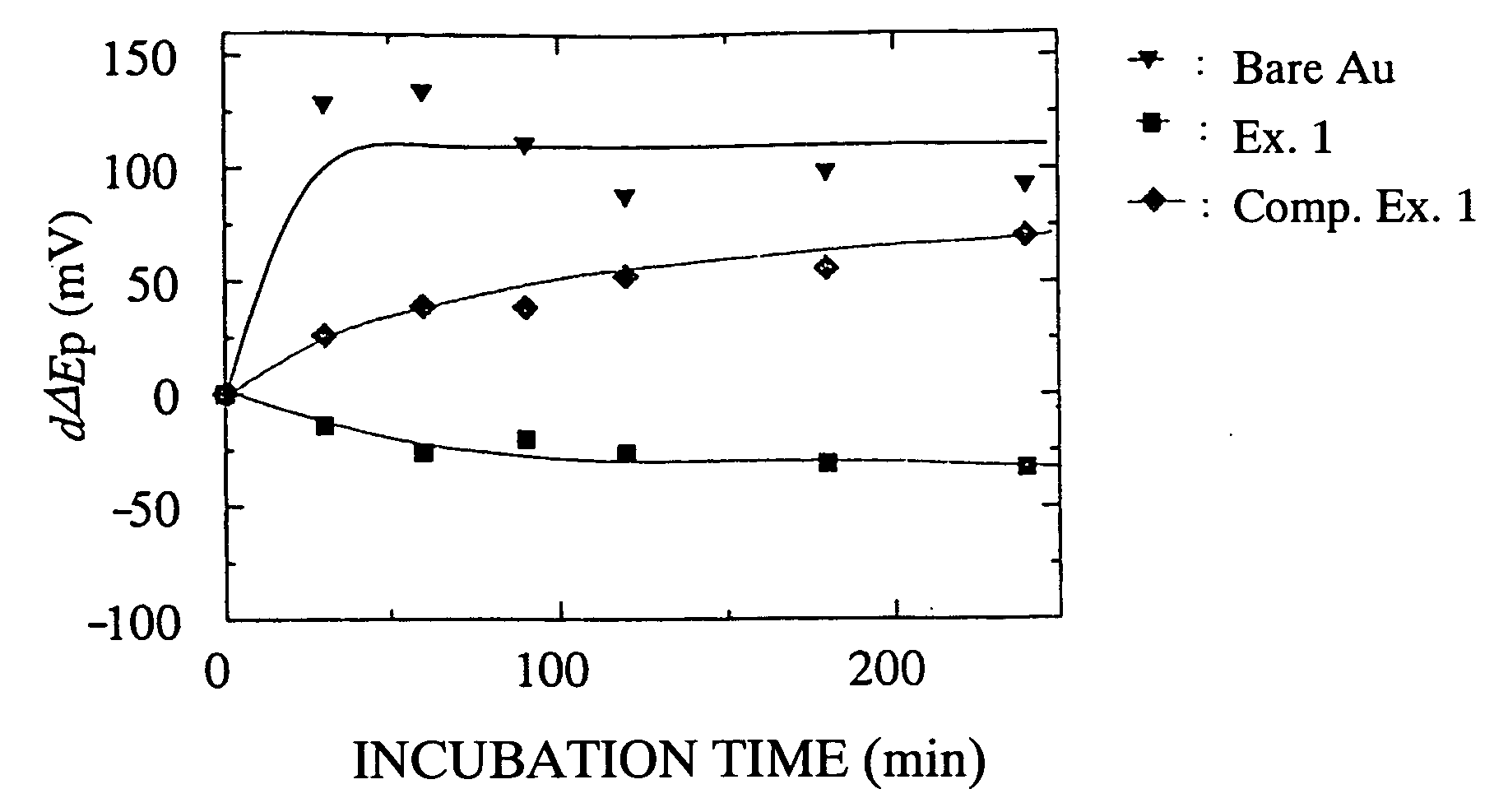
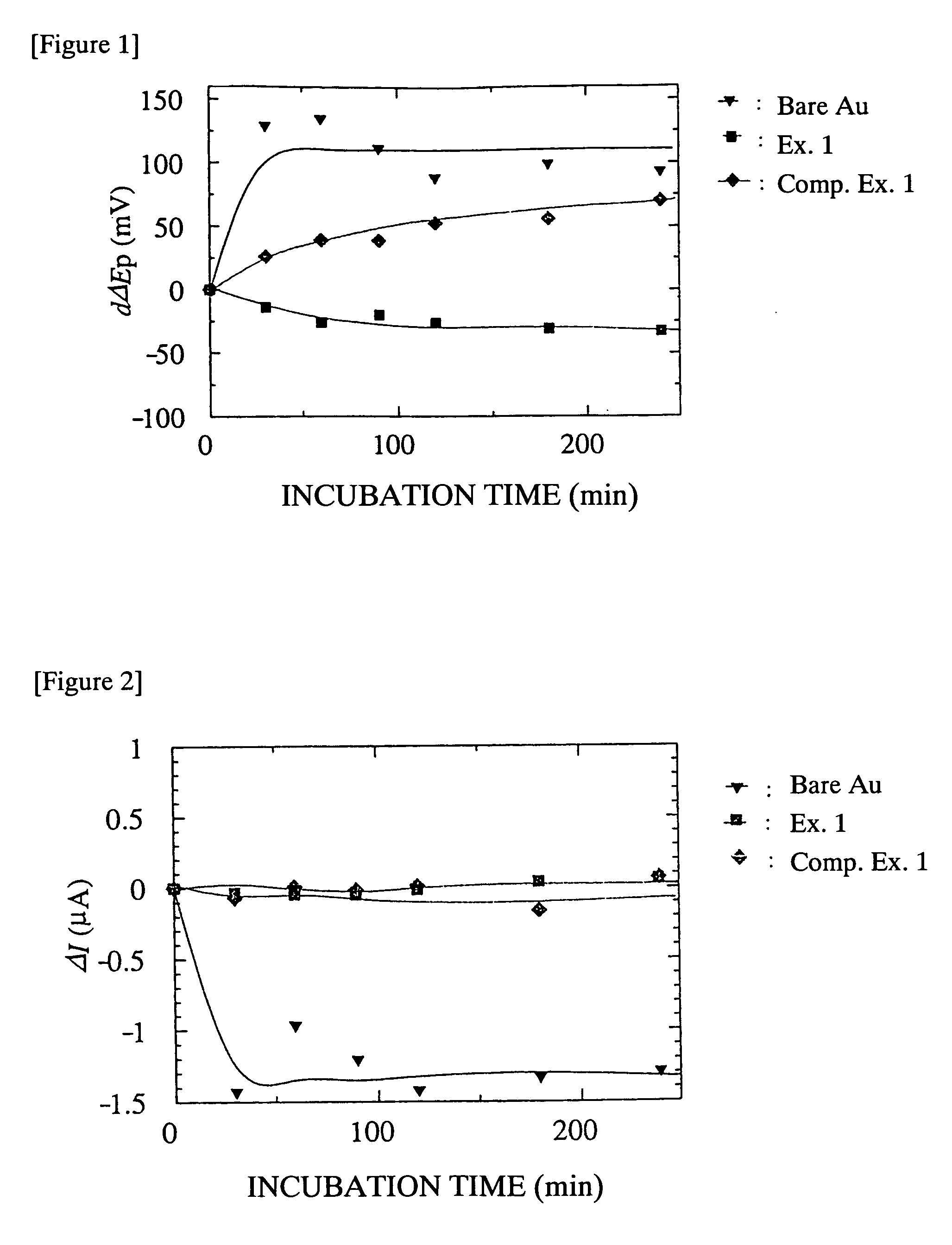
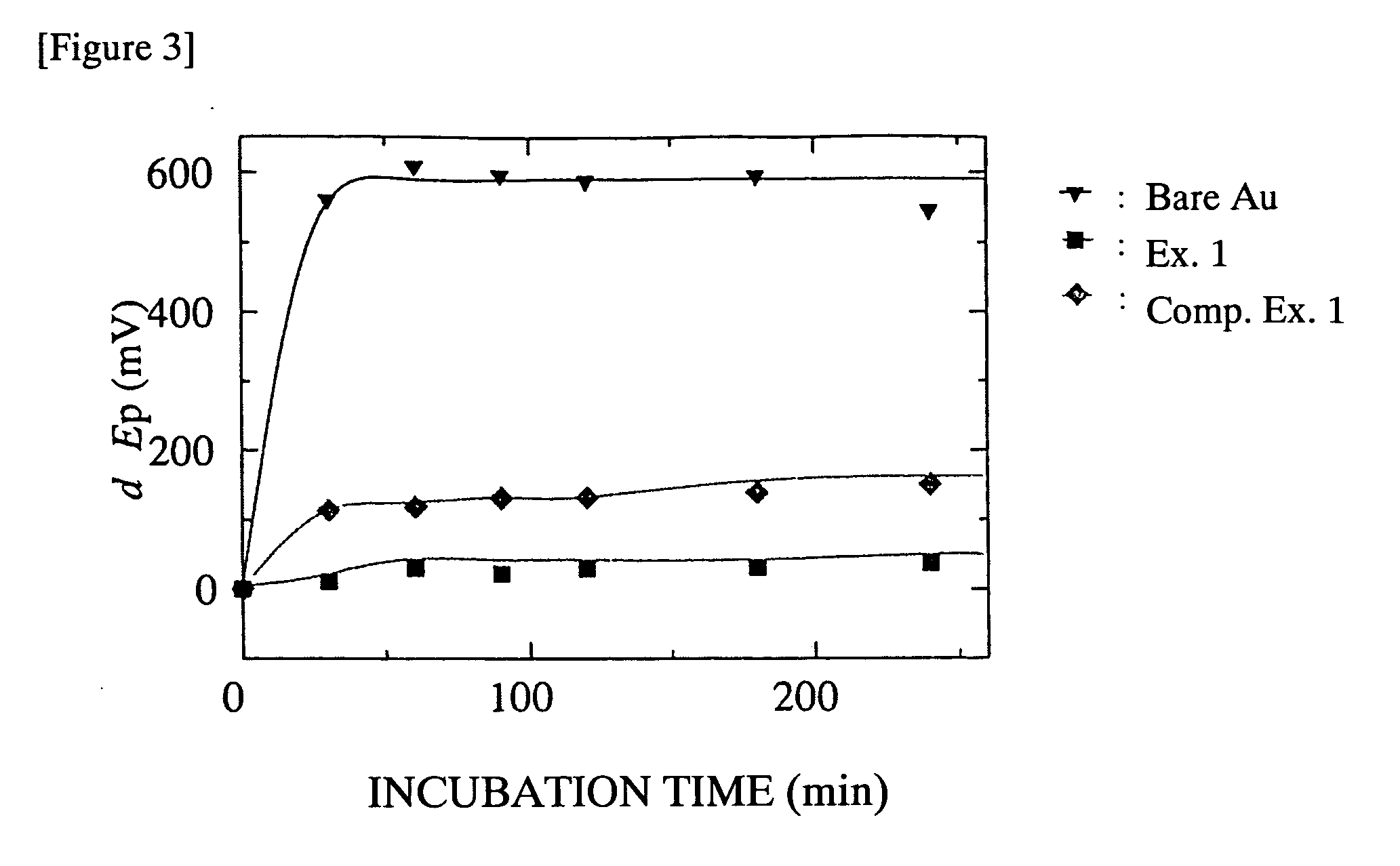
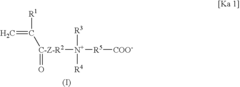
What Determines the Biocompatibility of Biomedical Polymers
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Biocompatibility Background and Objectives
Biomedical polymers have evolved significantly over the past several decades, transitioning from simple structural materials to sophisticated platforms capable of interfacing with biological systems. The concept of biocompatibility emerged in the 1940s when synthetic polymers were first considered for medical applications, but gained substantial momentum in the 1960s with the development of the first generation of biomaterials designed specifically for medical use.
The evolution of biomedical polymers has been characterized by three distinct generations. First-generation materials (1960s-1970s) focused primarily on achieving minimal toxicity and tissue reaction. Second-generation materials (1980s-1990s) introduced bioactive properties and controlled degradation profiles. Current third-generation materials aim to stimulate specific cellular responses at the molecular level, representing a paradigm shift from merely compatible materials to those that actively participate in healing processes.
