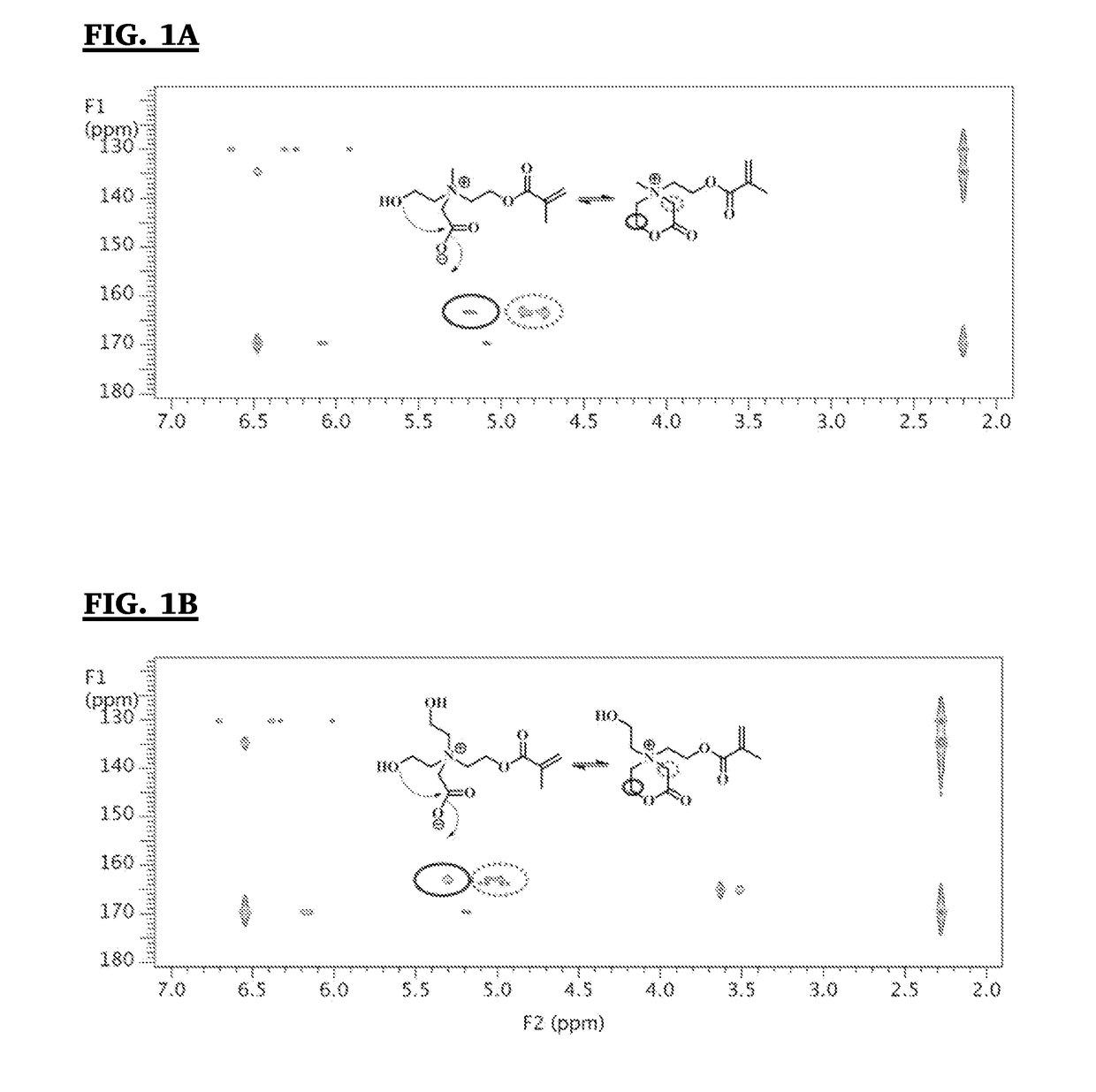
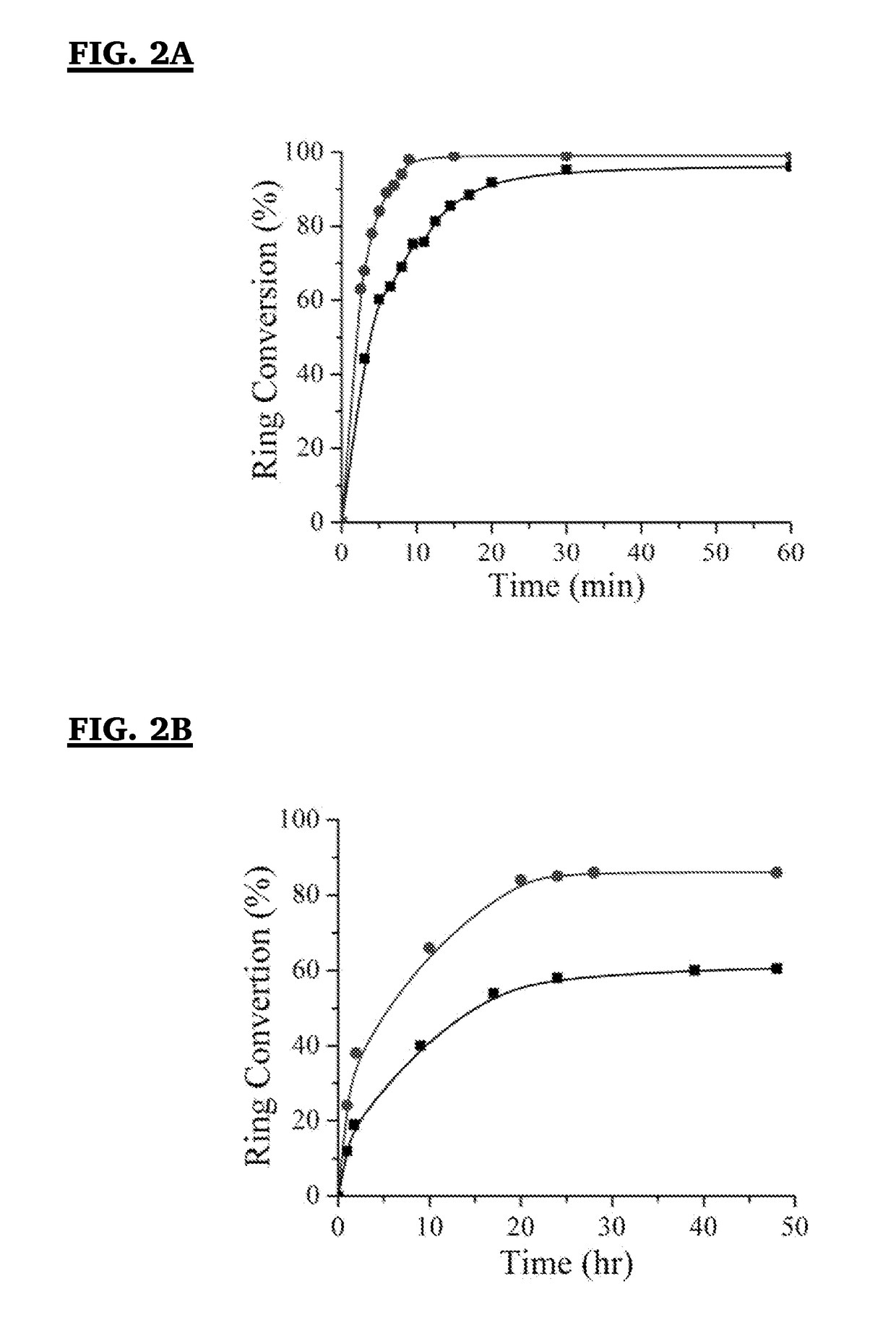
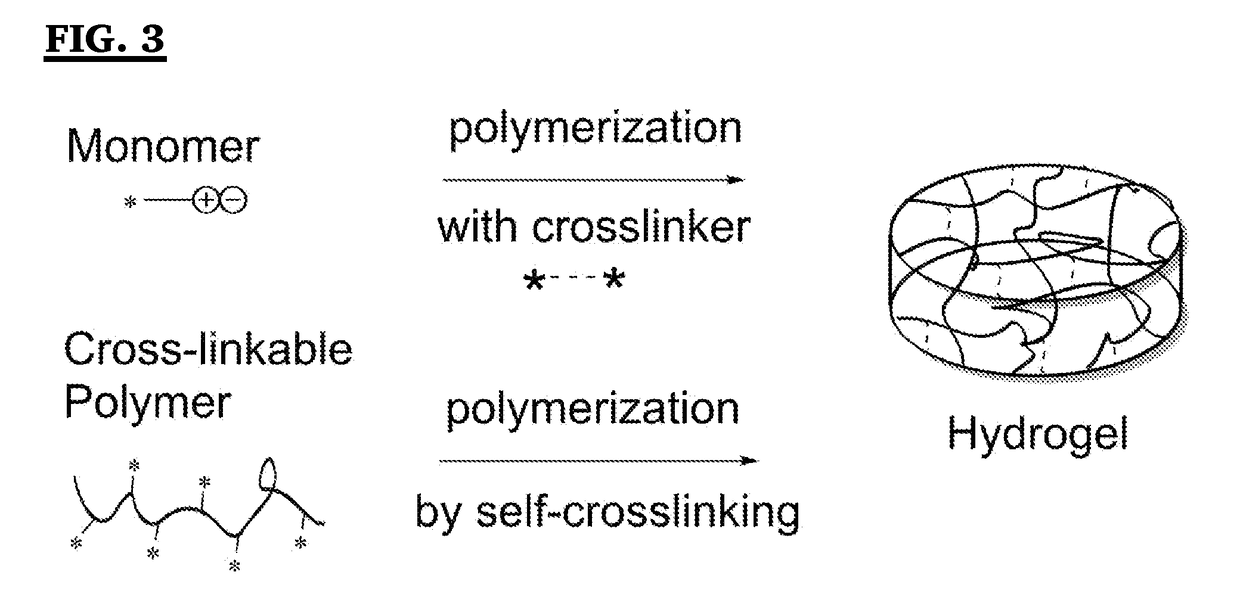
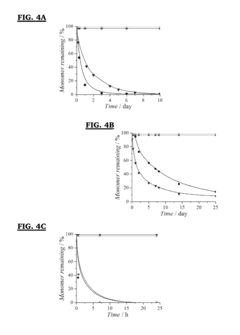
Analysis of Antifouling Properties in Biomedical Polymers
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Antifouling Background and Objectives
The field of biomedical polymers has witnessed significant evolution over the past four decades, transitioning from simple inert materials to sophisticated bioactive interfaces. Antifouling properties—the ability to resist protein adsorption, cell adhesion, and bacterial colonization—have emerged as critical characteristics for implantable devices, biosensors, and drug delivery systems. The historical trajectory began with polyethylene glycol (PEG) coatings in the 1980s, progressing through zwitterionic polymers in the early 2000s, and now exploring biomimetic and stimuli-responsive materials that can adapt to biological environments.
