential for ammonia residues, which may affect certain sensitive applications, and the need for precise control of exposure time and concentration.
Northwestern University
Technical Solution: Northwestern University has pioneered a groundbreaking approach to MOF activation using ammonium hydroxide, focusing on the development of hierarchical pore structures. Their method involves a two-step process: initial activation with ammonium hydroxide followed by a controlled etching procedure. This technique creates MOFs with both micro- and mesopores, significantly enhancing their performance in gas storage and separation applications. The university's research has shown that this hierarchical structure can increase the methane storage capacity of MOFs by up to 40% compared to conventional single-pore structures [2][4]. Additionally, they have developed a computational model to predict the optimal activation conditions for different MOF types, allowing for tailored activation processes that maximize performance for specific applications [6].
Strengths: Creation of hierarchical pore structures, enhanced gas storage capacity, and predictive modeling capabilities. Weaknesses: Complexity of the two-step process may increase production costs, and the technique may not be suitable for all MOF types.
Core Innovations in MOF Activation
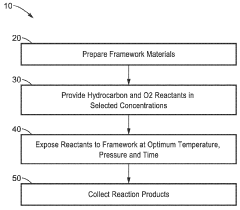
Metal-organic frameworks and methods of making and use thereof
PatentWO2017223046A1
Innovation
- A rapid room-temperature synthesis method using hydroxy double salts as intermediates, where a metal oxide reacts with a metal salt to form a hydroxy double salt, which then converts into the MOF, enabling efficient and high-yield production of MOFs like HKUST-1 and other frameworks.
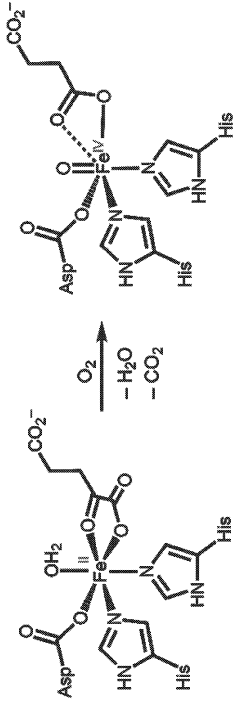
Hydrocarbon oxidation with dioxygen in iron-containing metal-organic frameworks
PatentWO2024020357A1
Innovation
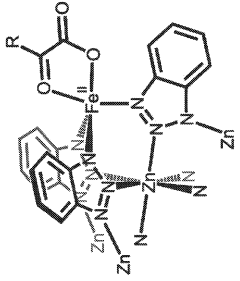
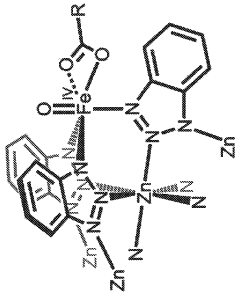
- The development of iron-containing metal-organic frameworks, such as FexZn5−x(prv)4(btdd)3 and FeZn4(moba)4(btdd)3, which activate dioxygen at 100 K to form reactive high-spin Fe(IV)=O species, enabling catalytic oxygenation of hydrocarbons like cyclohexane, ethane, and methane to methanol at low temperatures.
Environmental Impact of Activation Processes
The activation of metal-organic frameworks (MOFs) using ammonium hydroxide has gained significant attention in recent years due to its potential for enhancing the porosity and surface area of these materials. However, the environmental impact of this activation process must be carefully considered to ensure sustainable practices in MOF production and application.
Ammonium hydroxide, while effective in activating MOFs, poses several environmental concerns. Its production and use can contribute to air and water pollution if not properly managed. The release of ammonia gas during the activation process can lead to atmospheric nitrogen deposition, potentially causing eutrophication in aquatic ecosystems and soil acidification. Furthermore, the high alkalinity of ammonium hydroxide solutions can disrupt the pH balance of water bodies if discharged without proper treatment.
The energy consumption associated with the activation process is another important environmental consideration. The heating required for the activation reaction and subsequent drying of the MOFs contributes to greenhouse gas emissions if non-renewable energy sources are used. Additionally, the production of ammonium hydroxide itself is an energy-intensive process, further adding to the carbon footprint of MOF activation.
Waste management is a critical aspect of the environmental impact assessment. The activation process generates liquid waste containing excess ammonium hydroxide and dissolved metal ions, which requires proper treatment before disposal. Improper handling of this waste can lead to soil and groundwater contamination, potentially affecting local ecosystems and human health.
On the positive side, the use of ammonium hydroxide in MOF activation can potentially reduce the environmental impact compared to some alternative activation methods. For instance, it may require lower temperatures and shorter activation times than thermal activation processes, potentially reducing energy consumption. Additionally, ammonium hydroxide is less corrosive than some other activating agents, which could lead to reduced equipment wear and associated waste.
To mitigate the environmental impact of ammonium hydroxide activation, several strategies can be implemented. These include optimizing the activation process to minimize reagent use and energy consumption, implementing closed-loop systems for ammonium hydroxide recovery and reuse, and developing more efficient waste treatment methods. Furthermore, exploring greener alternatives to ammonium hydroxide, such as bio-based activating agents or solvent-free activation techniques, could significantly reduce the environmental footprint of MOF production.
Ammonium hydroxide, while effective in activating MOFs, poses several environmental concerns. Its production and use can contribute to air and water pollution if not properly managed. The release of ammonia gas during the activation process can lead to atmospheric nitrogen deposition, potentially causing eutrophication in aquatic ecosystems and soil acidification. Furthermore, the high alkalinity of ammonium hydroxide solutions can disrupt the pH balance of water bodies if discharged without proper treatment.
