How Biomedical Polymers Facilitate Microfluidics in Labs
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Evolution and Research Objectives
Biomedical polymers have undergone significant evolution since their initial introduction in laboratory settings in the mid-20th century. Early applications primarily focused on basic structural components, with materials like polyethylene and polyvinyl chloride serving rudimentary functions in laboratory equipment. The 1970s marked a pivotal shift with the development of more sophisticated polymers exhibiting enhanced biocompatibility and specialized properties, enabling their integration into more complex biomedical applications.
The 1990s witnessed a revolutionary advancement with the emergence of microfluidic technologies, where biomedical polymers began playing a crucial role in miniaturized laboratory systems. This period saw the introduction of polydimethylsiloxane (PDMS), which quickly became the gold standard material for microfluidic device fabrication due to its optical transparency, gas permeability, and ease of processing through soft lithography techniques.
Recent decades have been characterized by accelerated innovation in polymer chemistry and processing technologies. The development of stimuli-responsive polymers, biodegradable materials, and polymer nanocomposites has significantly expanded the functional capabilities of microfluidic systems. These advancements have enabled precise control over fluid dynamics at microscale levels, facilitating complex operations such as cell sorting, drug screening, and point-of-care diagnostics.
Current research trends indicate a growing focus on sustainable and environmentally friendly polymer alternatives, addressing concerns about waste generation in laboratory settings. Additionally, there is increasing interest in developing polymers with enhanced surface properties for specific biological interactions, improved optical characteristics for advanced detection methods, and greater chemical resistance for compatibility with diverse reagents.
The primary technical objectives in this field include developing polymers with superior mechanical stability under various operating conditions, enhancing biocompatibility for cell-based applications, and improving manufacturing scalability to facilitate broader adoption of microfluidic technologies. Researchers are particularly focused on creating polymers that can maintain precise microstructural features while offering flexibility in design and functionality.
Another critical research goal involves the development of multi-functional polymers capable of integrating sensing capabilities directly into microfluidic structures, potentially eliminating the need for external detection systems. This approach aims to create more compact, efficient, and cost-effective lab-on-a-chip devices that can revolutionize biomedical research and clinical diagnostics.
The convergence of advanced polymer science with microfluidic engineering represents a promising frontier for addressing complex biomedical challenges, with potential applications spanning from fundamental research to clinical implementation. The continued evolution of biomedical polymers is expected to play a pivotal role in shaping the future landscape of laboratory technologies and healthcare solutions.
The 1990s witnessed a revolutionary advancement with the emergence of microfluidic technologies, where biomedical polymers began playing a crucial role in miniaturized laboratory systems. This period saw the introduction of polydimethylsiloxane (PDMS), which quickly became the gold standard material for microfluidic device fabrication due to its optical transparency, gas permeability, and ease of processing through soft lithography techniques.
Recent decades have been characterized by accelerated innovation in polymer chemistry and processing technologies. The development of stimuli-responsive polymers, biodegradable materials, and polymer nanocomposites has significantly expanded the functional capabilities of microfluidic systems. These advancements have enabled precise control over fluid dynamics at microscale levels, facilitating complex operations such as cell sorting, drug screening, and point-of-care diagnostics.
Current research trends indicate a growing focus on sustainable and environmentally friendly polymer alternatives, addressing concerns about waste generation in laboratory settings. Additionally, there is increasing interest in developing polymers with enhanced surface properties for specific biological interactions, improved optical characteristics for advanced detection methods, and greater chemical resistance for compatibility with diverse reagents.
The primary technical objectives in this field include developing polymers with superior mechanical stability under various operating conditions, enhancing biocompatibility for cell-based applications, and improving manufacturing scalability to facilitate broader adoption of microfluidic technologies. Researchers are particularly focused on creating polymers that can maintain precise microstructural features while offering flexibility in design and functionality.
Another critical research goal involves the development of multi-functional polymers capable of integrating sensing capabilities directly into microfluidic structures, potentially eliminating the need for external detection systems. This approach aims to create more compact, efficient, and cost-effective lab-on-a-chip devices that can revolutionize biomedical research and clinical diagnostics.
The convergence of advanced polymer science with microfluidic engineering represents a promising frontier for addressing complex biomedical challenges, with potential applications spanning from fundamental research to clinical implementation. The continued evolution of biomedical polymers is expected to play a pivotal role in shaping the future landscape of laboratory technologies and healthcare solutions.
Market Analysis for Polymer-Based Microfluidic Systems
The global market for polymer-based microfluidic systems has experienced substantial growth over the past decade, driven primarily by increasing applications in diagnostics, drug discovery, and personalized medicine. Current market valuations indicate the global microfluidics market reached approximately 20 billion USD in 2022, with polymer-based systems accounting for over 40% of this value. Industry analysts project a compound annual growth rate of 15-18% through 2028, significantly outpacing many other biomedical technology sectors.