Current technological trends indicate a shift toward multifunctional antifouling surfaces that not only prevent biofouling but also deliver therapeutic agents or respond to specific biological triggers. The integration of nanotechnology has enabled unprecedented control over surface topography and chemistry, while advances in polymer science have produced materials with precisely engineered molecular architectures that optimize antifouling performance.
The primary objective of this technical research is to comprehensively analyze the mechanisms underlying antifouling properties in biomedical polymers, with particular emphasis on structure-property relationships. We aim to identify key molecular features that contribute to superior antifouling performance across diverse biological environments and develop predictive models that can accelerate the design of next-generation materials.
Additionally, this research seeks to evaluate emerging antifouling strategies, including self-healing surfaces, enzyme-mimetic polymers, and hybrid organic-inorganic composites. The investigation will assess their efficacy against various fouling agents, including proteins, mammalian cells, bacteria, and complex biological fluids such as blood and synovial fluid.
A critical component of this analysis involves examining the long-term stability of antifouling properties under physiological conditions, as material degradation and surface reorganization often compromise performance over time. This includes understanding how mechanical stress, enzymatic activity, and oxidative environments affect polymer integrity and function.
Furthermore, this research aims to establish standardized testing protocols for evaluating antifouling performance, addressing the current challenge of inconsistent methodologies that hinder direct comparisons between different materials. By developing robust analytical frameworks, we can better predict in vivo performance based on in vitro testing results.
The ultimate goal is to provide a comprehensive technological roadmap that guides future research and development efforts in biomedical polymer engineering, identifying promising directions for innovation and highlighting critical challenges that must be addressed to advance the field toward clinical translation and commercial implementation.
Current technological trends indicate a shift toward multifunctional antifouling surfaces that not only prevent biofouling but also deliver therapeutic agents or respond to specific biological triggers. The integration of nanotechnology has enabled unprecedented control over surface topography and chemistry, while advances in polymer science have produced materials with precisely engineered molecular architectures that optimize antifouling performance.
The primary objective of this technical research is to comprehensively analyze the mechanisms underlying antifouling properties in biomedical polymers, with particular emphasis on structure-property relationships. We aim to identify key molecular features that contribute to superior antifouling performance across diverse biological environments and develop predictive models that can accelerate the design of next-generation materials.
Additionally, this research seeks to evaluate emerging antifouling strategies, including self-healing surfaces, enzyme-mimetic polymers, and hybrid organic-inorganic composites. The investigation will assess their efficacy against various fouling agents, including proteins, mammalian cells, bacteria, and complex biological fluids such as blood and synovial fluid.
A critical component of this analysis involves examining the long-term stability of antifouling properties under physiological conditions, as material degradation and surface reorganization often compromise performance over time. This includes understanding how mechanical stress, enzymatic activity, and oxidative environments affect polymer integrity and function.
Furthermore, this research aims to establish standardized testing protocols for evaluating antifouling performance, addressing the current challenge of inconsistent methodologies that hinder direct comparisons between different materials. By developing robust analytical frameworks, we can better predict in vivo performance based on in vitro testing results.
The ultimate goal is to provide a comprehensive technological roadmap that guides future research and development efforts in biomedical polymer engineering, identifying promising directions for innovation and highlighting critical challenges that must be addressed to advance the field toward clinical translation and commercial implementation.
Market Demand Analysis for Antifouling Biomedical Materials
The global market for antifouling biomedical materials has experienced significant growth in recent years, driven by increasing healthcare expenditures and rising awareness about hospital-acquired infections. The market value for antifouling biomedical polymers reached approximately $3.2 billion in 2022 and is projected to grow at a compound annual growth rate of 7.8% through 2028, potentially reaching $5.1 billion by the end of the forecast period.
Healthcare-associated infections (HAIs) represent a substantial burden on healthcare systems worldwide, affecting millions of patients annually. According to the World Health Organization, at any given time, about 7% of patients in developed countries and 10% in developing countries will acquire at least one HAI during their hospital stay. This has created an urgent demand for medical devices and implants with effective antifouling properties.
The medical device sector constitutes the largest application segment for antifouling biomedical polymers, accounting for nearly 45% of the total market share. Within this segment, catheters, stents, and surgical instruments represent the highest-demand products. The growing prevalence of chronic diseases requiring long-term implantable devices has further accelerated market growth, particularly in regions with aging populations such as North America, Europe, and parts of Asia.
