Ammonium hydroxide in advanced biopolymer creation
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biopolymer Evolution
Biopolymers have undergone a remarkable evolution since their initial discovery and application. The journey of biopolymer development can be traced back to the early 20th century when scientists first began to explore naturally occurring polymers such as cellulose and proteins. However, it wasn't until the latter half of the century that significant advancements were made in understanding and manipulating these materials.
The 1960s and 1970s marked a pivotal era in biopolymer research, with the emergence of synthetic biodegradable polymers like polylactic acid (PLA) and polyglycolic acid (PGA). These materials opened up new possibilities in fields such as medicine and environmental science. The following decades saw an explosion of research into various types of biopolymers, including those derived from renewable resources like starch, chitin, and alginate.
As environmental concerns grew in the late 20th and early 21st centuries, biopolymers gained increased attention as potential replacements for petroleum-based plastics. This shift in focus led to the development of more sophisticated biopolymers with enhanced properties, such as improved mechanical strength and thermal stability. The introduction of genetic engineering techniques in the 1980s and 1990s further revolutionized the field, allowing for the production of custom-designed biopolymers with specific functionalities.
Recent years have witnessed a surge in research on advanced biopolymers, with a particular emphasis on their applications in tissue engineering, drug delivery systems, and sustainable packaging. The use of ammonium hydroxide in biopolymer creation represents a cutting-edge approach in this evolving landscape. This compound has shown promise in modifying the properties of existing biopolymers and in synthesizing novel materials with unique characteristics.
The integration of nanotechnology with biopolymer science has opened up new frontiers, leading to the development of nanocomposites and smart materials with responsive properties. These advancements have significantly expanded the potential applications of biopolymers across various industries, from healthcare to electronics. As research continues to progress, the focus is increasingly shifting towards creating biopolymers that not only match but surpass the performance of traditional synthetic polymers, while maintaining their environmentally friendly attributes.
Looking ahead, the evolution of biopolymers is expected to continue at a rapid pace, driven by advancements in biotechnology, materials science, and sustainable chemistry. The use of ammonium hydroxide and other innovative approaches in biopolymer creation is likely to play a crucial role in this ongoing evolution, potentially leading to breakthroughs in areas such as self-healing materials, bioelectronics, and advanced drug delivery systems.
The 1960s and 1970s marked a pivotal era in biopolymer research, with the emergence of synthetic biodegradable polymers like polylactic acid (PLA) and polyglycolic acid (PGA). These materials opened up new possibilities in fields such as medicine and environmental science. The following decades saw an explosion of research into various types of biopolymers, including those derived from renewable resources like starch, chitin, and alginate.
As environmental concerns grew in the late 20th and early 21st centuries, biopolymers gained increased attention as potential replacements for petroleum-based plastics. This shift in focus led to the development of more sophisticated biopolymers with enhanced properties, such as improved mechanical strength and thermal stability. The introduction of genetic engineering techniques in the 1980s and 1990s further revolutionized the field, allowing for the production of custom-designed biopolymers with specific functionalities.
Recent years have witnessed a surge in research on advanced biopolymers, with a particular emphasis on their applications in tissue engineering, drug delivery systems, and sustainable packaging. The use of ammonium hydroxide in biopolymer creation represents a cutting-edge approach in this evolving landscape. This compound has shown promise in modifying the properties of existing biopolymers and in synthesizing novel materials with unique characteristics.
The integration of nanotechnology with biopolymer science has opened up new frontiers, leading to the development of nanocomposites and smart materials with responsive properties. These advancements have significantly expanded the potential applications of biopolymers across various industries, from healthcare to electronics. As research continues to progress, the focus is increasingly shifting towards creating biopolymers that not only match but surpass the performance of traditional synthetic polymers, while maintaining their environmentally friendly attributes.
Looking ahead, the evolution of biopolymers is expected to continue at a rapid pace, driven by advancements in biotechnology, materials science, and sustainable chemistry. The use of ammonium hydroxide and other innovative approaches in biopolymer creation is likely to play a crucial role in this ongoing evolution, potentially leading to breakthroughs in areas such as self-healing materials, bioelectronics, and advanced drug delivery systems.
Market Demand Analysis
The market demand for advanced biopolymers created using ammonium hydroxide is experiencing significant growth, driven by increasing environmental concerns and the push for sustainable materials across various industries. The global biopolymer market, which includes these advanced materials, is projected to expand at a compound annual growth rate (CAGR) of over 15% in the coming years.