Understanding biocompatibility has similarly evolved from simplistic definitions centered on "doing no harm" to more nuanced perspectives that consider the dynamic interactions between materials and biological environments. Modern definitions encompass the material's ability to perform with an appropriate host response in specific applications, recognizing that biocompatibility is context-dependent rather than an inherent material property.
The field now faces increasing complexity as advances in regenerative medicine, drug delivery systems, and implantable devices demand polymers with precisely tailored biological interactions. This complexity is further amplified by regulatory frameworks that require comprehensive biocompatibility testing according to standards such as ISO 10993 and FDA guidelines.
The primary objective of this technical research is to systematically analyze the determinants of biocompatibility in biomedical polymers, examining both material-intrinsic factors and extrinsic variables that influence host responses. We aim to establish correlations between polymer chemistry, surface properties, processing methods, and resulting biological performance to develop predictive models for biocompatibility.
Additionally, this research seeks to identify emerging trends in biocompatible polymer design, including stimuli-responsive materials, biomimetic approaches, and cell-instructive polymers. By mapping the technological landscape, we intend to highlight promising research directions and potential breakthrough technologies that could address current limitations in biomedical polymer applications.
The ultimate goal is to provide a comprehensive framework for understanding, predicting, and enhancing biocompatibility of polymeric biomaterials, thereby accelerating the development of next-generation medical devices and therapies with improved clinical outcomes and reduced development timelines.
The evolution of biomedical polymers has been characterized by three distinct generations. First-generation materials (1960s-1970s) focused primarily on achieving minimal toxicity and tissue reaction. Second-generation materials (1980s-1990s) introduced bioactive properties and controlled degradation profiles. Current third-generation materials aim to stimulate specific cellular responses at the molecular level, representing a paradigm shift from merely compatible materials to those that actively participate in healing processes.
Understanding biocompatibility has similarly evolved from simplistic definitions centered on "doing no harm" to more nuanced perspectives that consider the dynamic interactions between materials and biological environments. Modern definitions encompass the material's ability to perform with an appropriate host response in specific applications, recognizing that biocompatibility is context-dependent rather than an inherent material property.