Geographical analysis reveals North America as the dominant market for antifouling biomedical materials, holding approximately 38% of the global market share. This dominance is attributed to advanced healthcare infrastructure, substantial R&D investments, and stringent regulations regarding infection control. The Asia-Pacific region, however, is emerging as the fastest-growing market with an estimated growth rate of 9.2% annually, driven by improving healthcare access, increasing medical tourism, and rising disposable incomes.
Consumer preferences are shifting toward minimally invasive procedures and outpatient care, creating demand for biomedical devices with enhanced biocompatibility and reduced fouling potential. Healthcare providers are increasingly willing to invest in premium antifouling materials that demonstrate clear cost-effectiveness through reduced infection rates and decreased length of hospital stays.
Industry surveys indicate that over 70% of hospital administrators consider infection prevention capabilities as a critical factor when procuring medical devices. This trend has prompted manufacturers to prioritize antifouling properties in their product development pipelines. The COVID-19 pandemic has further heightened awareness about infection control measures, creating additional market opportunities for antifouling biomedical materials.
Regulatory factors also significantly influence market demand. Stringent guidelines from bodies such as the FDA and European Medicines Agency regarding biocompatibility and infection control have accelerated the adoption of advanced antifouling technologies. Additionally, reimbursement policies favoring preventive healthcare measures have created financial incentives for healthcare facilities to invest in infection-resistant medical technologies.
Healthcare-associated infections (HAIs) represent a substantial burden on healthcare systems worldwide, affecting millions of patients annually. According to the World Health Organization, at any given time, about 7% of patients in developed countries and 10% in developing countries will acquire at least one HAI during their hospital stay. This has created an urgent demand for medical devices and implants with effective antifouling properties.
The medical device sector constitutes the largest application segment for antifouling biomedical polymers, accounting for nearly 45% of the total market share. Within this segment, catheters, stents, and surgical instruments represent the highest-demand products. The growing prevalence of chronic diseases requiring long-term implantable devices has further accelerated market growth, particularly in regions with aging populations such as North America, Europe, and parts of Asia.
Geographical analysis reveals North America as the dominant market for antifouling biomedical materials, holding approximately 38% of the global market share. This dominance is attributed to advanced healthcare infrastructure, substantial R&D investments, and stringent regulations regarding infection control. The Asia-Pacific region, however, is emerging as the fastest-growing market with an estimated growth rate of 9.2% annually, driven by improving healthcare access, increasing medical tourism, and rising disposable incomes.
Consumer preferences are shifting toward minimally invasive procedures and outpatient care, creating demand for biomedical devices with enhanced biocompatibility and reduced fouling potential. Healthcare providers are increasingly willing to invest in premium antifouling materials that demonstrate clear cost-effectiveness through reduced infection rates and decreased length of hospital stays.
Industry surveys indicate that over 70% of hospital administrators consider infection prevention capabilities as a critical factor when procuring medical devices. This trend has prompted manufacturers to prioritize antifouling properties in their product development pipelines. The COVID-19 pandemic has further heightened awareness about infection control measures, creating additional market opportunities for antifouling biomedical materials.
Regulatory factors also significantly influence market demand. Stringent guidelines from bodies such as the FDA and European Medicines Agency regarding biocompatibility and infection control have accelerated the adoption of advanced antifouling technologies. Additionally, reimbursement policies favoring preventive healthcare measures have created financial incentives for healthcare facilities to invest in infection-resistant medical technologies.
Current Antifouling Technology Status and Challenges
The global landscape of antifouling technologies for biomedical polymers presents a complex picture of significant advancements alongside persistent challenges. Currently, the field is dominated by several major approaches, each with distinct advantages and limitations. Hydrophilic polymer coatings, particularly those utilizing polyethylene glycol (PEG) derivatives, represent the most widely implemented solution due to their established efficacy in creating a hydration layer that prevents protein adsorption. However, recent studies have revealed concerning limitations regarding PEG's long-term stability in biological environments, with evidence of oxidative degradation occurring after prolonged exposure.
Zwitterionic polymers have emerged as promising alternatives, demonstrating superior resistance to nonspecific protein adsorption through their strong hydration capacity. Materials such as poly(carboxybetaine) and poly(sulfobetaine) have shown remarkable performance in laboratory settings, yet their widespread commercial implementation remains constrained by complex synthesis procedures and higher production costs compared to conventional materials.
Micro-structured surfaces that mimic natural antifouling mechanisms, such as the lotus leaf or shark skin, represent another innovative approach. These biomimetic solutions have demonstrated impressive initial results in controlled environments, but face significant challenges in maintaining their structural integrity and antifouling properties under dynamic physiological conditions, particularly in high-flow or high-stress environments like cardiovascular applications.
A critical technical barrier across the field remains the achievement of long-term stability. Most current antifouling solutions show performance degradation over time, with effectiveness typically diminishing after periods ranging from weeks to months depending on the specific application environment. This presents a particular challenge for permanently implanted medical devices where replacement involves significant patient risk.