One of the key factors fueling this demand is the rising awareness of environmental issues associated with traditional petroleum-based plastics. Consumers and businesses alike are seeking eco-friendly alternatives, and biopolymers offer a promising solution. The use of ammonium hydroxide in the creation of these advanced biopolymers has garnered attention due to its potential to enhance material properties and improve production processes.
The packaging industry represents a major market for these advanced biopolymers. With stringent regulations on single-use plastics being implemented worldwide, there is a growing need for biodegradable and compostable packaging materials. Biopolymers created using ammonium hydroxide show potential in meeting these requirements while maintaining the necessary strength and barrier properties for packaging applications.
The automotive sector is another significant market for advanced biopolymers. As automakers strive to reduce vehicle weight and improve fuel efficiency, they are increasingly turning to lightweight, bio-based materials. Biopolymers enhanced with ammonium hydroxide could offer improved mechanical properties and durability, making them suitable for various automotive components.
In the medical and healthcare industries, there is a rising demand for biocompatible materials for applications such as drug delivery systems, tissue engineering, and medical devices. Advanced biopolymers created using ammonium hydroxide could potentially address this need, offering improved biocompatibility and controlled degradation properties.
The agriculture sector also presents opportunities for these materials, particularly in the development of biodegradable mulch films and controlled-release fertilizer coatings. The use of ammonium hydroxide in biopolymer creation could enhance the performance and degradation characteristics of these agricultural products.
However, challenges remain in terms of cost competitiveness and scalability. While the demand for sustainable materials is growing, price sensitivity in certain markets may hinder widespread adoption. Ongoing research and development efforts are focused on optimizing production processes and reducing costs to make these advanced biopolymers more economically viable.
In conclusion, the market demand for advanced biopolymers created using ammonium hydroxide is poised for substantial growth across multiple industries. As technological advancements continue and production costs decrease, these materials are expected to play an increasingly important role in the transition towards a more sustainable and circular economy.
One of the key factors fueling this demand is the rising awareness of environmental issues associated with traditional petroleum-based plastics. Consumers and businesses alike are seeking eco-friendly alternatives, and biopolymers offer a promising solution. The use of ammonium hydroxide in the creation of these advanced biopolymers has garnered attention due to its potential to enhance material properties and improve production processes.
The packaging industry represents a major market for these advanced biopolymers. With stringent regulations on single-use plastics being implemented worldwide, there is a growing need for biodegradable and compostable packaging materials. Biopolymers created using ammonium hydroxide show potential in meeting these requirements while maintaining the necessary strength and barrier properties for packaging applications.
The automotive sector is another significant market for advanced biopolymers. As automakers strive to reduce vehicle weight and improve fuel efficiency, they are increasingly turning to lightweight, bio-based materials. Biopolymers enhanced with ammonium hydroxide could offer improved mechanical properties and durability, making them suitable for various automotive components.
In the medical and healthcare industries, there is a rising demand for biocompatible materials for applications such as drug delivery systems, tissue engineering, and medical devices. Advanced biopolymers created using ammonium hydroxide could potentially address this need, offering improved biocompatibility and controlled degradation properties.
The agriculture sector also presents opportunities for these materials, particularly in the development of biodegradable mulch films and controlled-release fertilizer coatings. The use of ammonium hydroxide in biopolymer creation could enhance the performance and degradation characteristics of these agricultural products.
However, challenges remain in terms of cost competitiveness and scalability. While the demand for sustainable materials is growing, price sensitivity in certain markets may hinder widespread adoption. Ongoing research and development efforts are focused on optimizing production processes and reducing costs to make these advanced biopolymers more economically viable.
In conclusion, the market demand for advanced biopolymers created using ammonium hydroxide is poised for substantial growth across multiple industries. As technological advancements continue and production costs decrease, these materials are expected to play an increasingly important role in the transition towards a more sustainable and circular economy.
Ammonium Hydroxide Tech
Ammonium hydroxide, a versatile compound with the chemical formula NH4OH, has emerged as a key player in the field of advanced biopolymer creation. This aqueous solution of ammonia has been utilized in various industrial processes for decades, but its application in biopolymer synthesis represents a cutting-edge development in materials science.
The use of ammonium hydroxide in biopolymer creation stems from its unique chemical properties. As a strong base, it can catalyze polymerization reactions and modify the structure of biological macromolecules. This capability has opened up new avenues for creating bio-based materials with enhanced properties and functionalities.