The field now faces increasing complexity as advances in regenerative medicine, drug delivery systems, and implantable devices demand polymers with precisely tailored biological interactions. This complexity is further amplified by regulatory frameworks that require comprehensive biocompatibility testing according to standards such as ISO 10993 and FDA guidelines.
The primary objective of this technical research is to systematically analyze the determinants of biocompatibility in biomedical polymers, examining both material-intrinsic factors and extrinsic variables that influence host responses. We aim to establish correlations between polymer chemistry, surface properties, processing methods, and resulting biological performance to develop predictive models for biocompatibility.
Additionally, this research seeks to identify emerging trends in biocompatible polymer design, including stimuli-responsive materials, biomimetic approaches, and cell-instructive polymers. By mapping the technological landscape, we intend to highlight promising research directions and potential breakthrough technologies that could address current limitations in biomedical polymer applications.
The ultimate goal is to provide a comprehensive framework for understanding, predicting, and enhancing biocompatibility of polymeric biomaterials, thereby accelerating the development of next-generation medical devices and therapies with improved clinical outcomes and reduced development timelines.
Market Analysis of Biocompatible Polymer Applications
The global biocompatible polymer market has experienced significant growth in recent years, driven primarily by increasing applications in medical devices, drug delivery systems, tissue engineering, and implantable materials. The market was valued at approximately 62.5 billion USD in 2022 and is projected to reach 125.7 billion USD by 2030, representing a compound annual growth rate (CAGR) of 9.1% during the forecast period.
Healthcare applications dominate the biocompatible polymer market, accounting for over 70% of the total market share. Within this segment, orthopedic implants represent the largest application area, followed by cardiovascular devices and drug delivery systems. The growing prevalence of chronic diseases, aging populations, and increasing demand for minimally invasive surgical procedures have substantially contributed to market expansion.
Geographically, North America holds the largest market share at approximately 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and growing medical tourism in countries like China, India, and South Korea.
The biodegradable polymer segment is experiencing particularly rapid growth, with a CAGR of 12.3%, outpacing non-biodegradable alternatives. This trend reflects increasing environmental concerns and regulatory pressures favoring sustainable materials. Polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers are the most widely used biodegradable polymers, collectively accounting for approximately 45% of the biodegradable polymer market.
Consumer demand is increasingly shifting toward customized biocompatible polymers with enhanced properties such as antimicrobial characteristics, improved mechanical strength, and controlled degradation rates. This trend is particularly evident in advanced wound care products and 3D-printed implants, where market growth rates exceed 15% annually.
Regulatory factors significantly influence market dynamics, with stringent approval processes in developed markets creating barriers to entry but also ensuring product quality and safety. The FDA in the United States and the European Medicines Agency have established comprehensive frameworks for evaluating biocompatible materials, which manufacturers must navigate to bring products to market.
Investment in research and development of novel biocompatible polymers has increased by approximately 11% annually over the past five years, with particular focus on smart polymers that respond to specific biological stimuli. This innovation pipeline suggests continued market expansion and diversification of applications in the coming decade.
Healthcare applications dominate the biocompatible polymer market, accounting for over 70% of the total market share. Within this segment, orthopedic implants represent the largest application area, followed by cardiovascular devices and drug delivery systems. The growing prevalence of chronic diseases, aging populations, and increasing demand for minimally invasive surgical procedures have substantially contributed to market expansion.
Geographically, North America holds the largest market share at approximately 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and growing medical tourism in countries like China, India, and South Korea.
The biodegradable polymer segment is experiencing particularly rapid growth, with a CAGR of 12.3%, outpacing non-biodegradable alternatives. This trend reflects increasing environmental concerns and regulatory pressures favoring sustainable materials. Polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers are the most widely used biodegradable polymers, collectively accounting for approximately 45% of the biodegradable polymer market.
Consumer demand is increasingly shifting toward customized biocompatible polymers with enhanced properties such as antimicrobial characteristics, improved mechanical strength, and controlled degradation rates. This trend is particularly evident in advanced wound care products and 3D-printed implants, where market growth rates exceed 15% annually.
Regulatory factors significantly influence market dynamics, with stringent approval processes in developed markets creating barriers to entry but also ensuring product quality and safety. The FDA in the United States and the European Medicines Agency have established comprehensive frameworks for evaluating biocompatible materials, which manufacturers must navigate to bring products to market.
Investment in research and development of novel biocompatible polymers has increased by approximately 11% annually over the past five years, with particular focus on smart polymers that respond to specific biological stimuli. This innovation pipeline suggests continued market expansion and diversification of applications in the coming decade.
Current Biocompatibility Assessment Challenges
Despite significant advancements in biocompatibility testing methodologies, the field faces several persistent challenges that impede accurate assessment of biomedical polymers. The current regulatory framework, primarily based on ISO 10993 standards, often fails to address the complexity of modern biomaterials and their diverse applications. These standards, while comprehensive, tend to emphasize acute toxicity over long-term performance and integration, creating a disconnect between testing outcomes and clinical reality.
