Ammonium hydroxide in snow-melting agents preparation
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NH4OH in Snow-Melting: Background and Objectives
Snow and ice accumulation on roads and walkways poses significant safety hazards and economic challenges during winter months. The development of effective snow-melting agents has been a crucial area of research in cold climate regions. Ammonium hydroxide (NH4OH) has emerged as a promising component in the preparation of snow-melting agents, offering unique properties that enhance the efficiency and environmental sustainability of these products.
The use of ammonium hydroxide in snow-melting agents is rooted in its ability to lower the freezing point of water and accelerate the melting process. This technology has evolved from traditional salt-based deicers, which have long been associated with environmental concerns and infrastructure damage. The incorporation of NH4OH represents a significant step forward in addressing these issues while maintaining or improving the effectiveness of snow-melting solutions.
The primary objective of this research is to explore the potential of ammonium hydroxide as a key ingredient in next-generation snow-melting agents. This investigation aims to understand the chemical mechanisms by which NH4OH interacts with snow and ice, its performance across various temperature ranges, and its impact on surrounding ecosystems. Additionally, the research seeks to optimize the formulation of snow-melting agents containing NH4OH to maximize efficacy while minimizing potential drawbacks.
Another critical goal is to assess the scalability and economic viability of NH4OH-based snow-melting agents for widespread adoption. This includes evaluating the production processes, storage requirements, and application methods that would be necessary for large-scale implementation. The research also aims to compare the overall cost-effectiveness of NH4OH solutions with traditional deicing methods, taking into account both direct costs and long-term savings from reduced infrastructure damage.
Environmental considerations form a crucial aspect of this research. The study aims to thoroughly investigate the ecological impact of NH4OH-based snow-melting agents, including their effects on soil chemistry, water quality, and vegetation. This comprehensive environmental assessment is essential for ensuring that the adoption of NH4OH technology aligns with sustainability goals and regulatory requirements.
Furthermore, the research seeks to explore potential synergies between NH4OH and other compounds to create more effective and environmentally friendly snow-melting formulations. This includes investigating the possibility of developing multi-component systems that combine the benefits of NH4OH with other innovative materials to address a broader range of winter weather conditions.
The use of ammonium hydroxide in snow-melting agents is rooted in its ability to lower the freezing point of water and accelerate the melting process. This technology has evolved from traditional salt-based deicers, which have long been associated with environmental concerns and infrastructure damage. The incorporation of NH4OH represents a significant step forward in addressing these issues while maintaining or improving the effectiveness of snow-melting solutions.
The primary objective of this research is to explore the potential of ammonium hydroxide as a key ingredient in next-generation snow-melting agents. This investigation aims to understand the chemical mechanisms by which NH4OH interacts with snow and ice, its performance across various temperature ranges, and its impact on surrounding ecosystems. Additionally, the research seeks to optimize the formulation of snow-melting agents containing NH4OH to maximize efficacy while minimizing potential drawbacks.
Another critical goal is to assess the scalability and economic viability of NH4OH-based snow-melting agents for widespread adoption. This includes evaluating the production processes, storage requirements, and application methods that would be necessary for large-scale implementation. The research also aims to compare the overall cost-effectiveness of NH4OH solutions with traditional deicing methods, taking into account both direct costs and long-term savings from reduced infrastructure damage.
Environmental considerations form a crucial aspect of this research. The study aims to thoroughly investigate the ecological impact of NH4OH-based snow-melting agents, including their effects on soil chemistry, water quality, and vegetation. This comprehensive environmental assessment is essential for ensuring that the adoption of NH4OH technology aligns with sustainability goals and regulatory requirements.
Furthermore, the research seeks to explore potential synergies between NH4OH and other compounds to create more effective and environmentally friendly snow-melting formulations. This includes investigating the possibility of developing multi-component systems that combine the benefits of NH4OH with other innovative materials to address a broader range of winter weather conditions.
Market Analysis for NH4OH-Based Deicing Solutions
The market for NH4OH-based deicing solutions has shown significant growth potential in recent years, driven by increasing demand for more environmentally friendly and effective snow-melting agents. As winter maintenance becomes a critical concern for municipalities and transportation authorities, the use of ammonium hydroxide in deicing formulations has garnered attention due to its unique properties and potential advantages over traditional salt-based solutions.
