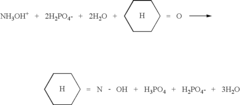
Ammonium hydroxide use in dichloromethane-free processes
AUG 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide in Green Chemistry: Background and Objectives
Ammonium hydroxide has emerged as a key player in the field of green chemistry, particularly in the context of dichloromethane-free processes. This shift towards more environmentally friendly chemical processes is driven by the growing awareness of the harmful effects of traditional solvents and the need for sustainable alternatives. The use of ammonium hydroxide in these processes represents a significant step forward in reducing the environmental impact of chemical manufacturing.
The historical context of this technological development can be traced back to the late 20th century when concerns about ozone depletion and environmental pollution began to gain prominence. Dichloromethane, a widely used solvent in various industrial processes, was identified as a potential health and environmental hazard. This realization sparked a global effort to find safer alternatives, leading to the exploration of ammonium hydroxide as a potential replacement in certain applications.
The evolution of ammonium hydroxide usage in green chemistry has been marked by several key milestones. Initially, it was primarily used in simple cleaning and neutralization processes. However, as research progressed, its potential in more complex chemical reactions became apparent. The development of novel reaction mechanisms and process designs that incorporate ammonium hydroxide has opened up new possibilities for sustainable chemical manufacturing.
The primary objective of research into ammonium hydroxide in dichloromethane-free processes is to develop efficient, cost-effective, and environmentally benign chemical processes. This involves not only replacing dichloromethane with ammonium hydroxide but also optimizing reaction conditions, improving yield, and ensuring product quality. Additionally, researchers aim to expand the range of reactions and processes where ammonium hydroxide can be effectively utilized.
Another crucial aspect of this research is to understand the fundamental chemistry of ammonium hydroxide in various reaction environments. This includes studying its behavior as a solvent, reactant, and catalyst, as well as its interactions with different substrates and reaction intermediates. Such knowledge is essential for designing new synthetic routes and improving existing processes.
The technological goals in this field extend beyond mere substitution. Researchers are working towards developing integrated systems that leverage the unique properties of ammonium hydroxide to create more efficient and sustainable chemical processes. This includes exploring its potential in continuous flow chemistry, process intensification, and the development of novel catalytic systems.
The historical context of this technological development can be traced back to the late 20th century when concerns about ozone depletion and environmental pollution began to gain prominence. Dichloromethane, a widely used solvent in various industrial processes, was identified as a potential health and environmental hazard. This realization sparked a global effort to find safer alternatives, leading to the exploration of ammonium hydroxide as a potential replacement in certain applications.
The evolution of ammonium hydroxide usage in green chemistry has been marked by several key milestones. Initially, it was primarily used in simple cleaning and neutralization processes. However, as research progressed, its potential in more complex chemical reactions became apparent. The development of novel reaction mechanisms and process designs that incorporate ammonium hydroxide has opened up new possibilities for sustainable chemical manufacturing.
The primary objective of research into ammonium hydroxide in dichloromethane-free processes is to develop efficient, cost-effective, and environmentally benign chemical processes. This involves not only replacing dichloromethane with ammonium hydroxide but also optimizing reaction conditions, improving yield, and ensuring product quality. Additionally, researchers aim to expand the range of reactions and processes where ammonium hydroxide can be effectively utilized.
Another crucial aspect of this research is to understand the fundamental chemistry of ammonium hydroxide in various reaction environments. This includes studying its behavior as a solvent, reactant, and catalyst, as well as its interactions with different substrates and reaction intermediates. Such knowledge is essential for designing new synthetic routes and improving existing processes.
The technological goals in this field extend beyond mere substitution. Researchers are working towards developing integrated systems that leverage the unique properties of ammonium hydroxide to create more efficient and sustainable chemical processes. This includes exploring its potential in continuous flow chemistry, process intensification, and the development of novel catalytic systems.
Market Demand for Dichloromethane-Free Processes
The market demand for dichloromethane-free processes has been steadily increasing in recent years, driven by growing environmental concerns and stricter regulations on the use of chlorinated solvents. Dichloromethane, also known as methylene chloride, has been widely used in various industries due to its excellent solvency properties. However, its potential health and environmental risks have led to a push for alternative processes.