A fundamental challenge lies in the limited correlation between in vitro and in vivo testing results. Cell culture models frequently fail to replicate the complex biological environment encountered by implanted materials, leading to discrepancies when materials transition to animal models or human applications. This translation gap represents a significant hurdle in predicting actual biocompatibility performance.
The dynamic nature of the host response presents another substantial challenge. Current assessment methods struggle to capture the temporal evolution of the foreign body reaction, which transitions through inflammation, foreign body giant cell formation, fibrosis, and potential integration phases. Static testing protocols often miss these critical dynamics, resulting in incomplete biocompatibility profiles.
Material degradation and leaching behaviors pose additional complications. Contemporary testing protocols inadequately address the long-term release of degradation products, additives, or processing residues that may accumulate in tissues over extended periods. This limitation is particularly problematic for biodegradable polymers, where degradation kinetics significantly influence biocompatibility outcomes.
Standardization issues further complicate assessment efforts. Variations in testing protocols between laboratories lead to inconsistent results, making cross-study comparisons challenging. The absence of universally accepted reference materials compounds this problem, as does the lack of standardized biological endpoints for specific applications.
Emerging technologies present novel challenges to established testing paradigms. Nanomaterials, surface-modified polymers, and composite materials exhibit unique biological interactions that conventional tests may not adequately evaluate. Similarly, 3D-printed polymeric structures with complex geometries and potentially entrapped processing agents require specialized assessment approaches currently underdeveloped.
Patient-specific factors represent perhaps the most overlooked aspect of biocompatibility assessment. Individual variations in immune response, metabolism, and physiological environment can dramatically alter material-tissue interactions. Current testing frameworks rarely account for these patient-specific variables, limiting the predictive value of standardized assessments for diverse patient populations.
A fundamental challenge lies in the limited correlation between in vitro and in vivo testing results. Cell culture models frequently fail to replicate the complex biological environment encountered by implanted materials, leading to discrepancies when materials transition to animal models or human applications. This translation gap represents a significant hurdle in predicting actual biocompatibility performance.
The dynamic nature of the host response presents another substantial challenge. Current assessment methods struggle to capture the temporal evolution of the foreign body reaction, which transitions through inflammation, foreign body giant cell formation, fibrosis, and potential integration phases. Static testing protocols often miss these critical dynamics, resulting in incomplete biocompatibility profiles.
Material degradation and leaching behaviors pose additional complications. Contemporary testing protocols inadequately address the long-term release of degradation products, additives, or processing residues that may accumulate in tissues over extended periods. This limitation is particularly problematic for biodegradable polymers, where degradation kinetics significantly influence biocompatibility outcomes.
Standardization issues further complicate assessment efforts. Variations in testing protocols between laboratories lead to inconsistent results, making cross-study comparisons challenging. The absence of universally accepted reference materials compounds this problem, as does the lack of standardized biological endpoints for specific applications.
Emerging technologies present novel challenges to established testing paradigms. Nanomaterials, surface-modified polymers, and composite materials exhibit unique biological interactions that conventional tests may not adequately evaluate. Similarly, 3D-printed polymeric structures with complex geometries and potentially entrapped processing agents require specialized assessment approaches currently underdeveloped.
Patient-specific factors represent perhaps the most overlooked aspect of biocompatibility assessment. Individual variations in immune response, metabolism, and physiological environment can dramatically alter material-tissue interactions. Current testing frameworks rarely account for these patient-specific variables, limiting the predictive value of standardized assessments for diverse patient populations.
Established Biocompatibility Enhancement Methodologies
01 Surface modification of biomedical polymers
Surface modification techniques can enhance the biocompatibility of polymers used in medical applications. These techniques include coating, grafting, and chemical treatment to alter the surface properties without affecting the bulk characteristics. Modified surfaces can reduce protein adsorption, prevent cell adhesion, or promote specific cellular interactions depending on the intended application. These modifications help minimize immune responses and improve integration with biological tissues.- Surface modification techniques for biocompatible polymers: Various surface modification techniques can be applied to biomedical polymers to enhance their biocompatibility. These techniques include plasma treatment, chemical grafting, and coating with bioactive molecules. Such modifications can alter the surface properties of polymers to reduce immune response, prevent protein adsorption, and improve cell adhesion, making them more suitable for implantation and interaction with biological tissues.
- Biodegradable polymer systems for medical applications: Biodegradable polymers offer significant advantages in medical applications as they can be metabolized and eliminated from the body after serving their purpose. These polymers can be engineered to degrade at controlled rates, releasing therapeutic agents or gradually transferring load-bearing responsibilities to healing tissues. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which have been extensively studied for applications in tissue engineering, drug delivery, and temporary implants.
- Hydrogel-based biomedical polymers: Hydrogels are three-dimensional networks of hydrophilic polymers that can absorb significant amounts of water while maintaining their structure. Their high water content and soft consistency make them similar to natural tissues, enhancing their biocompatibility. These materials can be designed with tunable mechanical properties, degradation rates, and response to stimuli such as pH or temperature. Hydrogels are widely used in contact lenses, wound dressings, drug delivery systems, and as scaffolds for tissue engineering due to their excellent biocompatibility and versatility.
