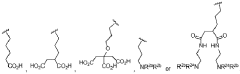
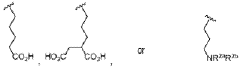
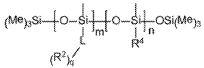
Ammonium hydroxide in optimizing nanostructured coating adhesion
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide in Nanocoating: Background and Objectives
Nanostructured coatings have emerged as a revolutionary technology in materials science, offering enhanced properties and functionalities to various surfaces. The use of ammonium hydroxide in optimizing the adhesion of these coatings represents a significant area of research with far-reaching implications across multiple industries.
The development of nanostructured coatings can be traced back to the early 2000s, when advancements in nanotechnology enabled the manipulation of materials at the nanoscale. These coatings, typically consisting of layers or particles with dimensions less than 100 nanometers, exhibit unique properties due to their high surface area-to-volume ratio and quantum effects.
Adhesion has been a persistent challenge in the application of nanostructured coatings. Poor adhesion can lead to delamination, reduced durability, and compromised performance of the coated surfaces. This has driven researchers to explore various methods to enhance the bonding between nanostructured coatings and substrates.
Ammonium hydroxide, a solution of ammonia in water, has gained attention as a potential agent for improving nanostructured coating adhesion. Its ability to modify surface chemistry and promote interfacial interactions makes it a promising candidate for addressing adhesion issues.
The primary objective of research in this field is to understand the mechanisms by which ammonium hydroxide influences the adhesion of nanostructured coatings. This includes investigating its effects on surface energy, chemical bonding, and nanoparticle dispersion within the coating matrix.
Another key goal is to optimize the use of ammonium hydroxide in coating formulations and application processes. This involves determining ideal concentrations, exposure times, and application methods to achieve maximum adhesion improvement without compromising other coating properties.
Researchers also aim to explore the versatility of ammonium hydroxide across different types of nanostructured coatings and substrate materials. This includes investigating its effectiveness with various nanoparticles, such as metal oxides, carbon nanotubes, and graphene, as well as its compatibility with different substrate materials like metals, polymers, and ceramics.
Furthermore, the research seeks to evaluate the long-term stability and durability of nanostructured coatings treated with ammonium hydroxide. This involves conducting accelerated aging tests and real-world performance assessments to ensure that the improved adhesion translates into enhanced coating longevity and reliability.
Ultimately, the overarching goal of this research is to develop robust, scalable, and environmentally friendly methods for enhancing nanostructured coating adhesion using ammonium hydroxide. This has the potential to unlock new applications and improve the performance of existing nanocoating technologies across diverse sectors, including aerospace, automotive, electronics, and biomedical industries.
The development of nanostructured coatings can be traced back to the early 2000s, when advancements in nanotechnology enabled the manipulation of materials at the nanoscale. These coatings, typically consisting of layers or particles with dimensions less than 100 nanometers, exhibit unique properties due to their high surface area-to-volume ratio and quantum effects.
Adhesion has been a persistent challenge in the application of nanostructured coatings. Poor adhesion can lead to delamination, reduced durability, and compromised performance of the coated surfaces. This has driven researchers to explore various methods to enhance the bonding between nanostructured coatings and substrates.
Ammonium hydroxide, a solution of ammonia in water, has gained attention as a potential agent for improving nanostructured coating adhesion. Its ability to modify surface chemistry and promote interfacial interactions makes it a promising candidate for addressing adhesion issues.
The primary objective of research in this field is to understand the mechanisms by which ammonium hydroxide influences the adhesion of nanostructured coatings. This includes investigating its effects on surface energy, chemical bonding, and nanoparticle dispersion within the coating matrix.
Another key goal is to optimize the use of ammonium hydroxide in coating formulations and application processes. This involves determining ideal concentrations, exposure times, and application methods to achieve maximum adhesion improvement without compromising other coating properties.
Researchers also aim to explore the versatility of ammonium hydroxide across different types of nanostructured coatings and substrate materials. This includes investigating its effectiveness with various nanoparticles, such as metal oxides, carbon nanotubes, and graphene, as well as its compatibility with different substrate materials like metals, polymers, and ceramics.