The pharmaceutical industry has been at the forefront of this shift, as dichloromethane is commonly used in drug manufacturing processes. With increasing pressure from regulatory bodies and consumers for greener production methods, pharmaceutical companies are actively seeking alternatives. This has created a significant market opportunity for dichloromethane-free processes, particularly those utilizing ammonium hydroxide.
In the paint and coatings industry, there is a growing demand for water-based formulations to replace solvent-based products. This transition is partly driven by the need to reduce volatile organic compound (VOC) emissions and improve worker safety. Dichloromethane-free processes that can effectively produce high-quality coatings are highly sought after in this sector.
The electronics industry is another key market for dichloromethane-free processes. As manufacturers strive to reduce their environmental footprint and comply with regulations such as the Restriction of Hazardous Substances (RoHS) directive, there is a strong interest in alternative cleaning and degreasing methods that do not rely on chlorinated solvents.
The adhesives and sealants market is also experiencing a shift towards more environmentally friendly production methods. Manufacturers are exploring dichloromethane-free processes to develop products that meet stringent environmental standards while maintaining performance characteristics. This trend is particularly evident in the construction and automotive sectors, where there is a growing emphasis on sustainable materials.
In the polymer processing industry, there is a rising demand for safer and more sustainable solvent systems. Dichloromethane has been traditionally used in the production of polycarbonates and other polymers, but manufacturers are now looking for alternative processes that can reduce health risks and environmental impact without compromising product quality.
The market potential for dichloromethane-free processes is further amplified by the global push towards circular economy principles. Industries are increasingly focusing on developing processes that minimize waste generation and enable easier recycling of materials. Processes that utilize ammonium hydroxide or other more environmentally benign substances align well with these sustainability goals.
As awareness of the potential risks associated with dichloromethane continues to grow, consumer preferences are shifting towards products manufactured using safer, more sustainable processes. This consumer-driven demand is creating additional pressure on industries to adopt dichloromethane-free alternatives, further expanding the market opportunity for innovative solutions in this space.
The pharmaceutical industry has been at the forefront of this shift, as dichloromethane is commonly used in drug manufacturing processes. With increasing pressure from regulatory bodies and consumers for greener production methods, pharmaceutical companies are actively seeking alternatives. This has created a significant market opportunity for dichloromethane-free processes, particularly those utilizing ammonium hydroxide.
In the paint and coatings industry, there is a growing demand for water-based formulations to replace solvent-based products. This transition is partly driven by the need to reduce volatile organic compound (VOC) emissions and improve worker safety. Dichloromethane-free processes that can effectively produce high-quality coatings are highly sought after in this sector.
The electronics industry is another key market for dichloromethane-free processes. As manufacturers strive to reduce their environmental footprint and comply with regulations such as the Restriction of Hazardous Substances (RoHS) directive, there is a strong interest in alternative cleaning and degreasing methods that do not rely on chlorinated solvents.
The adhesives and sealants market is also experiencing a shift towards more environmentally friendly production methods. Manufacturers are exploring dichloromethane-free processes to develop products that meet stringent environmental standards while maintaining performance characteristics. This trend is particularly evident in the construction and automotive sectors, where there is a growing emphasis on sustainable materials.
In the polymer processing industry, there is a rising demand for safer and more sustainable solvent systems. Dichloromethane has been traditionally used in the production of polycarbonates and other polymers, but manufacturers are now looking for alternative processes that can reduce health risks and environmental impact without compromising product quality.
The market potential for dichloromethane-free processes is further amplified by the global push towards circular economy principles. Industries are increasingly focusing on developing processes that minimize waste generation and enable easier recycling of materials. Processes that utilize ammonium hydroxide or other more environmentally benign substances align well with these sustainability goals.
As awareness of the potential risks associated with dichloromethane continues to grow, consumer preferences are shifting towards products manufactured using safer, more sustainable processes. This consumer-driven demand is creating additional pressure on industries to adopt dichloromethane-free alternatives, further expanding the market opportunity for innovative solutions in this space.
Current Challenges in Ammonium Hydroxide Utilization
The utilization of ammonium hydroxide in dichloromethane-free processes faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary obstacles is the corrosive nature of ammonium hydroxide, which can lead to equipment degradation and increased maintenance costs. This corrosivity necessitates the use of specialized materials and protective coatings, adding complexity and expense to process design and implementation.