- Biocompatibility testing methods for polymeric biomaterials: Standardized testing methods are essential for evaluating the biocompatibility of polymeric biomaterials before clinical use. These methods include in vitro cytotoxicity assays, hemolysis tests, protein adsorption studies, and in vivo implantation tests. Advanced techniques such as cell adhesion studies, inflammatory response assessments, and long-term degradation analyses provide comprehensive information about how polymeric materials interact with biological systems. These testing protocols help ensure that biomedical polymers meet safety and performance requirements for their intended applications.
- Polymer-based drug delivery systems: Polymers can be engineered to deliver therapeutic agents in a controlled manner, improving drug efficacy while reducing side effects. These systems can be designed to respond to specific physiological conditions, targeting drug release to particular tissues or cellular compartments. Biocompatible polymers used in drug delivery include polyethylene glycol (PEG), poly(lactic-co-glycolic acid) (PLGA), and various natural polymers like chitosan and alginate. The biocompatibility of these systems is crucial for preventing adverse reactions while maintaining therapeutic effectiveness over the required duration.
02 Biodegradable polymers for medical implants
Biodegradable polymers offer significant advantages for temporary medical implants as they eliminate the need for removal surgery. These materials gradually break down in the body into non-toxic components that can be metabolized or excreted. The degradation rate can be tailored by adjusting the polymer composition, molecular weight, and crystallinity. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which have demonstrated good biocompatibility in various clinical applications.Expand Specific Solutions03 Hydrogel polymers for biomedical applications
Hydrogels are water-swollen polymeric networks that mimic the properties of natural tissues, making them excellent candidates for biomedical applications. Their high water content and soft consistency provide good biocompatibility and reduced mechanical irritation to surrounding tissues. These materials can be designed with stimuli-responsive properties, allowing controlled release of therapeutic agents or dynamic changes in physical properties. Applications include wound dressings, drug delivery systems, tissue engineering scaffolds, and contact lenses.Expand Specific Solutions04 Biocompatibility testing methods for polymeric materials
Standardized testing protocols are essential for evaluating the biocompatibility of polymeric materials intended for medical use. These tests assess cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity according to international standards. Advanced methods include in vitro cell culture systems, ex vivo tissue models, and computational approaches to predict biocompatibility. These testing methodologies help ensure safety and efficacy of polymeric biomaterials before clinical application and regulatory approval.Expand Specific Solutions05 Polymer-based drug delivery systems
Polymeric materials serve as effective carriers for controlled drug delivery, offering advantages such as sustained release, targeted delivery, and protection of therapeutic agents. Biocompatible polymers can be formulated into various structures including nanoparticles, microspheres, and implantable devices to optimize drug release kinetics. Smart polymer systems respond to specific physiological conditions or external stimuli to release drugs at the right time and location. These systems enhance therapeutic efficacy while reducing side effects and improving patient compliance.Expand Specific Solutions
Leading Organizations in Biomedical Polymer Research
The biocompatibility of biomedical polymers market is in a growth phase, driven by increasing demand for implantable medical devices and tissue engineering applications. The global market size is estimated to exceed $10 billion, with annual growth rates of 8-10%. Leading players include established medical device manufacturers like Medtronic Vascular and Covidien (both Medtronic subsidiaries), who leverage extensive clinical experience in polymer applications. Academic institutions (Emory University, Zhejiang University) collaborate with specialized polymer companies such as Surmodics and Asahi Kasei to advance the technology. Material innovation leaders like Toyobo and Nippon Shokubai focus on developing novel biocompatible polymers with enhanced properties. The technology is approaching maturity for conventional applications, while emerging areas like biodegradable implants and drug-eluting polymers represent frontier research opportunities requiring further development.
Medtronic Vascular, Inc.
Technical Solution: Medtronic has developed proprietary surface modification technologies for their biomedical polymers, particularly focusing on their vascular implants. Their approach involves creating hydrophilic coatings that reduce protein adsorption and platelet adhesion, which are primary factors affecting biocompatibility. The company utilizes phosphorylcholine-based biomimetic coatings that mimic cell membrane surfaces to create "stealth" properties that help implants evade immune recognition. Additionally, Medtronic has pioneered controlled drug-eluting polymer systems that gradually release anti-inflammatory and anti-proliferative agents to modulate the foreign body response. Their research has demonstrated that surface topography at the micro and nano scale significantly influences cell adhesion and proliferation patterns, leading to the development of textured polymer surfaces with optimized cell interaction properties.
Strengths: Extensive clinical validation through their wide portfolio of implantable devices provides real-world biocompatibility data. Their proprietary surface modification technologies effectively reduce thrombogenicity in blood-contacting applications. Weaknesses: Their approaches often require complex manufacturing processes that increase production costs, and some coating technologies may have limited durability over extended implantation periods.