Furthermore, the research seeks to evaluate the long-term stability and durability of nanostructured coatings treated with ammonium hydroxide. This involves conducting accelerated aging tests and real-world performance assessments to ensure that the improved adhesion translates into enhanced coating longevity and reliability.
Ultimately, the overarching goal of this research is to develop robust, scalable, and environmentally friendly methods for enhancing nanostructured coating adhesion using ammonium hydroxide. This has the potential to unlock new applications and improve the performance of existing nanocoating technologies across diverse sectors, including aerospace, automotive, electronics, and biomedical industries.
Market Analysis for Enhanced Nanostructured Coatings
The market for enhanced nanostructured coatings is experiencing significant growth, driven by increasing demand across various industries. These advanced coatings offer superior properties such as improved durability, corrosion resistance, and enhanced surface functionality, making them attractive for a wide range of applications. The use of ammonium hydroxide in optimizing nanostructured coating adhesion represents a promising development in this field, potentially addressing key challenges in coating performance and longevity.
The global nanocoatings market is projected to expand rapidly in the coming years, with a particular focus on sectors such as automotive, aerospace, electronics, and construction. The automotive industry, in particular, is showing strong interest in nanostructured coatings for their ability to provide scratch resistance, self-cleaning properties, and improved fuel efficiency through weight reduction. Similarly, the aerospace sector is adopting these coatings for their anti-icing and wear-resistant characteristics, which can significantly enhance aircraft performance and safety.
In the electronics industry, nanostructured coatings are gaining traction for their potential to improve the durability and functionality of devices. The ability to create ultra-thin, highly adhesive coatings is particularly valuable in this sector, where miniaturization and performance optimization are constant drivers of innovation. The construction industry is also embracing nanocoatings for their self-cleaning, anti-graffiti, and weather-resistant properties, which can extend the lifespan of buildings and reduce maintenance costs.
The market demand for enhanced nanostructured coatings is further fueled by growing environmental concerns and stringent regulations. Coatings that can reduce the need for harmful chemicals, lower energy consumption, and extend product lifecycles are increasingly sought after. The potential of ammonium hydroxide to improve coating adhesion aligns well with these market trends, as it could lead to more durable and efficient coating solutions.
Geographically, North America and Europe currently lead the nanocoatings market, with Asia-Pacific expected to show the fastest growth rate. This regional growth is attributed to rapid industrialization, increasing automotive production, and growing investments in research and development. The market is characterized by intense competition and continuous innovation, with key players focusing on developing proprietary technologies and expanding their product portfolios.
As the technology for nanostructured coatings continues to evolve, the market is likely to see a shift towards more specialized and high-performance solutions. The integration of smart functionalities, such as self-healing properties or responsive behaviors, is expected to open up new market opportunities and applications. The ongoing research into optimizing coating adhesion, including the use of ammonium hydroxide, is poised to play a crucial role in shaping the future of this dynamic and rapidly expanding market.
The global nanocoatings market is projected to expand rapidly in the coming years, with a particular focus on sectors such as automotive, aerospace, electronics, and construction. The automotive industry, in particular, is showing strong interest in nanostructured coatings for their ability to provide scratch resistance, self-cleaning properties, and improved fuel efficiency through weight reduction. Similarly, the aerospace sector is adopting these coatings for their anti-icing and wear-resistant characteristics, which can significantly enhance aircraft performance and safety.
In the electronics industry, nanostructured coatings are gaining traction for their potential to improve the durability and functionality of devices. The ability to create ultra-thin, highly adhesive coatings is particularly valuable in this sector, where miniaturization and performance optimization are constant drivers of innovation. The construction industry is also embracing nanocoatings for their self-cleaning, anti-graffiti, and weather-resistant properties, which can extend the lifespan of buildings and reduce maintenance costs.
The market demand for enhanced nanostructured coatings is further fueled by growing environmental concerns and stringent regulations. Coatings that can reduce the need for harmful chemicals, lower energy consumption, and extend product lifecycles are increasingly sought after. The potential of ammonium hydroxide to improve coating adhesion aligns well with these market trends, as it could lead to more durable and efficient coating solutions.