Geographically, research leadership in this domain shows distinct patterns. North American institutions and companies lead in fundamental research and patent filings, particularly in novel polymer chemistry approaches. European entities demonstrate strength in biomimetic surface technologies, while East Asian research groups, particularly in Japan and South Korea, have made significant advances in zwitterionic materials and their clinical applications.
Regulatory hurdles present another significant challenge, with stringent biocompatibility requirements creating lengthy approval pathways for novel antifouling materials. This regulatory landscape has resulted in a notable gap between laboratory innovations and clinically approved solutions, with many promising technologies remaining in pre-clinical development stages for extended periods.
The integration of antifouling properties with other essential material characteristics—such as mechanical strength, flexibility, and drug-eluting capabilities—represents perhaps the most complex technical challenge. Current solutions often achieve antifouling properties at the expense of other critical material parameters, necessitating careful application-specific optimization.
Zwitterionic polymers have emerged as promising alternatives, demonstrating superior resistance to nonspecific protein adsorption through their strong hydration capacity. Materials such as poly(carboxybetaine) and poly(sulfobetaine) have shown remarkable performance in laboratory settings, yet their widespread commercial implementation remains constrained by complex synthesis procedures and higher production costs compared to conventional materials.
Micro-structured surfaces that mimic natural antifouling mechanisms, such as the lotus leaf or shark skin, represent another innovative approach. These biomimetic solutions have demonstrated impressive initial results in controlled environments, but face significant challenges in maintaining their structural integrity and antifouling properties under dynamic physiological conditions, particularly in high-flow or high-stress environments like cardiovascular applications.
A critical technical barrier across the field remains the achievement of long-term stability. Most current antifouling solutions show performance degradation over time, with effectiveness typically diminishing after periods ranging from weeks to months depending on the specific application environment. This presents a particular challenge for permanently implanted medical devices where replacement involves significant patient risk.
Geographically, research leadership in this domain shows distinct patterns. North American institutions and companies lead in fundamental research and patent filings, particularly in novel polymer chemistry approaches. European entities demonstrate strength in biomimetic surface technologies, while East Asian research groups, particularly in Japan and South Korea, have made significant advances in zwitterionic materials and their clinical applications.
Regulatory hurdles present another significant challenge, with stringent biocompatibility requirements creating lengthy approval pathways for novel antifouling materials. This regulatory landscape has resulted in a notable gap between laboratory innovations and clinically approved solutions, with many promising technologies remaining in pre-clinical development stages for extended periods.
The integration of antifouling properties with other essential material characteristics—such as mechanical strength, flexibility, and drug-eluting capabilities—represents perhaps the most complex technical challenge. Current solutions often achieve antifouling properties at the expense of other critical material parameters, necessitating careful application-specific optimization.
Current Antifouling Strategies for Biomedical Polymers
01 Hydrophilic polymer coatings for antifouling applications
Hydrophilic polymers such as polyethylene glycol (PEG) and its derivatives can be used as coatings on biomedical devices to prevent protein adsorption and bacterial adhesion. These polymers create a hydration layer that repels proteins and microorganisms, thereby reducing biofouling. The hydrophilic nature of these coatings makes them effective for various medical implants, catheters, and biosensors where maintaining clean surfaces is critical for proper function and reducing infection risks.- Hydrophilic polymer coatings for antifouling applications: Hydrophilic polymers such as polyethylene glycol (PEG) and its derivatives can be used as coatings on biomedical devices to prevent protein adsorption and bacterial adhesion. These polymers create a hydration layer that repels proteins and microorganisms, thereby reducing biofouling. The hydrophilic nature of these coatings makes them effective for various medical implants, catheters, and biosensors where maintaining clean surfaces is critical for proper function and reducing infection risks.
- Zwitterionic polymers for enhanced biocompatibility: Zwitterionic polymers containing both positive and negative charges in their structure demonstrate excellent antifouling properties in biomedical applications. These polymers, including phosphorylcholine-based materials and sulfobetaine-containing polymers, mimic cell membrane surfaces and strongly bind water molecules, creating a strong hydration barrier that prevents protein adsorption and cell attachment. Their unique charge balance provides superior long-term stability compared to neutral hydrophilic polymers in complex biological environments.
- Surface modification techniques for polymer antifouling properties: Various surface modification techniques can enhance the antifouling properties of biomedical polymers. These include plasma treatment, UV grafting, chemical vapor deposition, and layer-by-layer assembly. These methods allow for the introduction of functional groups or the attachment of antifouling molecules to polymer surfaces without altering their bulk properties. Surface roughness, charge density, and wettability can be precisely controlled to optimize the antifouling performance for specific biomedical applications.
- Polymer brush architectures for preventing biofouling: Polymer brushes, consisting of polymer chains tethered at one end to a surface, provide effective antifouling properties due to their unique architecture. When exposed to an aqueous environment, these brushes extend outward, creating a physical and energetic barrier against protein adsorption and cellular adhesion. The density, length, and composition of these polymer brushes can be tailored to optimize antifouling performance in various biomedical applications such as implantable devices, biosensors, and drug delivery systems.