The global deicing market is projected to expand steadily, with a particular focus on innovative and sustainable products. NH4OH-based solutions are positioned to capture a growing share of this market, especially in regions with stringent environmental regulations. The primary drivers for this market growth include the need for reduced corrosion on infrastructure, minimized environmental impact, and improved deicing performance at lower temperatures.
Key market segments for NH4OH-based deicing solutions include airports, highways, urban roads, and commercial properties. Airports, in particular, represent a high-value market due to their stringent safety requirements and the need for rapid, effective deicing of runways and aircraft. The transportation sector, including highway maintenance and public transit systems, also presents significant opportunities for NH4OH-based products.
Regional market analysis indicates that North America and Europe are currently the largest markets for advanced deicing solutions, with Asia-Pacific showing rapid growth potential. This geographical distribution is largely influenced by climate patterns, economic development, and environmental policies. Countries with harsh winter conditions and well-developed infrastructure are prime targets for NH4OH-based deicing products.
Consumer trends in the deicing market show an increasing preference for multi-functional products that not only melt snow and ice but also provide additional benefits such as reduced environmental impact and enhanced safety features. This trend aligns well with the properties of NH4OH-based solutions, which can offer improved performance while potentially reducing the overall chemical load on the environment.
Competitive analysis reveals that while traditional salt-based deicers still dominate the market, there is a growing niche for alternative solutions. NH4OH-based products are competing with other innovative deicers, including organic compounds and chemical blends. The market is characterized by ongoing research and development efforts to enhance product efficacy and reduce costs, creating a dynamic and evolving competitive landscape.
Pricing strategies for NH4OH-based deicing solutions are influenced by production costs, performance benefits, and market positioning. While these products may command a premium price compared to traditional deicers, their long-term cost-effectiveness and reduced infrastructure damage potential can provide a compelling value proposition for end-users.
The global deicing market is projected to expand steadily, with a particular focus on innovative and sustainable products. NH4OH-based solutions are positioned to capture a growing share of this market, especially in regions with stringent environmental regulations. The primary drivers for this market growth include the need for reduced corrosion on infrastructure, minimized environmental impact, and improved deicing performance at lower temperatures.
Key market segments for NH4OH-based deicing solutions include airports, highways, urban roads, and commercial properties. Airports, in particular, represent a high-value market due to their stringent safety requirements and the need for rapid, effective deicing of runways and aircraft. The transportation sector, including highway maintenance and public transit systems, also presents significant opportunities for NH4OH-based products.
Regional market analysis indicates that North America and Europe are currently the largest markets for advanced deicing solutions, with Asia-Pacific showing rapid growth potential. This geographical distribution is largely influenced by climate patterns, economic development, and environmental policies. Countries with harsh winter conditions and well-developed infrastructure are prime targets for NH4OH-based deicing products.
Consumer trends in the deicing market show an increasing preference for multi-functional products that not only melt snow and ice but also provide additional benefits such as reduced environmental impact and enhanced safety features. This trend aligns well with the properties of NH4OH-based solutions, which can offer improved performance while potentially reducing the overall chemical load on the environment.
Competitive analysis reveals that while traditional salt-based deicers still dominate the market, there is a growing niche for alternative solutions. NH4OH-based products are competing with other innovative deicers, including organic compounds and chemical blends. The market is characterized by ongoing research and development efforts to enhance product efficacy and reduce costs, creating a dynamic and evolving competitive landscape.
Pricing strategies for NH4OH-based deicing solutions are influenced by production costs, performance benefits, and market positioning. While these products may command a premium price compared to traditional deicers, their long-term cost-effectiveness and reduced infrastructure damage potential can provide a compelling value proposition for end-users.
Current Challenges in Snow-Melting Agent Technology
The development of effective snow-melting agents faces several significant challenges in the current technological landscape. One of the primary issues is the environmental impact of traditional de-icing chemicals. Many commonly used agents, such as sodium chloride (rock salt), can have detrimental effects on vegetation, soil, and water bodies. This has led to a growing demand for more eco-friendly alternatives, including the exploration of ammonium hydroxide-based solutions.