Geographically, North America and Europe currently lead the nanocoatings market, with Asia-Pacific expected to show the fastest growth rate. This regional growth is attributed to rapid industrialization, increasing automotive production, and growing investments in research and development. The market is characterized by intense competition and continuous innovation, with key players focusing on developing proprietary technologies and expanding their product portfolios.
As the technology for nanostructured coatings continues to evolve, the market is likely to see a shift towards more specialized and high-performance solutions. The integration of smart functionalities, such as self-healing properties or responsive behaviors, is expected to open up new market opportunities and applications. The ongoing research into optimizing coating adhesion, including the use of ammonium hydroxide, is poised to play a crucial role in shaping the future of this dynamic and rapidly expanding market.
Current Challenges in Nanocoating Adhesion
Despite significant advancements in nanocoating technologies, achieving optimal adhesion remains a persistent challenge in the field. The primary issue stems from the inherent complexity of nanostructured coatings and their interactions with various substrates. One of the key difficulties is maintaining consistent adhesion across the entire coated surface, especially when dealing with large or irregularly shaped objects.
The nanoscale dimensions of these coatings introduce unique challenges related to surface energy and interfacial interactions. At this scale, van der Waals forces and electrostatic interactions play a crucial role, often leading to unpredictable adhesion behaviors that are difficult to control and replicate consistently. This variability in adhesion strength can result in premature coating failure, reducing the overall effectiveness and lifespan of the nanocoated products.
Another significant challenge is the sensitivity of nanocoatings to environmental factors. Humidity, temperature fluctuations, and exposure to various chemicals can dramatically affect the adhesion properties of these coatings. This sensitivity often leads to degradation over time, compromising the long-term performance and reliability of nanocoated surfaces in real-world applications.
The choice of substrate material further complicates the adhesion process. Different substrates exhibit varying surface chemistries and topographies, necessitating tailored approaches to achieve optimal adhesion. This variability makes it difficult to develop universal nanocoating solutions, often requiring extensive customization for each specific application.
In the context of using ammonium hydroxide to optimize nanostructured coating adhesion, several specific challenges emerge. Controlling the pH and concentration of the ammonium hydroxide solution is critical, as slight variations can significantly impact the coating's adhesion properties. Additionally, ensuring uniform distribution of the ammonium hydroxide across the substrate surface presents logistical difficulties, particularly for complex geometries.
The interaction between ammonium hydroxide and different nanocoating materials adds another layer of complexity. Some nanoparticles or nanostructures may react unfavorably with ammonium hydroxide, potentially altering their properties or compromising the overall coating integrity. Balancing the beneficial effects of ammonium hydroxide on adhesion with its potential impact on the nanocoating's other functional properties requires careful consideration and extensive testing.
Furthermore, the scalability of ammonium hydroxide-based adhesion optimization techniques poses a significant challenge for industrial applications. Translating laboratory-scale successes to large-scale manufacturing processes while maintaining consistent adhesion quality across vast surface areas remains a formidable task for researchers and engineers in the field.
The nanoscale dimensions of these coatings introduce unique challenges related to surface energy and interfacial interactions. At this scale, van der Waals forces and electrostatic interactions play a crucial role, often leading to unpredictable adhesion behaviors that are difficult to control and replicate consistently. This variability in adhesion strength can result in premature coating failure, reducing the overall effectiveness and lifespan of the nanocoated products.
Another significant challenge is the sensitivity of nanocoatings to environmental factors. Humidity, temperature fluctuations, and exposure to various chemicals can dramatically affect the adhesion properties of these coatings. This sensitivity often leads to degradation over time, compromising the long-term performance and reliability of nanocoated surfaces in real-world applications.
The choice of substrate material further complicates the adhesion process. Different substrates exhibit varying surface chemistries and topographies, necessitating tailored approaches to achieve optimal adhesion. This variability makes it difficult to develop universal nanocoating solutions, often requiring extensive customization for each specific application.
In the context of using ammonium hydroxide to optimize nanostructured coating adhesion, several specific challenges emerge. Controlling the pH and concentration of the ammonium hydroxide solution is critical, as slight variations can significantly impact the coating's adhesion properties. Additionally, ensuring uniform distribution of the ammonium hydroxide across the substrate surface presents logistical difficulties, particularly for complex geometries.