Another challenge lies in the volatility of ammonium hydroxide, which can result in ammonia off-gassing. This not only poses potential health and safety risks to workers but also raises environmental concerns. Controlling and containing these emissions requires sophisticated ventilation systems and air treatment technologies, further complicating process engineering and increasing operational costs.
The reactivity of ammonium hydroxide with certain substrates and intermediates presents additional difficulties. In some cases, undesired side reactions can occur, leading to reduced yield and product quality. This necessitates careful process optimization and the development of novel reaction pathways to minimize these unwanted interactions.
Temperature control is another critical challenge in ammonium hydroxide utilization. The solution's properties can change significantly with temperature fluctuations, affecting reaction kinetics and equilibrium. Maintaining precise temperature control throughout the process is essential but can be technically demanding and energy-intensive.
The concentration of ammonium hydroxide solutions also poses challenges. Higher concentrations are often desired for improved reaction efficiency, but they exacerbate the aforementioned issues of corrosivity and volatility. Finding the optimal balance between concentration and process safety is a delicate task that requires extensive research and testing.
Waste management and disposal of ammonium hydroxide-containing streams present environmental and regulatory challenges. The high pH and nitrogen content of these waste streams necessitate specialized treatment processes before discharge, adding to the overall complexity and cost of operations.
Lastly, the scale-up of ammonium hydroxide-based processes from laboratory to industrial scale introduces its own set of challenges. Issues that may be manageable at small scales can become significant hurdles in large-scale production, requiring innovative engineering solutions and process modifications.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, process engineering, and chemical technology. Ongoing research efforts are focused on developing novel materials resistant to ammonium hydroxide corrosion, improving containment and emission control technologies, and exploring alternative reaction pathways that minimize the drawbacks associated with ammonium hydroxide use while maintaining its benefits in dichloromethane-free processes.
Another challenge lies in the volatility of ammonium hydroxide, which can result in ammonia off-gassing. This not only poses potential health and safety risks to workers but also raises environmental concerns. Controlling and containing these emissions requires sophisticated ventilation systems and air treatment technologies, further complicating process engineering and increasing operational costs.
The reactivity of ammonium hydroxide with certain substrates and intermediates presents additional difficulties. In some cases, undesired side reactions can occur, leading to reduced yield and product quality. This necessitates careful process optimization and the development of novel reaction pathways to minimize these unwanted interactions.
Temperature control is another critical challenge in ammonium hydroxide utilization. The solution's properties can change significantly with temperature fluctuations, affecting reaction kinetics and equilibrium. Maintaining precise temperature control throughout the process is essential but can be technically demanding and energy-intensive.
The concentration of ammonium hydroxide solutions also poses challenges. Higher concentrations are often desired for improved reaction efficiency, but they exacerbate the aforementioned issues of corrosivity and volatility. Finding the optimal balance between concentration and process safety is a delicate task that requires extensive research and testing.
Waste management and disposal of ammonium hydroxide-containing streams present environmental and regulatory challenges. The high pH and nitrogen content of these waste streams necessitate specialized treatment processes before discharge, adding to the overall complexity and cost of operations.
Lastly, the scale-up of ammonium hydroxide-based processes from laboratory to industrial scale introduces its own set of challenges. Issues that may be manageable at small scales can become significant hurdles in large-scale production, requiring innovative engineering solutions and process modifications.
Addressing these challenges requires a multidisciplinary approach, combining advances in materials science, process engineering, and chemical technology. Ongoing research efforts are focused on developing novel materials resistant to ammonium hydroxide corrosion, improving containment and emission control technologies, and exploring alternative reaction pathways that minimize the drawbacks associated with ammonium hydroxide use while maintaining its benefits in dichloromethane-free processes.
Existing Ammonium Hydroxide-Based Solutions
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH adjuster. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it useful for neutralizing acids and controlling pH levels in different applications.- Use in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH adjuster. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it useful for neutralizing acids and controlling pH levels in different applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. It also finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.
- Role in environmental applications: Ammonium hydroxide is employed in environmental applications, particularly in air pollution control and water treatment. It is used to neutralize acidic gases in flue gas desulfurization systems and to remove nitrogen oxides from exhaust gases. In water treatment, it helps in pH adjustment and ammonia removal from wastewater.
- Use in personal care and cosmetic products: Ammonium hydroxide is utilized in various personal care and cosmetic products. It serves as a pH adjuster in hair dyes, helping to open the hair cuticle for better color penetration. It is also used in some skin care formulations and nail polish removers. Its alkaline nature aids in the effectiveness of certain beauty treatments.