Asahi Kasei Corp.
Technical Solution: Asahi Kasei has developed advanced biocompatible polymer technologies focusing on hemocompatibility for blood-contacting medical devices. Their flagship approach involves cellulose-based hollow fiber membranes with modified surface properties for hemodialysis applications. These membranes incorporate precisely controlled pore structures and surface chemistries to minimize protein adsorption and complement activation. Asahi Kasei has pioneered the use of polyvinylpyrrolidone (PVP) blending and grafting techniques to create hydrophilic surfaces that resist protein fouling while maintaining mechanical integrity. Their research has demonstrated that controlling the microstructure of polymer surfaces at the nanometer scale significantly impacts blood compatibility by influencing protein conformation upon adsorption. The company has also developed proprietary manufacturing processes that eliminate potentially leachable additives and residual chemicals that could trigger adverse biological responses. Recent innovations include polymers with zwitterionic functional groups that create a tightly bound water layer at the material surface, effectively preventing protein adhesion and platelet activation.
Strengths: Their technologies demonstrate exceptional hemocompatibility with minimal complement activation and protein adsorption, making them ideal for blood-contacting applications. Their manufacturing processes ensure high purity materials with minimal leachables. Weaknesses: The specialized surface modifications may add significant cost to the final products, and some of their approaches may have limited applicability beyond blood-contacting devices.
Critical Biocompatibility Determinants Analysis
Biocompatible Material
PatentActiveUS20080262181A1
Innovation
- A biocompatible material is developed using a polymer composed of an amino acid-type betaine monomer and a polymerizable monomer, with a weight ratio ranging from 1/99 to 100/1, which minimizes interaction with proteins and blood cells, allowing for advanced medical device and artificial organ development.
Biocompatible polymers for coating or fabricating implantable medical devices
PatentInactiveEP2413987A2
Innovation
- Development of biocompatible polymers comprising hydroxyalkyl methacrylate and acrylate monomers, such as 4-hydroxybutyl methacrylate and hexyl methacrylate, for coating or fabricating implantable medical devices, which provide controlled drug release and improved biocompatibility by minimizing immune reactions and cytotoxicity.
Regulatory Framework for Biomedical Materials
The regulatory landscape governing biomedical polymers is complex and multifaceted, with frameworks varying across different regions while sharing fundamental principles focused on safety and efficacy. In the United States, the Food and Drug Administration (FDA) classifies biomedical materials into three categories based on risk levels, with polymeric materials typically evaluated through the 510(k) or Premarket Approval (PMA) pathways depending on their intended use and risk profile.
The European Union implements the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for clinical evidence and post-market surveillance of biomedical materials. These regulations emphasize the importance of biocompatibility testing according to ISO 10993 standards, which outline specific protocols for evaluating cytotoxicity, sensitization, irritation, and systemic toxicity of polymeric materials.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established its own regulatory framework that incorporates elements from both US and EU systems while adding Japan-specific requirements. Similarly, China's National Medical Products Administration (NMPA) has developed comprehensive regulations for biomedical materials with particular emphasis on local testing requirements.
International harmonization efforts, led by organizations such as the International Medical Device Regulators Forum (IMDRF), aim to standardize biocompatibility testing methodologies and acceptance criteria across different jurisdictions. These initiatives have resulted in the development of globally recognized standards like ISO 10993 and ASTM F748, which provide specific guidelines for evaluating the biocompatibility of polymers intended for medical applications.
Regulatory frameworks increasingly incorporate risk-based approaches that consider the nature and duration of body contact when determining testing requirements. For example, polymers used in transient contact devices face less stringent requirements compared to those used in long-term implantable devices. This tiered approach helps balance the need for comprehensive safety evaluation with practical considerations of time and resource constraints.
Recent regulatory trends show increased focus on the chemical characterization of polymeric materials, with authorities requiring more detailed information about extractables and leachables. Additionally, there is growing emphasis on evaluating the long-term stability and degradation profiles of biodegradable polymers, with regulatory bodies demanding robust data on degradation products and their potential biological effects.
Compliance with these regulatory frameworks represents a significant challenge for developers of biomedical polymers, requiring substantial investment in testing and documentation. However, early engagement with regulatory authorities through pre-submission consultations can help navigate these complex requirements more efficiently and increase the likelihood of successful market authorization.
The European Union implements the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for clinical evidence and post-market surveillance of biomedical materials. These regulations emphasize the importance of biocompatibility testing according to ISO 10993 standards, which outline specific protocols for evaluating cytotoxicity, sensitization, irritation, and systemic toxicity of polymeric materials.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established its own regulatory framework that incorporates elements from both US and EU systems while adding Japan-specific requirements. Similarly, China's National Medical Products Administration (NMPA) has developed comprehensive regulations for biomedical materials with particular emphasis on local testing requirements.