The interaction between ammonium hydroxide and different nanocoating materials adds another layer of complexity. Some nanoparticles or nanostructures may react unfavorably with ammonium hydroxide, potentially altering their properties or compromising the overall coating integrity. Balancing the beneficial effects of ammonium hydroxide on adhesion with its potential impact on the nanocoating's other functional properties requires careful consideration and extensive testing.
Furthermore, the scalability of ammonium hydroxide-based adhesion optimization techniques poses a significant challenge for industrial applications. Translating laboratory-scale successes to large-scale manufacturing processes while maintaining consistent adhesion quality across vast surface areas remains a formidable task for researchers and engineers in the field.
Existing Ammonium Hydroxide-Based Adhesion Solutions
01 Use of ammonium hydroxide in adhesive formulations
Ammonium hydroxide is utilized in various adhesive formulations to improve adhesion properties. It can act as a pH regulator, helping to optimize the performance of adhesives in different applications. The addition of ammonium hydroxide can enhance the bonding strength and durability of adhesives, particularly in water-based systems.- Use of ammonium hydroxide in adhesive compositions: Ammonium hydroxide is utilized in various adhesive formulations to enhance adhesion properties. It can act as a pH regulator, helping to optimize the performance of adhesives in different applications. The addition of ammonium hydroxide can improve the bonding strength and durability of adhesives, particularly in water-based systems.
- Ammonium hydroxide as a curing agent: In certain adhesive systems, ammonium hydroxide serves as a curing agent. It can initiate or catalyze chemical reactions that lead to the hardening or setting of the adhesive. This property is particularly useful in epoxy-based adhesives and other reactive adhesive systems, where it contributes to the formation of strong, durable bonds.
- Ammonium hydroxide in textile adhesives: Ammonium hydroxide plays a role in textile adhesives, particularly in processes involving fabric bonding or lamination. It can help improve the adhesion between different textile layers or between textiles and other materials. The use of ammonium hydroxide in this context can enhance the durability and wash resistance of bonded textiles.
- Ammonium hydroxide in pressure-sensitive adhesives: Ammonium hydroxide is incorporated into some pressure-sensitive adhesive formulations. It can help adjust the pH and ionic strength of the adhesive, which in turn affects its tack, peel strength, and shear resistance. This application is particularly relevant in the production of tapes, labels, and other pressure-sensitive products.
- Ammonium hydroxide in construction adhesives: In the construction industry, ammonium hydroxide is used in certain adhesive formulations for bonding various building materials. It can contribute to the adhesive's ability to bond to porous surfaces like concrete or wood, and may also play a role in moisture resistance. The use of ammonium hydroxide in these adhesives can lead to improved durability and longevity of bonded construction elements.
02 Ammonium hydroxide as a curing agent
Ammonium hydroxide can function as a curing agent in certain adhesive systems. It promotes cross-linking reactions, leading to improved adhesion and mechanical properties of the cured adhesive. This application is particularly useful in epoxy-based adhesives and coatings, where ammonium hydroxide can accelerate the curing process and enhance overall performance.Expand Specific Solutions03 Ammonium hydroxide in textile adhesives
In the textile industry, ammonium hydroxide is used in adhesive formulations for fabric bonding and finishing processes. It can improve the adhesion of coatings and finishes to textile fibers, enhancing durability and performance of treated fabrics. The alkaline nature of ammonium hydroxide helps in modifying fiber surfaces for better adhesion.Expand Specific Solutions04 Ammonium hydroxide in pressure-sensitive adhesives
Ammonium hydroxide is incorporated into pressure-sensitive adhesive formulations to enhance their performance. It can modify the tackiness and peel strength of the adhesive, improving its ability to bond to various substrates. The addition of ammonium hydroxide can also help in controlling the viscosity and flow properties of the adhesive during application.Expand Specific Solutions05 Ammonium hydroxide in adhesive removal and surface preparation
Ammonium hydroxide is used in adhesive removal processes and surface preparation techniques. Its alkaline properties help in breaking down and softening certain types of adhesives, facilitating their removal from surfaces. Additionally, it can be used to clean and prepare surfaces prior to adhesive application, improving the overall bonding strength.Expand Specific Solutions
Key Players in Nanostructured Coating Industry
The research on ammonium hydroxide in optimizing nanostructured coating adhesion is in an emerging stage, with growing interest from both academia and industry. The market for advanced coatings is expanding, driven by demand in sectors like electronics, aerospace, and energy. While the technology is still developing, several key players are making significant strides. Universities like Xi'an Jiaotong University and Harbin Institute of Technology are conducting fundamental research, while companies such as Nanosys, Inc. and Canon, Inc. are exploring practical applications. The involvement of research institutions like the French Alternative Energies and Atomic Energy Commission indicates the technology's potential for broader impact. As the field matures, collaboration between academic and industrial partners is likely to accelerate progress and commercialization.