- Stimuli-responsive polymers with switchable antifouling properties: Stimuli-responsive polymers can change their physical or chemical properties in response to environmental triggers such as temperature, pH, light, or electrical signals. These smart materials offer switchable antifouling properties that can be activated on demand. For example, thermoresponsive polymers like poly(N-isopropylacrylamide) undergo conformational changes at specific temperatures, allowing for controlled protein adsorption or release. This dynamic behavior enables advanced biomedical applications including controlled drug delivery, cell sheet engineering, and self-cleaning surfaces.
02 Zwitterionic polymers for enhanced biocompatibility
Zwitterionic polymers contain both positive and negative charges in their structure, creating a strong hydration layer that effectively prevents protein adsorption and cell attachment. These polymers, including phosphorylcholine-based materials and sulfobetaine-containing polymers, demonstrate excellent antifouling properties while maintaining biocompatibility. They can be applied as coatings on medical devices or incorporated into the bulk material to create surfaces that resist biofouling in complex biological environments.Expand Specific Solutions03 Polymer brush architectures for improved antifouling performance
Polymer brushes consist of polymer chains tethered at one end to a surface, creating a dense barrier against fouling agents. These architectures can be designed with controlled density, thickness, and composition to optimize antifouling performance. Various grafting techniques, including "grafting-to" and "grafting-from" approaches, allow for the creation of robust antifouling surfaces with long-term stability in biological environments. The extended conformation of polymer brushes provides steric hindrance against protein adsorption and bacterial attachment.Expand Specific Solutions04 Stimuli-responsive polymers for switchable antifouling properties
Stimuli-responsive polymers can change their physical properties in response to environmental triggers such as temperature, pH, or light. These smart materials can switch between fouling and antifouling states on demand, allowing for controlled protein adsorption or cell attachment when needed. Applications include biosensors, drug delivery systems, and tissue engineering scaffolds where selective biological interactions are desired. The reversible nature of these materials provides unique advantages for biomedical devices requiring dynamic surface properties.Expand Specific Solutions05 Nanostructured polymer surfaces for enhanced antifouling
Nanostructured polymer surfaces with controlled topography can significantly enhance antifouling performance by combining physical and chemical antifouling mechanisms. These surfaces can be created through various techniques including nanoimprinting, phase separation, and self-assembly processes. The combination of nanoscale features with antifouling polymer chemistry creates synergistic effects that reduce protein adsorption and bacterial attachment more effectively than smooth surfaces. Applications include medical implants, marine coatings, and biosensors where long-term fouling resistance is critical.Expand Specific Solutions
Key Industry Players in Biomedical Antifouling Materials
The antifouling properties in biomedical polymers market is currently in a growth phase, with increasing demand driven by healthcare applications requiring contamination-resistant surfaces. The global market is expanding steadily, estimated at approximately $2-3 billion annually with projected CAGR of 7-9%. Research institutions like University of Washington, Northwestern University, and University of Akron are leading academic innovation, while companies including Nitto Denko, 3M Innovative Properties, and Boston Scientific Scimed represent commercial technology maturity. The competitive landscape features collaboration between academic centers and industry players, with recent advances in environmentally friendly antifouling solutions. Asian institutions, particularly from China (Nanjing University, South China University of Technology) are increasingly contributing significant research alongside traditional Western leaders.
University of Washington
Technical Solution: The University of Washington has pioneered groundbreaking research in zwitterionic polymer systems for biomedical antifouling applications. Their approach centers on the development of ultra-low fouling zwitterionic materials that maintain a strong hydration layer through electrostatic interactions, effectively preventing protein adsorption and subsequent biological fouling. The research team has developed novel polymerization techniques that allow precise control over the density and distribution of zwitterionic groups, optimizing antifouling performance for specific applications. Their studies have demonstrated that these materials can reduce protein adsorption by over 98% compared to conventional polymers in complex biological fluids. The university has also developed innovative surface grafting methods that enable the attachment of zwitterionic polymers to various substrate materials, including metals, ceramics, and other polymers, greatly expanding the potential applications. Recent work has focused on stimuli-responsive zwitterionic systems that can change their properties in response to environmental triggers, creating "smart" surfaces that adapt to changing physiological conditions. Their research has shown that these materials maintain their antifouling properties for extended periods under physiological conditions, with in vitro studies demonstrating effectiveness for over 30 days in blood plasma.
Strengths: World-leading expertise in zwitterionic chemistry and polymer science has resulted in highly effective antifouling materials with exceptional performance in complex biological environments. Their academic approach facilitates fundamental understanding of fouling mechanisms. Weaknesses: As an academic institution, they may face challenges in scaling up technologies for commercial production, and their solutions may require industrial partnerships to reach practical application.
Nitto Denko Corp.
Technical Solution: Nitto Denko has developed innovative antifouling polymer technologies specifically designed for biomedical applications. Their approach centers on the development of specialized hydrogel coatings with controlled water content and mechanical properties that resist protein adsorption and cell adhesion. These hydrogels incorporate methacrylate-based polymers with precisely engineered network structures that maintain hydration while providing mechanical stability. Nitto's research has demonstrated that optimizing the mesh size of these networks can effectively prevent fouling molecules from penetrating the surface while allowing smaller beneficial molecules to pass through. The company has also pioneered the development of amphiphilic block copolymers that self-assemble into nanoscale domains on device surfaces, creating heterogeneous interfaces that disrupt protein adsorption mechanisms. Their proprietary surface modification techniques enable covalent attachment of these polymers to various substrate materials, ensuring long-term stability under physiological conditions. Recent innovations include stimuli-responsive antifouling coatings that can change their properties in response to environmental triggers such as pH or temperature, allowing for "smart" biomedical interfaces that adapt to changing physiological conditions.