International harmonization efforts, led by organizations such as the International Medical Device Regulators Forum (IMDRF), aim to standardize biocompatibility testing methodologies and acceptance criteria across different jurisdictions. These initiatives have resulted in the development of globally recognized standards like ISO 10993 and ASTM F748, which provide specific guidelines for evaluating the biocompatibility of polymers intended for medical applications.
Regulatory frameworks increasingly incorporate risk-based approaches that consider the nature and duration of body contact when determining testing requirements. For example, polymers used in transient contact devices face less stringent requirements compared to those used in long-term implantable devices. This tiered approach helps balance the need for comprehensive safety evaluation with practical considerations of time and resource constraints.
Recent regulatory trends show increased focus on the chemical characterization of polymeric materials, with authorities requiring more detailed information about extractables and leachables. Additionally, there is growing emphasis on evaluating the long-term stability and degradation profiles of biodegradable polymers, with regulatory bodies demanding robust data on degradation products and their potential biological effects.
Compliance with these regulatory frameworks represents a significant challenge for developers of biomedical polymers, requiring substantial investment in testing and documentation. However, early engagement with regulatory authorities through pre-submission consultations can help navigate these complex requirements more efficiently and increase the likelihood of successful market authorization.
Host-Material Interaction Mechanisms
The interaction between host biological systems and implanted polymeric materials represents a complex interplay of physical, chemical, and biological processes that ultimately determine biocompatibility outcomes. At the molecular level, when a polymer material contacts biological fluids, protein adsorption occurs within seconds to minutes, forming a conditioning film that mediates subsequent cellular responses. This protein layer's composition and conformation significantly influence how the host recognizes and responds to the foreign material.
Surface properties of polymers play a crucial role in these interactions. Hydrophilicity/hydrophobicity balance affects protein adsorption patterns, with hydrophobic surfaces typically attracting more proteins but often in denatured conformations. Surface charge influences electrostatic interactions with biological components, while surface roughness and topography can modulate cellular adhesion, proliferation, and differentiation behaviors at the interface.
The inflammatory response represents a critical host-material interaction mechanism. Upon implantation, polymeric materials trigger an acute inflammatory response characterized by neutrophil recruitment, followed by monocytes that differentiate into macrophages. These macrophages may fuse to form foreign body giant cells when unable to phagocytose larger implants. The persistence and intensity of this inflammatory cascade significantly impact long-term biocompatibility and material integration.
Complement activation represents another key interaction pathway, where polymer surfaces can trigger this proteolytic cascade through classical, alternative, or lectin pathways. The resulting complement fragments serve as opsonins facilitating phagocytosis and amplify inflammatory responses through anaphylatoxin production, directly affecting material biocompatibility.
Material degradation mechanisms constitute important dynamic aspects of host-material interactions. Hydrolytic and enzymatic degradation of polymers can release monomers, oligomers, or additives that may trigger local or systemic toxicity. Additionally, oxidative degradation mediated by reactive oxygen species produced during inflammatory responses can alter material properties over time, creating a feedback loop that influences ongoing host responses.
The formation of a fibrous capsule represents the end-stage host response to many implanted polymers. This encapsulation process involves fibroblast recruitment, collagen deposition, and matrix remodeling, effectively isolating the material from surrounding tissues. The thickness and composition of this capsule reflect the cumulative host-material interactions and significantly impact the functional performance of implanted devices.
Surface properties of polymers play a crucial role in these interactions. Hydrophilicity/hydrophobicity balance affects protein adsorption patterns, with hydrophobic surfaces typically attracting more proteins but often in denatured conformations. Surface charge influences electrostatic interactions with biological components, while surface roughness and topography can modulate cellular adhesion, proliferation, and differentiation behaviors at the interface.
The inflammatory response represents a critical host-material interaction mechanism. Upon implantation, polymeric materials trigger an acute inflammatory response characterized by neutrophil recruitment, followed by monocytes that differentiate into macrophages. These macrophages may fuse to form foreign body giant cells when unable to phagocytose larger implants. The persistence and intensity of this inflammatory cascade significantly impact long-term biocompatibility and material integration.
Complement activation represents another key interaction pathway, where polymer surfaces can trigger this proteolytic cascade through classical, alternative, or lectin pathways. The resulting complement fragments serve as opsonins facilitating phagocytosis and amplify inflammatory responses through anaphylatoxin production, directly affecting material biocompatibility.
Material degradation mechanisms constitute important dynamic aspects of host-material interactions. Hydrolytic and enzymatic degradation of polymers can release monomers, oligomers, or additives that may trigger local or systemic toxicity. Additionally, oxidative degradation mediated by reactive oxygen species produced during inflammatory responses can alter material properties over time, creating a feedback loop that influences ongoing host responses.
The formation of a fibrous capsule represents the end-stage host response to many implanted polymers. This encapsulation process involves fibroblast recruitment, collagen deposition, and matrix remodeling, effectively isolating the material from surrounding tissues. The thickness and composition of this capsule reflect the cumulative host-material interactions and significantly impact the functional performance of implanted devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!