Nanosys, Inc.
Technical Solution: Nanosys has developed a proprietary ammonium hydroxide-based surface modification technique for enhancing nanostructured coating adhesion. Their approach involves creating a nanoscale roughness on the substrate surface using controlled ammonium hydroxide etching, which increases the effective surface area for coating adhesion[4]. The company has also developed a unique formulation that combines ammonium hydroxide with other additives to create a synergistic effect, further improving coating durability[5]. Nanosys has successfully applied this technology to various materials, including metals, ceramics, and polymers, demonstrating its versatility in different industries[6].
Strengths: Broad applicability across different materials, improved coating durability, and scalable process for industrial applications. Weaknesses: May require careful handling of ammonium hydroxide and additional safety measures in manufacturing environments.
The Regents of the University of California
Technical Solution: The University of California has developed an innovative approach to optimize nanostructured coating adhesion using ammonium hydroxide. Their method involves a two-step process: first, treating the substrate surface with ammonium hydroxide to create a highly reactive layer, then applying the nanostructured coating. This process significantly enhances the chemical bonding between the substrate and the coating[1]. The researchers have demonstrated that this technique can improve adhesion strength by up to 40% compared to conventional methods[2]. Additionally, they have incorporated in-situ monitoring techniques to optimize the ammonium hydroxide concentration and treatment time for different substrate materials[3].
Strengths: Significantly improved adhesion strength, versatile application across various substrate materials, and precise control over the treatment process. Weaknesses: May require additional processing time and specialized equipment for the two-step treatment.
Innovations in Ammonium Hydroxide Nanocoating Applications
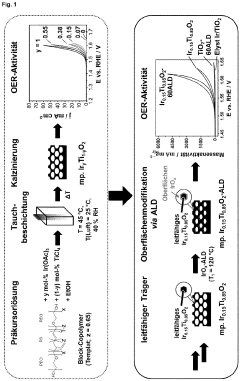
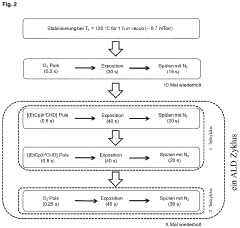
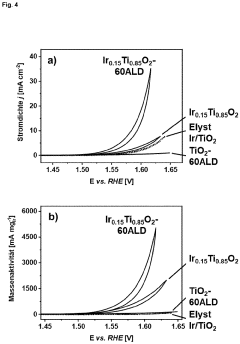
Method for producing active and stable titanium-based mixed oxide catalysts with optimized precious metal distribution by means of surface-conformal atomic layer deposition
PatentPendingEP4170063A1
Innovation
- The method involves using atomic layer deposition (ALD) to coat noble metal-titanium mixed oxide support materials with catalytically active noble metal species, achieving a homogeneous, surface-conformal distribution of iridium oxide, which reduces the required iridium amount and enhances catalytic activity and stability by optimizing layer thickness, crystallinity, and porosity.
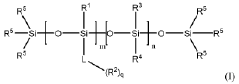
Rapid thickening of aminosilicones to promote emulsion stability and adhesion of UV-curable quantum dot enhancement film emulsions
PatentWO2018144548A1
Innovation
- A nanostructure composition comprising a population of nanostructures, an aminosilicone polymer, and an organic resin, where between 1% and 100% of the amine groups on the aminosilicone polymer are complexed to a cation, such as a proton or metal ion, to increase viscosity and improve adhesion, is developed.