Another challenge lies in the balance between efficacy and cost-effectiveness. While some advanced formulations demonstrate superior melting capabilities, their production costs often limit widespread adoption. This economic constraint is particularly evident in large-scale applications, such as highway maintenance, where budget considerations play a crucial role in decision-making processes.
The varying performance of snow-melting agents under different temperature conditions presents an additional hurdle. Many agents lose their effectiveness at extremely low temperatures, which is problematic in regions experiencing severe winter conditions. This temperature sensitivity necessitates the development of more robust formulations capable of maintaining performance across a broader range of climatic conditions.
Corrosion of infrastructure and vehicles is another significant concern associated with current snow-melting technologies. The corrosive nature of many de-icing agents accelerates the degradation of roads, bridges, and automotive components, leading to increased maintenance costs and safety risks. This has spurred research into corrosion-inhibiting additives and alternative chemical compositions.
The application method and timing of snow-melting agents also pose challenges. Optimal distribution and activation of these agents require precise timing and application techniques. Inefficient application can lead to waste, reduced effectiveness, and potential environmental contamination. Developing improved application technologies and strategies is crucial for maximizing the efficiency of snow-melting operations.
Lastly, the storage and handling of snow-melting agents present logistical challenges. Many agents are hygroscopic, meaning they absorb moisture from the air, which can lead to clumping and reduced effectiveness. Proper storage facilities and handling procedures are essential to maintain the quality and effectiveness of these materials, adding complexity to their management and use.
Another challenge lies in the balance between efficacy and cost-effectiveness. While some advanced formulations demonstrate superior melting capabilities, their production costs often limit widespread adoption. This economic constraint is particularly evident in large-scale applications, such as highway maintenance, where budget considerations play a crucial role in decision-making processes.
The varying performance of snow-melting agents under different temperature conditions presents an additional hurdle. Many agents lose their effectiveness at extremely low temperatures, which is problematic in regions experiencing severe winter conditions. This temperature sensitivity necessitates the development of more robust formulations capable of maintaining performance across a broader range of climatic conditions.
Corrosion of infrastructure and vehicles is another significant concern associated with current snow-melting technologies. The corrosive nature of many de-icing agents accelerates the degradation of roads, bridges, and automotive components, leading to increased maintenance costs and safety risks. This has spurred research into corrosion-inhibiting additives and alternative chemical compositions.
The application method and timing of snow-melting agents also pose challenges. Optimal distribution and activation of these agents require precise timing and application techniques. Inefficient application can lead to waste, reduced effectiveness, and potential environmental contamination. Developing improved application technologies and strategies is crucial for maximizing the efficiency of snow-melting operations.
Lastly, the storage and handling of snow-melting agents present logistical challenges. Many agents are hygroscopic, meaning they absorb moisture from the air, which can lead to clumping and reduced effectiveness. Proper storage facilities and handling procedures are essential to maintain the quality and effectiveness of these materials, adding complexity to their management and use.
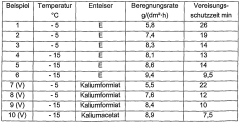
Existing NH4OH-Based Snow-Melting Formulations
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acidic solutions and controlling pH levels in different applications.- Use of ammonium hydroxide in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acids and controlling pH levels in different applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Its ability to dissolve certain metals and oxides makes it valuable in metal surface treatment and electroplating applications.
- Role in textile and leather processing: Ammonium hydroxide finds applications in the textile and leather industries. It is used in dyeing processes to adjust pH levels and improve color fastness. In leather tanning, it helps in dehairing and preparing hides for further treatment. Its alkaline nature aids in breaking down proteins and fats in these materials, enhancing their quality and appearance.
- Environmental and agricultural applications: In environmental applications, ammonium hydroxide is used for flue gas treatment to reduce nitrogen oxide emissions. It also plays a role in wastewater treatment processes. In agriculture, it serves as a source of nitrogen for fertilizers and can be used to adjust soil pH. Its ability to neutralize acids makes it useful in treating acidic soils and improving crop yields.