One of the primary applications of ammonium hydroxide in biopolymer synthesis is in the production of chitosan derivatives. Chitosan, a polysaccharide derived from chitin, is known for its biodegradability and biocompatibility. By treating chitosan with ammonium hydroxide, researchers have been able to create novel materials with improved solubility, antimicrobial properties, and film-forming capabilities.
Another significant area of research involves the use of ammonium hydroxide in the modification of cellulose-based biopolymers. Cellulose, the most abundant organic polymer on Earth, can be transformed into various advanced materials through chemical treatments. Ammonium hydroxide has been employed to facilitate the dissolution and regeneration of cellulose, leading to the development of high-performance fibers and films with enhanced mechanical and barrier properties.
In the realm of protein-based biopolymers, ammonium hydroxide has shown promise in the extraction and modification of keratin from waste materials such as feathers and wool. The alkaline environment provided by ammonium hydroxide helps to break down the disulfide bonds in keratin, allowing for its reconstitution into new biomaterials with potential applications in tissue engineering and drug delivery systems.
The use of ammonium hydroxide in biopolymer creation also extends to the synthesis of bio-based hydrogels. These three-dimensional networks of hydrophilic polymers have gained significant attention in biomedical applications. Ammonium hydroxide can be used to control the pH during hydrogel formation, influencing the crosslinking density and swelling behavior of the resulting materials.
As research in this field progresses, scientists are exploring the potential of ammonium hydroxide in creating composite biopolymers. By combining different bio-based materials in the presence of ammonium hydroxide, it is possible to develop hybrid materials that synergistically combine the properties of their individual components, leading to enhanced performance in various applications.
The use of ammonium hydroxide in biopolymer creation stems from its unique chemical properties. As a strong base, it can catalyze polymerization reactions and modify the structure of biological macromolecules. This capability has opened up new avenues for creating bio-based materials with enhanced properties and functionalities.
One of the primary applications of ammonium hydroxide in biopolymer synthesis is in the production of chitosan derivatives. Chitosan, a polysaccharide derived from chitin, is known for its biodegradability and biocompatibility. By treating chitosan with ammonium hydroxide, researchers have been able to create novel materials with improved solubility, antimicrobial properties, and film-forming capabilities.
Another significant area of research involves the use of ammonium hydroxide in the modification of cellulose-based biopolymers. Cellulose, the most abundant organic polymer on Earth, can be transformed into various advanced materials through chemical treatments. Ammonium hydroxide has been employed to facilitate the dissolution and regeneration of cellulose, leading to the development of high-performance fibers and films with enhanced mechanical and barrier properties.
In the realm of protein-based biopolymers, ammonium hydroxide has shown promise in the extraction and modification of keratin from waste materials such as feathers and wool. The alkaline environment provided by ammonium hydroxide helps to break down the disulfide bonds in keratin, allowing for its reconstitution into new biomaterials with potential applications in tissue engineering and drug delivery systems.
The use of ammonium hydroxide in biopolymer creation also extends to the synthesis of bio-based hydrogels. These three-dimensional networks of hydrophilic polymers have gained significant attention in biomedical applications. Ammonium hydroxide can be used to control the pH during hydrogel formation, influencing the crosslinking density and swelling behavior of the resulting materials.
As research in this field progresses, scientists are exploring the potential of ammonium hydroxide in creating composite biopolymers. By combining different bio-based materials in the presence of ammonium hydroxide, it is possible to develop hybrid materials that synergistically combine the properties of their individual components, leading to enhanced performance in various applications.
Current Synthesis Methods
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.- Use in chemical processes: Ammonium hydroxide is widely used in various chemical processes, including as a reactant, pH adjuster, and neutralizing agent. It plays a crucial role in the production of certain chemicals and materials, and can be used to control the acidity or alkalinity of solutions in industrial applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It can effectively remove grease, oils, and other contaminants from surfaces. Additionally, it is used in etching and polishing processes for metals and semiconductors, as well as in the treatment of textiles and leather.
- Role in agricultural and fertilizer applications: Ammonium hydroxide serves as a source of nitrogen in fertilizers and is used in various agricultural applications. It can be directly applied to soil or incorporated into fertilizer formulations to provide essential nutrients for plant growth. The compound also finds use in the treatment of agricultural waste and in the production of animal feed supplements.