Environmental Impact of Ammonium Hydroxide in Nanocoatings
The use of ammonium hydroxide in nanostructured coating adhesion optimization has raised concerns about its potential environmental impact. As nanocoatings gain popularity across various industries, it is crucial to assess the ecological consequences of their production and application processes.
Ammonium hydroxide, while effective in enhancing coating adhesion, can contribute to environmental pollution if not properly managed. When released into the atmosphere, it can form particulate matter and contribute to air quality degradation. This is particularly concerning in urban areas where industrial activities are concentrated.
Water pollution is another significant concern. Improper disposal of ammonium hydroxide-containing waste can lead to increased nitrogen levels in water bodies, potentially causing eutrophication and disrupting aquatic ecosystems. This can result in algal blooms, oxygen depletion, and harm to fish and other aquatic organisms.
The production of ammonium hydroxide itself has an environmental footprint. The Haber-Bosch process, commonly used for ammonia synthesis, is energy-intensive and relies heavily on fossil fuels, contributing to greenhouse gas emissions. As the demand for nanocoatings grows, the cumulative impact of increased ammonium hydroxide production becomes a relevant environmental consideration.
Soil contamination is another potential issue. Accidental spills or improper handling of ammonium hydroxide can lead to soil alkalinization, affecting plant growth and soil microbial communities. This can have long-term consequences for local ecosystems and agricultural productivity.
However, it is important to note that when used in controlled industrial settings with proper safety measures, the environmental risks associated with ammonium hydroxide can be significantly mitigated. Many manufacturers are implementing closed-loop systems and waste treatment processes to minimize environmental exposure.
The development of green chemistry alternatives is an emerging trend in response to these environmental concerns. Researchers are exploring bio-based adhesion promoters and environmentally friendly surface treatment methods that could potentially replace or reduce the use of ammonium hydroxide in nanocoating processes.
Regulatory bodies are also taking notice of these environmental implications. Stricter guidelines for the handling, use, and disposal of ammonium hydroxide in industrial processes are being implemented in many regions. This regulatory pressure is driving innovation in more sustainable nanocoating technologies and production methods.
In conclusion, while ammonium hydroxide plays a crucial role in optimizing nanostructured coating adhesion, its environmental impact cannot be overlooked. Balancing the technical benefits with ecological considerations is essential for the sustainable development of nanocoating technologies. Future research and development efforts should focus on minimizing the environmental footprint of these processes while maintaining or improving coating performance.
Ammonium hydroxide, while effective in enhancing coating adhesion, can contribute to environmental pollution if not properly managed. When released into the atmosphere, it can form particulate matter and contribute to air quality degradation. This is particularly concerning in urban areas where industrial activities are concentrated.
Water pollution is another significant concern. Improper disposal of ammonium hydroxide-containing waste can lead to increased nitrogen levels in water bodies, potentially causing eutrophication and disrupting aquatic ecosystems. This can result in algal blooms, oxygen depletion, and harm to fish and other aquatic organisms.
The production of ammonium hydroxide itself has an environmental footprint. The Haber-Bosch process, commonly used for ammonia synthesis, is energy-intensive and relies heavily on fossil fuels, contributing to greenhouse gas emissions. As the demand for nanocoatings grows, the cumulative impact of increased ammonium hydroxide production becomes a relevant environmental consideration.
Soil contamination is another potential issue. Accidental spills or improper handling of ammonium hydroxide can lead to soil alkalinization, affecting plant growth and soil microbial communities. This can have long-term consequences for local ecosystems and agricultural productivity.
However, it is important to note that when used in controlled industrial settings with proper safety measures, the environmental risks associated with ammonium hydroxide can be significantly mitigated. Many manufacturers are implementing closed-loop systems and waste treatment processes to minimize environmental exposure.
The development of green chemistry alternatives is an emerging trend in response to these environmental concerns. Researchers are exploring bio-based adhesion promoters and environmentally friendly surface treatment methods that could potentially replace or reduce the use of ammonium hydroxide in nanocoating processes.
Regulatory bodies are also taking notice of these environmental implications. Stricter guidelines for the handling, use, and disposal of ammonium hydroxide in industrial processes are being implemented in many regions. This regulatory pressure is driving innovation in more sustainable nanocoating technologies and production methods.