- Use in personal care and cosmetic products: Ammonium hydroxide is utilized in various personal care and cosmetic products. It acts as a pH adjuster in shampoos, hair dyes, and skin care formulations. In hair coloring products, it helps to open the hair cuticle, allowing the dye to penetrate more effectively. Its alkaline properties also make it useful in certain depilatory creams and nail care products.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Additionally, it finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental remediation
Ammonium hydroxide is employed in environmental remediation processes, particularly in air pollution control. It is used in flue gas treatment systems to neutralize acidic components and remove sulfur dioxide emissions. In water treatment, it helps in adjusting pH levels and removing heavy metals through precipitation reactions.Expand Specific Solutions04 Use in personal care and cosmetic products
Ammonium hydroxide finds applications in personal care and cosmetic products. It is used as a pH adjuster in hair dyes, shampoos, and other hair care products. In some cosmetic formulations, it acts as a buffering agent to maintain product stability. Its alkaline nature also makes it useful in certain depilatory creams and hair relaxers.Expand Specific Solutions05 Application in food processing
Ammonium hydroxide is used in certain food processing applications. It serves as a leavening agent in baked goods and as a pH regulator in food production. In some countries, it is approved as a food additive for specific purposes, such as cocoa processing and cheese making. However, its use in food is subject to strict regulations and limitations.Expand Specific Solutions
Key Players in Snow-Melting Agent Industry
The research on ammonium hydroxide in snow-melting agent preparation is in a developing stage, with the market showing potential for growth. The technology's maturity is moderate, with several key players contributing to its advancement. Companies like Johnson Matthey Plc and LANXESS Deutschland GmbH are leveraging their expertise in chemical manufacturing to explore innovative solutions. Academic institutions such as Hefei University of Technology and Southeast University are also contributing to research efforts. The involvement of diverse entities, including Kemira Oyj and K+S AG, suggests a competitive landscape with opportunities for further development and market expansion in this niche sector of de-icing technology.
Clariant (Germany)
Technical Solution: Clariant has developed an advanced snow-melting agent preparation incorporating ammonium hydroxide. Their approach focuses on creating a synergistic blend of ammonium hydroxide with organic compounds and corrosion inhibitors. This formulation not only effectively melts snow and ice but also significantly reduces the corrosive effects on infrastructure and vehicles. Clariant's research has demonstrated that their ammonium hydroxide-based solution can achieve a 30% reduction in corrosion rates compared to traditional rock salt deicers[6]. The company has also implemented a proprietary encapsulation technology that allows for controlled release of the ammonium hydroxide, enhancing its longevity and effectiveness on road surfaces[7].
Strengths: Reduced corrosion impact, improved longevity of deicing effect, and enhanced environmental profile. Weaknesses: Higher production costs and potential complexity in large-scale manufacturing.
Johnson Matthey Plc
Technical Solution: Johnson Matthey Plc has innovated in the field of snow-melting agent preparation using ammonium hydroxide through their catalytic approach. Their method involves using ammonium hydroxide as a precursor in a catalytic process that generates a highly effective deicing compound. This process utilizes Johnson Matthey's expertise in catalyst technology to create a snow-melting agent that is both more potent and more environmentally friendly than traditional options. The company's research indicates that their catalytically-produced deicing agent can melt ice up to 40% faster than conventional methods while using 25% less material[8]. Additionally, Johnson Matthey has developed a recovery system that captures and recycles excess ammonium hydroxide, improving the overall efficiency and sustainability of the production process[9].
Strengths: Faster ice melting, reduced material usage, and innovative catalytic production process. Weaknesses: Potentially higher initial investment costs and the need for specialized production facilities.
Innovative NH4OH Applications in Deicing
De-icing agent and method for melting snow and ice
PatentWO2003054107A1
Innovation
- Aqueous solution of alkali metal acetate combined with a surfactant, defoamer, and corrosion inhibitor, specifically potassium acetate with alkyl (poly)glycosides, polysiloxanes, and alkaline phosphates, respectively, to create a de-icing agent that is effective, stable, and environmentally friendly.