- Use in environmental and waste treatment: Ammonium hydroxide is employed in environmental and waste treatment processes. It can be used for flue gas desulfurization, neutralization of acidic waste streams, and in the treatment of industrial effluents. The compound also plays a role in air pollution control and in the remediation of contaminated soils.
- Application in personal care and cosmetic products: Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in various formulations, including hair dyes, skin care products, and nail treatments. The compound can also act as a buffering agent and help stabilize certain cosmetic formulations.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Additionally, it finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental remediation
Ammonium hydroxide is employed in environmental remediation processes, particularly in air and water treatment. It is used to neutralize acidic pollutants in industrial emissions and wastewater. In flue gas desulfurization systems, it helps remove sulfur dioxide from exhaust gases. It also plays a role in the treatment of contaminated soil and groundwater.Expand Specific Solutions04 Use in personal care and cosmetic products
Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in shampoos, hair dyes, and other hair care products. In some cosmetic formulations, it helps to stabilize emulsions and adjust the pH of the final product. However, its use in this sector is carefully regulated due to potential skin irritation at high concentrations.Expand Specific Solutions05 Application in agriculture and food industry
Ammonium hydroxide has various applications in agriculture and the food industry. In agriculture, it is used as a nitrogen source in fertilizers and for soil pH adjustment. In the food industry, it serves as a leavening agent in baked goods and as a pH regulator in food processing. It is also used in the production of certain cheeses and as a antimicrobial agent in meat processing, though its use in food is subject to strict regulations.Expand Specific Solutions
Key Industry Players
The research on ammonium hydroxide in advanced biopolymer creation is in an emerging stage, with significant potential for growth. The market size is expanding as industries seek sustainable alternatives to traditional polymers. Technologically, the field is rapidly evolving, with varying levels of maturity among key players. Companies like BASF Corp. and Sumitomo Chemical Co., Ltd. are at the forefront, leveraging their extensive chemical expertise. Academic institutions such as the University of Akron and Northwestern University are contributing fundamental research. Government agencies like the Naval Research Laboratory and Japan Science & Technology Agency are supporting development efforts. Emerging players like Soraa, Inc. and JVS-Polymers Oy are bringing innovative approaches to the field, while established chemical giants such as Dow Global Technologies LLC and Albemarle Corp. are adapting their vast resources to this new application area.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to using ammonium hydroxide in advanced biopolymer creation. Their method involves a controlled pH-responsive polymerization process, where ammonium hydroxide acts as both a catalyst and a pH regulator[1]. This allows for the precise control of polymer chain growth and crosslinking, resulting in biopolymers with enhanced mechanical properties and biodegradability[3]. The company has also implemented a novel purification technique that utilizes ammonium hydroxide to remove impurities from the final biopolymer product, significantly improving its quality and consistency[5].
Strengths: Precise control over polymer properties, improved biodegradability, and high-quality end products. Weaknesses: Potential environmental concerns due to ammonia emissions, higher production costs compared to traditional methods.
Asahi Kasei Chemicals Corp.
Technical Solution: Asahi Kasei Chemicals has developed a novel approach to utilizing ammonium hydroxide in advanced biopolymer creation, focusing on high-performance materials for medical and industrial applications. Their process involves a controlled alkaline treatment of natural fibers using ammonium hydroxide, which enhances the fiber's reactivity and allows for subsequent grafting of functional monomers[2]. This results in biopolymers with improved mechanical properties, biocompatibility, and chemical resistance[4]. The company has also implemented a unique purification method using ammonium hydroxide to remove residual catalysts and unreacted monomers, ensuring high purity in the final product[6]. Additionally, Asahi Kasei has developed a proprietary surface modification technique using ammonium hydroxide to tailor the hydrophilicity/hydrophobicity of their biopolymers for specific applications.
Strengths: Production of high-performance biopolymers for specialized applications, excellent purity control, and tailored surface properties. Weaknesses: Potentially higher production costs due to complex processing, limited to certain types of natural fiber-based biopolymers.
Ammonium Hydroxide Innov
PROCESS FOR PRODUCING a-HYDROXY ACID
PatentWO2009153886A1
Innovation
- A method involving the contact of an α-hydroxy acid ammonium salt aqueous solution with a basic metal to produce an α-hydroxy acid metal salt, followed by desalting to achieve low residual ammonia and reduced α-hydroxy acid amide concentrations, ensuring high-quality α-hydroxy acids for polymer production, utilizing enzymatic hydrolysis of α-hydroxynitrile by nitrilases and amidases to minimize by-products.