In conclusion, while ammonium hydroxide plays a crucial role in optimizing nanostructured coating adhesion, its environmental impact cannot be overlooked. Balancing the technical benefits with ecological considerations is essential for the sustainable development of nanocoating technologies. Future research and development efforts should focus on minimizing the environmental footprint of these processes while maintaining or improving coating performance.
Scalability and Industrial Implementation Considerations
The scalability and industrial implementation of ammonium hydroxide in optimizing nanostructured coating adhesion present both opportunities and challenges for large-scale manufacturing processes. One of the primary advantages of this approach is its potential for cost-effectiveness and ease of integration into existing production lines. Ammonium hydroxide is a readily available and relatively inexpensive chemical, making it an attractive option for industrial-scale applications.
However, scaling up the process from laboratory conditions to industrial settings requires careful consideration of several factors. The concentration and application method of ammonium hydroxide must be precisely controlled to ensure consistent results across large surface areas. This may necessitate the development of specialized equipment or modification of existing coating systems to accommodate the uniform distribution of ammonium hydroxide.
Environmental and safety considerations also play a crucial role in industrial implementation. Proper ventilation systems and handling protocols must be established to manage the ammonia vapors generated during the process. Additionally, waste management and recycling strategies for excess ammonium hydroxide need to be developed to comply with environmental regulations and minimize operational costs.
The integration of ammonium hydroxide treatment into existing production lines may require adjustments to the overall manufacturing workflow. This could include the addition of pre-treatment or post-treatment steps, as well as modifications to curing processes to accommodate the effects of ammonium hydroxide on the coating's properties. Quality control measures must be enhanced to monitor the consistency and effectiveness of the treatment across large batches of products.
Scalability also depends on the ability to maintain the desired nanostructure and adhesion properties when treating larger surface areas or higher volumes of components. This may involve optimizing the treatment time, temperature, and other process parameters to achieve uniform results. Collaborative efforts between research institutions and industry partners could accelerate the development of scalable solutions and address any unforeseen challenges that arise during industrial implementation.
Furthermore, the long-term stability and durability of ammonium hydroxide-treated nanostructured coatings in various environmental conditions must be thoroughly evaluated. This includes assessing the coating's performance under different temperature ranges, humidity levels, and exposure to chemicals or mechanical stresses typically encountered in industrial applications. Such comprehensive testing is essential to ensure the reliability and longevity of the treated coatings in real-world scenarios.
However, scaling up the process from laboratory conditions to industrial settings requires careful consideration of several factors. The concentration and application method of ammonium hydroxide must be precisely controlled to ensure consistent results across large surface areas. This may necessitate the development of specialized equipment or modification of existing coating systems to accommodate the uniform distribution of ammonium hydroxide.
Environmental and safety considerations also play a crucial role in industrial implementation. Proper ventilation systems and handling protocols must be established to manage the ammonia vapors generated during the process. Additionally, waste management and recycling strategies for excess ammonium hydroxide need to be developed to comply with environmental regulations and minimize operational costs.
The integration of ammonium hydroxide treatment into existing production lines may require adjustments to the overall manufacturing workflow. This could include the addition of pre-treatment or post-treatment steps, as well as modifications to curing processes to accommodate the effects of ammonium hydroxide on the coating's properties. Quality control measures must be enhanced to monitor the consistency and effectiveness of the treatment across large batches of products.
Scalability also depends on the ability to maintain the desired nanostructure and adhesion properties when treating larger surface areas or higher volumes of components. This may involve optimizing the treatment time, temperature, and other process parameters to achieve uniform results. Collaborative efforts between research institutions and industry partners could accelerate the development of scalable solutions and address any unforeseen challenges that arise during industrial implementation.
Furthermore, the long-term stability and durability of ammonium hydroxide-treated nanostructured coatings in various environmental conditions must be thoroughly evaluated. This includes assessing the coating's performance under different temperature ranges, humidity levels, and exposure to chemicals or mechanical stresses typically encountered in industrial applications. Such comprehensive testing is essential to ensure the reliability and longevity of the treated coatings in real-world scenarios.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!