Strengths: Exceptional expertise in polymer chemistry and surface modification technologies enables creation of highly specialized antifouling surfaces. Their established manufacturing infrastructure facilitates scale-up and commercialization. Weaknesses: Some of their more advanced technologies require complex processing steps that may limit broad application, and their solutions may be more expensive than conventional approaches due to specialized materials and processing requirements.
Critical Patents and Research in Antifouling Surfaces
Switchable antimicrobial and antifouling carboxybetaine-based hydrogels and elastomers with enhanced mechanical properties
PatentActiveUS20170362458A1
Innovation
- Development of switchable zwitterionic polymer hydrogels and elastomers that can switch between antimicrobial and antifouling states based on pH conditions, incorporating carboxybetaine groups with ethanol, propanol, or butanol moieties for enhanced mechanical properties and biocompatibility.
Peptidomimetic polymers for antifouling surfaces
PatentInactiveUS20060241281A1
Innovation
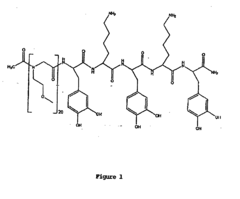
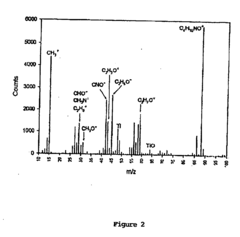
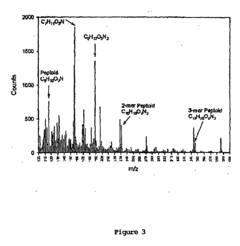
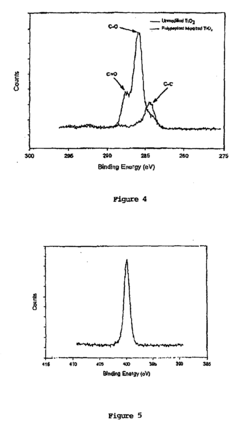
- Development of chimeric peptidomimetic polymers comprising a peptide or polypeptide anchor moiety coupled with a peptoid component, utilizing dihydroxyphenyl derivatives and ether-linked alkyl groups for robust anchorage and resistance to protein and cell fouling, inspired by mussel adhesive proteins, which are synthesized using a versatile solid-phase approach.
Biocompatibility and Safety Considerations
The biocompatibility and safety profile of antifouling polymers represents a critical consideration in their development for biomedical applications. When these materials interface with biological systems, they must not elicit adverse reactions such as inflammation, immune responses, or toxicity. The evaluation of biocompatibility follows standardized protocols outlined in ISO 10993, which encompasses cytotoxicity, sensitization, irritation, and systemic toxicity assessments.
Primary biocompatibility concerns for antifouling polymers include leaching of unreacted monomers, degradation products, and additives that may cause local or systemic toxicity. Particularly concerning are low molecular weight components that might migrate from the polymer matrix into surrounding tissues or the bloodstream. The degradation kinetics of these materials must be thoroughly characterized to predict long-term safety profiles, especially for implantable devices where material stability is paramount.
Hemocompatibility represents a specialized aspect of biocompatibility particularly relevant to antifouling polymers intended for blood-contacting applications. These materials must resist platelet adhesion, prevent complement activation, and avoid initiating coagulation cascades. Surface properties such as charge distribution, hydrophilicity, and topography significantly influence these interactions and must be optimized to minimize thrombogenic potential.
Regulatory frameworks for antifouling polymers vary by application and geographical region, with more stringent requirements for long-term implantable devices compared to external-use products. The FDA in the United States and the EMA in Europe have established specific guidelines for the evaluation of materials intended for different biomedical applications, with particular emphasis on the risk assessment of novel materials or those with limited clinical history.
Recent advances in biocompatibility testing include the development of organ-on-chip technologies and advanced in vitro models that better simulate the complex biological environment. These approaches provide more predictive assessments of material performance while reducing reliance on animal testing, aligning with global efforts to implement the 3Rs principle (Replacement, Reduction, Refinement) in biomedical research.
The long-term safety of antifouling polymers remains an active area of investigation, particularly regarding the potential for chronic inflammation, foreign body responses, and material fatigue. Post-market surveillance and clinical follow-up studies provide valuable data on real-world performance and help identify rare adverse events that may not be detected during pre-clinical evaluation. This continuous monitoring process informs iterative improvements in material design and processing techniques.
Primary biocompatibility concerns for antifouling polymers include leaching of unreacted monomers, degradation products, and additives that may cause local or systemic toxicity. Particularly concerning are low molecular weight components that might migrate from the polymer matrix into surrounding tissues or the bloodstream. The degradation kinetics of these materials must be thoroughly characterized to predict long-term safety profiles, especially for implantable devices where material stability is paramount.