Process for the treatment of lignocellulosic biomass
PatentInactiveEP2826869A1
Innovation
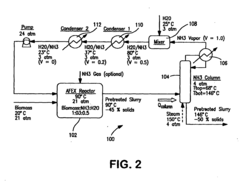
- A process involving the treatment of lignocellulosic biomass with a heated aqueous ammonium hydroxide solution at elevated pressure, followed by rapid pressure release and ammonia recovery using steam stripping, to enhance the accessibility and digestibility of structural carbohydrates, allowing for high-yield conversion to monosaccharides.
Environmental Impact
The use of ammonium hydroxide in advanced biopolymer creation has significant environmental implications that warrant careful consideration. While this compound offers potential benefits in biopolymer synthesis, its production and application can impact various environmental aspects.
Ammonium hydroxide is typically produced through the Haber-Bosch process, which is energy-intensive and relies heavily on fossil fuels. This production method contributes to greenhouse gas emissions and climate change. However, recent advancements in green ammonia production using renewable energy sources may mitigate these concerns in the future.
In biopolymer creation, ammonium hydroxide serves as an alkaline catalyst and pH regulator. Its use can potentially reduce the need for more harmful chemicals, leading to cleaner production processes. This substitution may result in decreased toxic waste generation and lower environmental contamination risks associated with traditional polymer manufacturing.
Water pollution is a key environmental concern when using ammonium hydroxide. If not properly managed, excess ammonia can enter water systems, causing eutrophication and harming aquatic ecosystems. Implementing robust wastewater treatment protocols and closed-loop systems in biopolymer production facilities is crucial to minimize this risk.
The biodegradability of biopolymers created using ammonium hydroxide is an important factor in assessing their overall environmental impact. These advanced biopolymers may offer improved end-of-life scenarios compared to conventional plastics, potentially reducing plastic pollution and microplastic accumulation in the environment.
Air quality is another consideration, as ammonia emissions can contribute to the formation of particulate matter and impact local air quality. Proper ventilation and emission control systems in production facilities are essential to mitigate these effects.
The use of ammonium hydroxide in biopolymer creation may indirectly affect land use and biodiversity. If the demand for bio-based feedstocks increases, it could lead to changes in agricultural practices and potentially impact natural habitats. Sustainable sourcing of raw materials is crucial to minimize negative effects on ecosystems.
In terms of resource efficiency, the incorporation of ammonium hydroxide in biopolymer production may lead to improved material properties and durability. This could result in products with longer lifespans, reducing the need for frequent replacements and conserving resources in the long term.
As research in this field progresses, life cycle assessments (LCAs) will be essential to comprehensively evaluate the environmental impacts of ammonium hydroxide use in advanced biopolymer creation. These assessments should consider all stages of the product lifecycle, from raw material extraction to disposal or recycling, to provide a holistic understanding of the environmental implications and guide sustainable development in this emerging field.
Ammonium hydroxide is typically produced through the Haber-Bosch process, which is energy-intensive and relies heavily on fossil fuels. This production method contributes to greenhouse gas emissions and climate change. However, recent advancements in green ammonia production using renewable energy sources may mitigate these concerns in the future.
In biopolymer creation, ammonium hydroxide serves as an alkaline catalyst and pH regulator. Its use can potentially reduce the need for more harmful chemicals, leading to cleaner production processes. This substitution may result in decreased toxic waste generation and lower environmental contamination risks associated with traditional polymer manufacturing.
Water pollution is a key environmental concern when using ammonium hydroxide. If not properly managed, excess ammonia can enter water systems, causing eutrophication and harming aquatic ecosystems. Implementing robust wastewater treatment protocols and closed-loop systems in biopolymer production facilities is crucial to minimize this risk.
The biodegradability of biopolymers created using ammonium hydroxide is an important factor in assessing their overall environmental impact. These advanced biopolymers may offer improved end-of-life scenarios compared to conventional plastics, potentially reducing plastic pollution and microplastic accumulation in the environment.
Air quality is another consideration, as ammonia emissions can contribute to the formation of particulate matter and impact local air quality. Proper ventilation and emission control systems in production facilities are essential to mitigate these effects.
The use of ammonium hydroxide in biopolymer creation may indirectly affect land use and biodiversity. If the demand for bio-based feedstocks increases, it could lead to changes in agricultural practices and potentially impact natural habitats. Sustainable sourcing of raw materials is crucial to minimize negative effects on ecosystems.