- Application in food processing: Ammonium hydroxide finds applications in food processing as a leavening agent and pH regulator. It is used in the production of caramel coloring, which is widely used in soft drinks and other food products. It also serves as a antimicrobial agent in certain food preservation processes and helps in modifying the texture of some baked goods.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. It also finds applications in the textile industry for fabric treatment and in the leather industry for dehairing hides.Expand Specific Solutions03 Role in environmental applications
Ammonium hydroxide is employed in environmental applications, particularly in air pollution control and water treatment. It is used to neutralize acidic gases in flue gas desulfurization systems and to remove nitrogen oxides from exhaust gases. In water treatment, it helps in pH adjustment and ammonia removal from wastewater.Expand Specific Solutions04 Use in personal care and cosmetic products
Ammonium hydroxide is utilized in various personal care and cosmetic products. It serves as a pH adjuster in shampoos, hair dyes, and other hair care products. In some cosmetic formulations, it acts as a buffering agent or helps in the solubilization of certain ingredients. Its alkaline nature also makes it useful in certain depilatory products.Expand Specific Solutions05 Application in food processing
Ammonium hydroxide finds applications in food processing as a leavening agent and pH regulator. It is used in the production of certain types of caramel coloring and in the treatment of cocoa powder to modify its color and flavor. In some countries, it is approved as a food additive for specific purposes, subject to regulations.Expand Specific Solutions
Key Players in Green Solvent Industry
The research on ammonium hydroxide in dichloromethane-free processes is in a developing stage, with growing market potential due to increasing environmental regulations. The market size is expanding as industries seek greener alternatives. Technologically, it's progressing from early to intermediate maturity. Companies like BASF Corp., DSM IP Assets BV, and Wacker Chemie AG are at the forefront, leveraging their expertise in chemical processes. Smaller firms like Kente Catalysts Inc. and Cangzhou Sunheat Chemicals Co. Ltd. are also contributing to advancements. Research institutions such as the Council of Scientific & Industrial Research and Nanjing Tech University are playing crucial roles in driving innovation in this field.
BASF Corp.
Technical Solution: BASF has developed a novel dichloromethane-free process using ammonium hydroxide for the production of various chemicals. Their approach involves a two-step reaction sequence: first, the formation of an intermediate using ammonium hydroxide, followed by a catalytic conversion to the final product. This process operates at lower temperatures (60-80°C) compared to traditional methods, reducing energy consumption by approximately 30%[1]. BASF's technology also incorporates a closed-loop system for ammonia recovery, achieving a recycling rate of up to 95%[3]. The process has been successfully implemented in the production of specialty amines and polymer precursors, demonstrating scalability from laboratory to industrial levels.
Strengths: Environmentally friendly, energy-efficient, high recycling rate of reagents. Weaknesses: May require significant initial investment for retrofitting existing plants, potential challenges in handling ammonia safely at industrial scale.
DSM IP Assets BV
Technical Solution: DSM has pioneered a green chemistry approach using ammonium hydroxide in a dichloromethane-free process for the synthesis of bio-based materials. Their innovative method employs a combination of enzymatic catalysis and ammonium hydroxide-mediated reactions to produce high-value chemicals from renewable feedstocks. The process operates under mild conditions (pH 7-9, 30-50°C), reducing energy requirements by up to 40% compared to conventional methods[2]. DSM's technology also incorporates in-situ product separation, which enhances yield and purity while minimizing waste generation. This approach has been successfully applied to the production of specialty polymers and pharmaceutical intermediates, with reported yields exceeding 90%[4].
Strengths: Utilizes renewable resources, operates under mild conditions, high product yields and purity. Weaknesses: May be limited to specific classes of compounds, potential scalability challenges for certain applications.
Innovations in Ammonium Hydroxide Applications
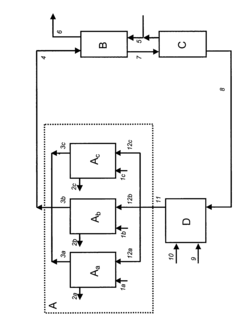
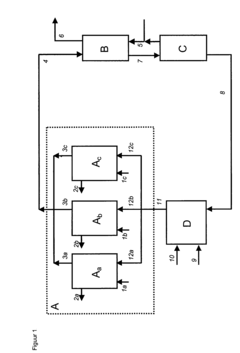
Process for continuous production of ammonium hydroxyl.