Hemocompatibility represents a specialized aspect of biocompatibility particularly relevant to antifouling polymers intended for blood-contacting applications. These materials must resist platelet adhesion, prevent complement activation, and avoid initiating coagulation cascades. Surface properties such as charge distribution, hydrophilicity, and topography significantly influence these interactions and must be optimized to minimize thrombogenic potential.
Regulatory frameworks for antifouling polymers vary by application and geographical region, with more stringent requirements for long-term implantable devices compared to external-use products. The FDA in the United States and the EMA in Europe have established specific guidelines for the evaluation of materials intended for different biomedical applications, with particular emphasis on the risk assessment of novel materials or those with limited clinical history.
Recent advances in biocompatibility testing include the development of organ-on-chip technologies and advanced in vitro models that better simulate the complex biological environment. These approaches provide more predictive assessments of material performance while reducing reliance on animal testing, aligning with global efforts to implement the 3Rs principle (Replacement, Reduction, Refinement) in biomedical research.
The long-term safety of antifouling polymers remains an active area of investigation, particularly regarding the potential for chronic inflammation, foreign body responses, and material fatigue. Post-market surveillance and clinical follow-up studies provide valuable data on real-world performance and help identify rare adverse events that may not be detected during pre-clinical evaluation. This continuous monitoring process informs iterative improvements in material design and processing techniques.
Regulatory Framework for Medical Polymer Materials
The regulatory landscape governing biomedical polymers with antifouling properties is complex and multifaceted, requiring manufacturers to navigate various international and regional frameworks. In the United States, the Food and Drug Administration (FDA) oversees medical polymers through different regulatory pathways depending on their intended use and risk classification. Polymers with antifouling properties often fall under Class II or III medical devices, requiring either 510(k) clearance or Premarket Approval (PMA), with specific considerations for surface-modifying technologies that prevent biofouling.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which place stringent requirements on biomedical polymers. These regulations emphasize comprehensive technical documentation, clinical evaluation, and post-market surveillance. Notably, polymers with antifouling properties must demonstrate both efficacy in preventing biological adhesion and safety regarding potential leachables and degradation products.
International standards play a crucial role in harmonizing regulatory approaches. ISO 10993 series for biological evaluation of medical devices is particularly relevant, with ISO 10993-1 providing a framework for risk assessment and ISO 10993-4 specifically addressing interactions with blood, which is critical for antifouling applications. Additionally, ASTM F2582 provides standard test methods for detecting protein fouling on materials, serving as an important benchmark for regulatory submissions.
Environmental regulations are increasingly impacting the development of antifouling polymers. Many traditional antifouling compounds face restrictions due to environmental concerns, pushing the industry toward more sustainable alternatives. The EU's REACH regulation and similar frameworks worldwide require thorough safety assessments of chemical components used in polymer formulations, particularly for novel antifouling agents.
Regulatory bodies are also adapting to emerging technologies in this field. Nanotechnology-enhanced polymers, biomimetic surfaces, and stimuli-responsive materials present unique regulatory challenges. The FDA has developed guidance documents for nanotechnology applications, while the EU has established the Scientific Committee on Emerging and Newly Identified Health Risks to address novel materials and technologies.
Compliance with these regulations requires extensive testing and documentation. Manufacturers must demonstrate biocompatibility, hemocompatibility, and durability of antifouling properties over the intended product lifecycle. Additionally, they must validate manufacturing processes to ensure consistent quality and performance of the antifouling surfaces, with particular attention to sterilization compatibility and shelf-life stability.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which place stringent requirements on biomedical polymers. These regulations emphasize comprehensive technical documentation, clinical evaluation, and post-market surveillance. Notably, polymers with antifouling properties must demonstrate both efficacy in preventing biological adhesion and safety regarding potential leachables and degradation products.
International standards play a crucial role in harmonizing regulatory approaches. ISO 10993 series for biological evaluation of medical devices is particularly relevant, with ISO 10993-1 providing a framework for risk assessment and ISO 10993-4 specifically addressing interactions with blood, which is critical for antifouling applications. Additionally, ASTM F2582 provides standard test methods for detecting protein fouling on materials, serving as an important benchmark for regulatory submissions.
Environmental regulations are increasingly impacting the development of antifouling polymers. Many traditional antifouling compounds face restrictions due to environmental concerns, pushing the industry toward more sustainable alternatives. The EU's REACH regulation and similar frameworks worldwide require thorough safety assessments of chemical components used in polymer formulations, particularly for novel antifouling agents.
Regulatory bodies are also adapting to emerging technologies in this field. Nanotechnology-enhanced polymers, biomimetic surfaces, and stimuli-responsive materials present unique regulatory challenges. The FDA has developed guidance documents for nanotechnology applications, while the EU has established the Scientific Committee on Emerging and Newly Identified Health Risks to address novel materials and technologies.
Compliance with these regulations requires extensive testing and documentation. Manufacturers must demonstrate biocompatibility, hemocompatibility, and durability of antifouling properties over the intended product lifecycle. Additionally, they must validate manufacturing processes to ensure consistent quality and performance of the antifouling surfaces, with particular attention to sterilization compatibility and shelf-life stability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!