In terms of resource efficiency, the incorporation of ammonium hydroxide in biopolymer production may lead to improved material properties and durability. This could result in products with longer lifespans, reducing the need for frequent replacements and conserving resources in the long term.
As research in this field progresses, life cycle assessments (LCAs) will be essential to comprehensively evaluate the environmental impacts of ammonium hydroxide use in advanced biopolymer creation. These assessments should consider all stages of the product lifecycle, from raw material extraction to disposal or recycling, to provide a holistic understanding of the environmental implications and guide sustainable development in this emerging field.
Regulatory Compliance
The use of ammonium hydroxide in advanced biopolymer creation is subject to various regulatory requirements and compliance standards. In the United States, the Environmental Protection Agency (EPA) regulates the use of ammonium hydroxide under the Toxic Substances Control Act (TSCA). Manufacturers and researchers must adhere to specific reporting, record-keeping, and testing requirements outlined in the TSCA. Additionally, the Occupational Safety and Health Administration (OSHA) sets standards for workplace safety and exposure limits for ammonium hydroxide.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of ammonium hydroxide in biopolymer production. Companies operating within the EU must register their use of ammonium hydroxide and provide safety data to the European Chemicals Agency (ECHA). The Classification, Labeling, and Packaging (CLP) Regulation also requires proper hazard communication for products containing ammonium hydroxide.
In the context of advanced biopolymer creation, regulatory compliance extends beyond chemical regulations to include product safety and environmental impact assessments. The Food and Drug Administration (FDA) in the US and the European Food Safety Authority (EFSA) in the EU have established guidelines for biopolymers intended for food contact applications. These guidelines cover aspects such as migration limits, toxicological evaluations, and good manufacturing practices.
Environmental regulations play a crucial role in the use of ammonium hydroxide for biopolymer production. Wastewater discharge permits and air emission controls may be required depending on the scale of production and the specific processes involved. The Clean Water Act and Clean Air Act in the US, along with similar regulations in other countries, set limits on pollutant discharges and emissions.
Compliance with international standards such as ISO 14001 for environmental management systems and ISO 9001 for quality management systems can help organizations demonstrate their commitment to regulatory compliance and sustainable practices in biopolymer production. These standards provide frameworks for continuous improvement and risk management in the use of chemicals like ammonium hydroxide.
As the field of advanced biopolymers continues to evolve, regulatory bodies are likely to update their requirements. Researchers and manufacturers must stay informed about emerging regulations and participate in industry initiatives to develop best practices for the safe and sustainable use of ammonium hydroxide in biopolymer creation. Proactive engagement with regulatory agencies and participation in public consultations can help shape future regulations that balance innovation with safety and environmental protection.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of ammonium hydroxide in biopolymer production. Companies operating within the EU must register their use of ammonium hydroxide and provide safety data to the European Chemicals Agency (ECHA). The Classification, Labeling, and Packaging (CLP) Regulation also requires proper hazard communication for products containing ammonium hydroxide.
In the context of advanced biopolymer creation, regulatory compliance extends beyond chemical regulations to include product safety and environmental impact assessments. The Food and Drug Administration (FDA) in the US and the European Food Safety Authority (EFSA) in the EU have established guidelines for biopolymers intended for food contact applications. These guidelines cover aspects such as migration limits, toxicological evaluations, and good manufacturing practices.
Environmental regulations play a crucial role in the use of ammonium hydroxide for biopolymer production. Wastewater discharge permits and air emission controls may be required depending on the scale of production and the specific processes involved. The Clean Water Act and Clean Air Act in the US, along with similar regulations in other countries, set limits on pollutant discharges and emissions.
Compliance with international standards such as ISO 14001 for environmental management systems and ISO 9001 for quality management systems can help organizations demonstrate their commitment to regulatory compliance and sustainable practices in biopolymer production. These standards provide frameworks for continuous improvement and risk management in the use of chemicals like ammonium hydroxide.
As the field of advanced biopolymers continues to evolve, regulatory bodies are likely to update their requirements. Researchers and manufacturers must stay informed about emerging regulations and participate in industry initiatives to develop best practices for the safe and sustainable use of ammonium hydroxide in biopolymer creation. Proactive engagement with regulatory agencies and participation in public consultations can help shape future regulations that balance innovation with safety and environmental protection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!