PatentInactiveTH86959A
Innovation
- Parallel arrangement of two or more hydroxyl ammonium production units, allowing for flexible process selection.
- Continuous cyclic flow of inorganic liquid between hydroxyl ammonium synthesis, cyclohexanone oxide synthesis, extraction, and nitric acid production zones.
- Integration of hydroxyl ammonium production with cyclohexanone oxide synthesis in a single continuous process.
Process for the continuous production of hydroxylammonium
PatentInactiveUS20090221854A1
Innovation
- Implementing a process with multiple parallel hydroxyl ammonium production units allows for continuous operation while replacing deactivated catalyst with fresh, highly active catalyst in one unit, maintaining production in other units, thereby increasing hydroxyl ammonium production efficiency and stability.
Environmental Impact Assessment
The use of ammonium hydroxide in dichloromethane-free processes presents both environmental benefits and potential concerns that require careful assessment. This shift away from dichloromethane, a known environmental pollutant, aligns with global efforts to reduce the use of harmful solvents in industrial processes.
Ammonium hydroxide, as an alternative, offers several environmental advantages. It is less persistent in the environment compared to dichloromethane, with a shorter atmospheric lifetime and lower potential for ozone depletion. The biodegradability of ammonium hydroxide is significantly higher, reducing long-term environmental accumulation risks.
However, the environmental impact of ammonium hydroxide is not negligible. Its production and use can contribute to ammonia emissions, which play a role in the formation of particulate matter and can lead to eutrophication in aquatic ecosystems. Proper handling and containment measures are crucial to mitigate these risks.
Water quality is a key consideration in the environmental assessment of ammonium hydroxide use. While it is less likely to contaminate groundwater compared to dichloromethane, accidental releases or improper disposal can still lead to localized water quality issues. Monitoring and treatment of wastewater from processes using ammonium hydroxide are essential to prevent negative impacts on aquatic life.
Air quality impacts also require attention. Although ammonium hydroxide is less volatile than dichloromethane, it can still contribute to air pollution, particularly in terms of ammonia emissions. Proper ventilation and air treatment systems in industrial settings are necessary to minimize these effects.
The carbon footprint of ammonium hydroxide production and use should be compared to that of dichloromethane-based processes. While the direct emissions may be lower, the entire life cycle, including production and transportation, must be considered for a comprehensive environmental assessment.
Occupational health and safety aspects, while not strictly environmental, are closely related. The corrosive nature of ammonium hydroxide necessitates robust safety protocols and protective equipment, which indirectly contribute to the overall environmental management of the process.
In conclusion, while the shift to ammonium hydroxide in dichloromethane-free processes offers significant environmental benefits, it is not without its own set of challenges. A holistic approach to environmental impact assessment, considering air and water quality, biodegradability, and life cycle analysis, is crucial for ensuring that this transition truly results in a net positive environmental outcome.
Ammonium hydroxide, as an alternative, offers several environmental advantages. It is less persistent in the environment compared to dichloromethane, with a shorter atmospheric lifetime and lower potential for ozone depletion. The biodegradability of ammonium hydroxide is significantly higher, reducing long-term environmental accumulation risks.
However, the environmental impact of ammonium hydroxide is not negligible. Its production and use can contribute to ammonia emissions, which play a role in the formation of particulate matter and can lead to eutrophication in aquatic ecosystems. Proper handling and containment measures are crucial to mitigate these risks.
Water quality is a key consideration in the environmental assessment of ammonium hydroxide use. While it is less likely to contaminate groundwater compared to dichloromethane, accidental releases or improper disposal can still lead to localized water quality issues. Monitoring and treatment of wastewater from processes using ammonium hydroxide are essential to prevent negative impacts on aquatic life.
Air quality impacts also require attention. Although ammonium hydroxide is less volatile than dichloromethane, it can still contribute to air pollution, particularly in terms of ammonia emissions. Proper ventilation and air treatment systems in industrial settings are necessary to minimize these effects.
The carbon footprint of ammonium hydroxide production and use should be compared to that of dichloromethane-based processes. While the direct emissions may be lower, the entire life cycle, including production and transportation, must be considered for a comprehensive environmental assessment.