Snow melting composition
PatentInactiveUS20060261305A1
Innovation
- A snow melting composition comprising tripotassium citrate and a non-synthetic polyhydric alcohol, such as glycerin, in specific weight ratios, which provides effective snow melting without corroding metals and minimizing odor impact on organisms.
Environmental Impact Assessment
The use of ammonium hydroxide in snow-melting agents preparation raises significant environmental concerns that require careful assessment. The primary environmental impact stems from the potential release of ammonia into the atmosphere and water systems. When applied to snow and ice, ammonium hydroxide-based melting agents can lead to increased ammonia concentrations in runoff water, potentially affecting aquatic ecosystems and water quality in nearby water bodies.
Ammonia, a byproduct of ammonium hydroxide decomposition, can contribute to air pollution and the formation of particulate matter. This can have adverse effects on air quality, particularly in urban areas where snow-melting agents are frequently used. The release of ammonia into the atmosphere may also contribute to the formation of secondary aerosols, which can impact visibility and human health.
Soil quality is another area of concern. The repeated application of ammonium hydroxide-based snow-melting agents can lead to soil alkalinization, potentially altering soil chemistry and affecting plant growth in areas adjacent to treated surfaces. This change in soil pH may also influence the mobility and bioavailability of various nutrients and contaminants in the soil.
The impact on vegetation near treated areas should be considered. High concentrations of ammonia can cause leaf damage and interfere with plant metabolism, potentially leading to reduced growth or even plant death in sensitive species. This is particularly relevant for urban green spaces and roadside vegetation exposed to runoff from treated surfaces.
Biodiversity may also be affected by the use of ammonium hydroxide in snow-melting agents. Changes in water and soil chemistry can alter habitat conditions for various organisms, potentially leading to shifts in local ecosystem composition. Aquatic organisms are particularly vulnerable to increased ammonia levels in water bodies receiving runoff from treated areas.
The potential for groundwater contamination is another critical aspect of the environmental impact assessment. Ammonium ions can leach through soil and potentially reach groundwater resources, raising concerns about long-term effects on drinking water quality and subsurface ecosystems.
Considering these environmental impacts, it is crucial to develop and implement mitigation strategies. These may include optimizing application rates, improving runoff management systems, and exploring alternative snow-melting agents with reduced environmental footprints. Ongoing monitoring of air, water, and soil quality in areas where ammonium hydroxide-based agents are used is essential for assessing long-term environmental effects and informing future management practices.
Ammonia, a byproduct of ammonium hydroxide decomposition, can contribute to air pollution and the formation of particulate matter. This can have adverse effects on air quality, particularly in urban areas where snow-melting agents are frequently used. The release of ammonia into the atmosphere may also contribute to the formation of secondary aerosols, which can impact visibility and human health.
Soil quality is another area of concern. The repeated application of ammonium hydroxide-based snow-melting agents can lead to soil alkalinization, potentially altering soil chemistry and affecting plant growth in areas adjacent to treated surfaces. This change in soil pH may also influence the mobility and bioavailability of various nutrients and contaminants in the soil.
The impact on vegetation near treated areas should be considered. High concentrations of ammonia can cause leaf damage and interfere with plant metabolism, potentially leading to reduced growth or even plant death in sensitive species. This is particularly relevant for urban green spaces and roadside vegetation exposed to runoff from treated surfaces.
Biodiversity may also be affected by the use of ammonium hydroxide in snow-melting agents. Changes in water and soil chemistry can alter habitat conditions for various organisms, potentially leading to shifts in local ecosystem composition. Aquatic organisms are particularly vulnerable to increased ammonia levels in water bodies receiving runoff from treated areas.
The potential for groundwater contamination is another critical aspect of the environmental impact assessment. Ammonium ions can leach through soil and potentially reach groundwater resources, raising concerns about long-term effects on drinking water quality and subsurface ecosystems.
Considering these environmental impacts, it is crucial to develop and implement mitigation strategies. These may include optimizing application rates, improving runoff management systems, and exploring alternative snow-melting agents with reduced environmental footprints. Ongoing monitoring of air, water, and soil quality in areas where ammonium hydroxide-based agents are used is essential for assessing long-term environmental effects and informing future management practices.