The energy consumption associated with the activation process is another important environmental consideration. The heating required for the activation reaction and subsequent drying of the MOFs contributes to greenhouse gas emissions if non-renewable energy sources are used. Additionally, the production of ammonium hydroxide itself is an energy-intensive process, further adding to the carbon footprint of MOF activation.
Waste management is a critical aspect of the environmental impact assessment. The activation process generates liquid waste containing excess ammonium hydroxide and dissolved metal ions, which requires proper treatment before disposal. Improper handling of this waste can lead to soil and groundwater contamination, potentially affecting local ecosystems and human health.
On the positive side, the use of ammonium hydroxide in MOF activation can potentially reduce the environmental impact compared to some alternative activation methods. For instance, it may require lower temperatures and shorter activation times than thermal activation processes, potentially reducing energy consumption. Additionally, ammonium hydroxide is less corrosive than some other activating agents, which could lead to reduced equipment wear and associated waste.
To mitigate the environmental impact of ammonium hydroxide activation, several strategies can be implemented. These include optimizing the activation process to minimize reagent use and energy consumption, implementing closed-loop systems for ammonium hydroxide recovery and reuse, and developing more efficient waste treatment methods. Furthermore, exploring greener alternatives to ammonium hydroxide, such as bio-based activating agents or solvent-free activation techniques, could significantly reduce the environmental footprint of MOF production.
Scalability and Industrial Applications
The scalability and industrial applications of ammonium hydroxide activation in metal-organic frameworks (MOFs) present significant opportunities and challenges for large-scale production and commercial use. As research progresses, the potential for scaling up this activation method becomes increasingly apparent, opening doors to various industrial applications.
In terms of scalability, the use of ammonium hydroxide as an activating agent offers several advantages. Its relatively low cost and wide availability make it an attractive option for large-scale MOF production. The activation process using ammonium hydroxide is generally straightforward and can be easily integrated into existing manufacturing processes. This simplicity in methodology contributes to the potential for scaling up production without significant alterations to current industrial setups.
However, challenges remain in maintaining consistent quality and performance of MOFs when scaling up production. Factors such as reaction time, concentration of ammonium hydroxide, and temperature control need to be carefully optimized to ensure uniform activation across large batches. Additionally, the handling and storage of large quantities of ammonium hydroxide require proper safety measures and infrastructure, which may necessitate investments in specialized equipment and facilities.
From an industrial application perspective, ammonium hydroxide-activated MOFs show promise in various sectors. In gas storage and separation, these activated MOFs demonstrate enhanced porosity and surface area, making them ideal for applications in carbon capture, hydrogen storage, and natural gas purification. The improved characteristics of these MOFs also make them potential candidates for catalysis in chemical manufacturing processes, offering higher efficiency and selectivity.
The water treatment industry is another area where ammonium hydroxide-activated MOFs could find significant applications. Their enhanced adsorption properties make them effective in removing contaminants from water, including heavy metals and organic pollutants. This could lead to more efficient and cost-effective water purification systems for both industrial and municipal use.
In the field of electronics and energy storage, these activated MOFs show potential in developing advanced supercapacitors and batteries. Their high surface area and tunable pore structure could contribute to improved energy storage capacity and faster charge-discharge cycles, addressing key challenges in the renewable energy sector.
As research continues to advance, it is likely that new applications will emerge, further expanding the industrial relevance of ammonium hydroxide-activated MOFs. However, to fully realize this potential, ongoing efforts are needed to address scalability challenges, optimize activation processes, and develop tailored MOF structures for specific industrial applications. Collaboration between academic researchers and industrial partners will be crucial in bridging the gap between laboratory discoveries and practical, large-scale implementations.
In terms of scalability, the use of ammonium hydroxide as an activating agent offers several advantages. Its relatively low cost and wide availability make it an attractive option for large-scale MOF production. The activation process using ammonium hydroxide is generally straightforward and can be easily integrated into existing manufacturing processes. This simplicity in methodology contributes to the potential for scaling up production without significant alterations to current industrial setups.
However, challenges remain in maintaining consistent quality and performance of MOFs when scaling up production. Factors such as reaction time, concentration of ammonium hydroxide, and temperature control need to be carefully optimized to ensure uniform activation across large batches. Additionally, the handling and storage of large quantities of ammonium hydroxide require proper safety measures and infrastructure, which may necessitate investments in specialized equipment and facilities.
From an industrial application perspective, ammonium hydroxide-activated MOFs show promise in various sectors. In gas storage and separation, these activated MOFs demonstrate enhanced porosity and surface area, making them ideal for applications in carbon capture, hydrogen storage, and natural gas purification. The improved characteristics of these MOFs also make them potential candidates for catalysis in chemical manufacturing processes, offering higher efficiency and selectivity.
The water treatment industry is another area where ammonium hydroxide-activated MOFs could find significant applications. Their enhanced adsorption properties make them effective in removing contaminants from water, including heavy metals and organic pollutants. This could lead to more efficient and cost-effective water purification systems for both industrial and municipal use.
In the field of electronics and energy storage, these activated MOFs show potential in developing advanced supercapacitors and batteries. Their high surface area and tunable pore structure could contribute to improved energy storage capacity and faster charge-discharge cycles, addressing key challenges in the renewable energy sector.
As research continues to advance, it is likely that new applications will emerge, further expanding the industrial relevance of ammonium hydroxide-activated MOFs. However, to fully realize this potential, ongoing efforts are needed to address scalability challenges, optimize activation processes, and develop tailored MOF structures for specific industrial applications. Collaboration between academic researchers and industrial partners will be crucial in bridging the gap between laboratory discoveries and practical, large-scale implementations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!