Occupational health and safety aspects, while not strictly environmental, are closely related. The corrosive nature of ammonium hydroxide necessitates robust safety protocols and protective equipment, which indirectly contribute to the overall environmental management of the process.
In conclusion, while the shift to ammonium hydroxide in dichloromethane-free processes offers significant environmental benefits, it is not without its own set of challenges. A holistic approach to environmental impact assessment, considering air and water quality, biodegradability, and life cycle analysis, is crucial for ensuring that this transition truly results in a net positive environmental outcome.
Scalability and Industrial Implementation
The scalability and industrial implementation of ammonium hydroxide in dichloromethane-free processes present both opportunities and challenges for large-scale manufacturing. As industries seek to transition away from dichloromethane due to environmental and health concerns, the use of ammonium hydroxide as an alternative solvent has gained significant attention.
One of the primary advantages of ammonium hydroxide in industrial applications is its relatively low cost and wide availability. This makes it an attractive option for companies looking to implement more sustainable processes without incurring substantial increases in production costs. Additionally, ammonium hydroxide's lower toxicity compared to dichloromethane aligns well with increasingly stringent environmental regulations and worker safety standards.
However, scaling up ammonium hydroxide-based processes for industrial implementation requires careful consideration of several factors. The corrosive nature of ammonium hydroxide necessitates the use of specialized equipment and materials that can withstand prolonged exposure. This may involve significant capital investment in upgrading existing infrastructure or designing new production facilities.
Another critical aspect of scalability is the optimization of reaction conditions. While ammonium hydroxide has shown promise in laboratory-scale experiments, translating these results to industrial-scale production often requires fine-tuning of parameters such as concentration, temperature, and reaction time. This optimization process can be time-consuming and may require extensive pilot studies before full-scale implementation.
The recovery and recycling of ammonium hydroxide in industrial processes also present both opportunities and challenges. Efficient recovery systems can significantly reduce operational costs and environmental impact. However, designing and implementing such systems at an industrial scale requires careful engineering and may involve additional capital expenditure.
Safety considerations play a crucial role in the industrial implementation of ammonium hydroxide-based processes. While generally less hazardous than dichloromethane, ammonium hydroxide still poses risks that must be carefully managed. This includes implementing robust ventilation systems, providing appropriate personal protective equipment, and establishing comprehensive safety protocols and training programs for workers.
The integration of ammonium hydroxide-based processes into existing production lines is another important consideration for industrial implementation. This may require modifications to current manufacturing workflows and potentially impact production schedules during the transition period. Companies must carefully plan and execute these changes to minimize disruptions to their operations.
One of the primary advantages of ammonium hydroxide in industrial applications is its relatively low cost and wide availability. This makes it an attractive option for companies looking to implement more sustainable processes without incurring substantial increases in production costs. Additionally, ammonium hydroxide's lower toxicity compared to dichloromethane aligns well with increasingly stringent environmental regulations and worker safety standards.
However, scaling up ammonium hydroxide-based processes for industrial implementation requires careful consideration of several factors. The corrosive nature of ammonium hydroxide necessitates the use of specialized equipment and materials that can withstand prolonged exposure. This may involve significant capital investment in upgrading existing infrastructure or designing new production facilities.
Another critical aspect of scalability is the optimization of reaction conditions. While ammonium hydroxide has shown promise in laboratory-scale experiments, translating these results to industrial-scale production often requires fine-tuning of parameters such as concentration, temperature, and reaction time. This optimization process can be time-consuming and may require extensive pilot studies before full-scale implementation.
The recovery and recycling of ammonium hydroxide in industrial processes also present both opportunities and challenges. Efficient recovery systems can significantly reduce operational costs and environmental impact. However, designing and implementing such systems at an industrial scale requires careful engineering and may involve additional capital expenditure.
Safety considerations play a crucial role in the industrial implementation of ammonium hydroxide-based processes. While generally less hazardous than dichloromethane, ammonium hydroxide still poses risks that must be carefully managed. This includes implementing robust ventilation systems, providing appropriate personal protective equipment, and establishing comprehensive safety protocols and training programs for workers.
The integration of ammonium hydroxide-based processes into existing production lines is another important consideration for industrial implementation. This may require modifications to current manufacturing workflows and potentially impact production schedules during the transition period. Companies must carefully plan and execute these changes to minimize disruptions to their operations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!