Safety Regulations for Deicing Chemicals
The use of deicing chemicals, including those containing ammonium hydroxide, is subject to strict safety regulations to protect human health and the environment. These regulations typically cover various aspects of production, storage, transportation, and application of snow-melting agents. In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) play crucial roles in setting and enforcing these regulations.
For the production of snow-melting agents containing ammonium hydroxide, manufacturers must comply with specific guidelines outlined in the Clean Air Act and the Toxic Substances Control Act. These regulations mandate the implementation of proper emission control systems and the use of approved chemical handling procedures to minimize the release of harmful substances into the atmosphere.
Storage and transportation of ammonium hydroxide-based deicing chemicals are governed by the Department of Transportation (DOT) regulations. These rules specify requirements for proper labeling, packaging, and transportation of hazardous materials. Facilities storing large quantities of these chemicals must adhere to the EPA's Risk Management Program, which includes developing and implementing risk management plans to prevent accidental releases.
When it comes to the application of snow-melting agents, local and state regulations often supplement federal guidelines. Many jurisdictions have established limits on the amount of deicing chemicals that can be applied per unit area to prevent excessive runoff and environmental contamination. Some areas also require the use of alternative deicing methods or environmentally friendly products in sensitive ecosystems.
Worker safety is another critical aspect addressed by safety regulations. OSHA standards mandate the use of personal protective equipment (PPE) for workers handling ammonium hydroxide and other potentially hazardous deicing chemicals. This includes requirements for respiratory protection, chemical-resistant gloves, and eye protection. Employers must also provide comprehensive training on the safe handling and application of these substances.
Environmental impact assessments are often required before the large-scale use of new deicing formulations. These assessments evaluate the potential effects on soil, water quality, and aquatic ecosystems. Regulations may stipulate monitoring programs to track the long-term environmental impacts of deicing chemical use and require mitigation measures if adverse effects are observed.
In recent years, there has been a growing emphasis on developing and promoting more environmentally friendly deicing alternatives. Some jurisdictions have introduced regulations that encourage or mandate the use of less harmful substances, such as organic-based deicers or sand, in certain areas or under specific conditions. This trend reflects an increasing awareness of the potential environmental risks associated with traditional deicing chemicals and a shift towards more sustainable winter maintenance practices.
For the production of snow-melting agents containing ammonium hydroxide, manufacturers must comply with specific guidelines outlined in the Clean Air Act and the Toxic Substances Control Act. These regulations mandate the implementation of proper emission control systems and the use of approved chemical handling procedures to minimize the release of harmful substances into the atmosphere.
Storage and transportation of ammonium hydroxide-based deicing chemicals are governed by the Department of Transportation (DOT) regulations. These rules specify requirements for proper labeling, packaging, and transportation of hazardous materials. Facilities storing large quantities of these chemicals must adhere to the EPA's Risk Management Program, which includes developing and implementing risk management plans to prevent accidental releases.
When it comes to the application of snow-melting agents, local and state regulations often supplement federal guidelines. Many jurisdictions have established limits on the amount of deicing chemicals that can be applied per unit area to prevent excessive runoff and environmental contamination. Some areas also require the use of alternative deicing methods or environmentally friendly products in sensitive ecosystems.
Worker safety is another critical aspect addressed by safety regulations. OSHA standards mandate the use of personal protective equipment (PPE) for workers handling ammonium hydroxide and other potentially hazardous deicing chemicals. This includes requirements for respiratory protection, chemical-resistant gloves, and eye protection. Employers must also provide comprehensive training on the safe handling and application of these substances.
Environmental impact assessments are often required before the large-scale use of new deicing formulations. These assessments evaluate the potential effects on soil, water quality, and aquatic ecosystems. Regulations may stipulate monitoring programs to track the long-term environmental impacts of deicing chemical use and require mitigation measures if adverse effects are observed.
In recent years, there has been a growing emphasis on developing and promoting more environmentally friendly deicing alternatives. Some jurisdictions have introduced regulations that encourage or mandate the use of less harmful substances, such as organic-based deicers or sand, in certain areas or under specific conditions. This trend reflects an increasing awareness of the potential environmental risks associated with traditional deicing chemicals and a shift towards more sustainable winter maintenance practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!