Demand for polymer-based microfluidic systems stems from several key market segments. The pharmaceutical and biotechnology sector represents the largest consumer base, utilizing these systems for high-throughput screening, drug delivery testing, and cellular analysis. Healthcare diagnostics follows closely, with point-of-care testing and molecular diagnostics driving adoption. Academic and research institutions constitute the third major market segment, employing microfluidic technologies for fundamental research and educational purposes.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and India making substantial investments in biotechnology infrastructure and manufacturing capabilities for microfluidic devices.
The cost advantage of polymers compared to traditional glass or silicon substrates represents a significant market driver. Polydimethylsiloxane (PDMS) remains the most widely used polymer, commanding roughly 65% of the polymer microfluidics market due to its optical transparency, gas permeability, and biocompatibility. Thermoplastics such as polymethyl methacrylate (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC) collectively account for approximately 30% of the market.
Market challenges include standardization issues, integration with existing laboratory workflows, and scaling manufacturing processes while maintaining quality. The fragmented nature of the market, with numerous small and medium enterprises competing alongside larger corporations, creates both competitive pressure and innovation opportunities.
Customer demand increasingly focuses on complete solutions rather than individual components, driving manufacturers toward integrated systems that combine microfluidic chips with detection systems, software, and reagents. This trend toward turnkey solutions is reshaping the competitive landscape, favoring companies with broader technological capabilities and application expertise.
Demand for polymer-based microfluidic systems stems from several key market segments. The pharmaceutical and biotechnology sector represents the largest consumer base, utilizing these systems for high-throughput screening, drug delivery testing, and cellular analysis. Healthcare diagnostics follows closely, with point-of-care testing and molecular diagnostics driving adoption. Academic and research institutions constitute the third major market segment, employing microfluidic technologies for fundamental research and educational purposes.
Geographically, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and India making substantial investments in biotechnology infrastructure and manufacturing capabilities for microfluidic devices.
The cost advantage of polymers compared to traditional glass or silicon substrates represents a significant market driver. Polydimethylsiloxane (PDMS) remains the most widely used polymer, commanding roughly 65% of the polymer microfluidics market due to its optical transparency, gas permeability, and biocompatibility. Thermoplastics such as polymethyl methacrylate (PMMA), polycarbonate (PC), and cyclic olefin copolymer (COC) collectively account for approximately 30% of the market.
Market challenges include standardization issues, integration with existing laboratory workflows, and scaling manufacturing processes while maintaining quality. The fragmented nature of the market, with numerous small and medium enterprises competing alongside larger corporations, creates both competitive pressure and innovation opportunities.
Customer demand increasingly focuses on complete solutions rather than individual components, driving manufacturers toward integrated systems that combine microfluidic chips with detection systems, software, and reagents. This trend toward turnkey solutions is reshaping the competitive landscape, favoring companies with broader technological capabilities and application expertise.
Current Polymer Technologies and Microfluidic Challenges
The polymer landscape for microfluidic applications has evolved significantly over the past decade, with polydimethylsiloxane (PDMS) remaining the dominant material due to its excellent optical transparency, gas permeability, and ease of fabrication through soft lithography. However, PDMS presents notable limitations including hydrophobicity, absorption of small hydrophobic molecules, and challenges in mass production scaling. These limitations have driven research toward alternative polymers such as cyclic olefin copolymer (COC), polymethyl methacrylate (PMMA), and polyethylene terephthalate (PET), each offering specific advantages for different microfluidic applications.
Current fabrication technologies for polymer-based microfluidic devices include soft lithography, hot embossing, injection molding, and more recently, 3D printing. While soft lithography offers high resolution and flexibility for prototyping, it faces scalability challenges. Injection molding provides excellent mass production capabilities but requires significant initial investment. 3D printing has emerged as a promising rapid prototyping method but still struggles with resolution limitations and material compatibility issues.
Surface modification represents a critical challenge in polymer microfluidics, particularly for biomedical applications requiring specific surface properties. Techniques such as plasma treatment, chemical functionalization, and layer-by-layer deposition are employed to modify polymer surfaces, though achieving long-term stability of these modifications remains problematic. The hydrophobic recovery of PDMS surfaces, for instance, often limits the shelf life of modified devices.
Integration challenges persist at the interface between polymer microfluidics and other system components. World-to-chip interfaces—connections between the macro world and microfluidic channels—often suffer from leakage, dead volume, and compatibility issues. Additionally, integrating sensors, electrodes, and other functional elements while maintaining the integrity of polymer structures presents significant engineering challenges.
Biocompatibility concerns arise when polymer microfluidic devices interact with biological samples. Material leaching, non-specific protein adsorption, and cellular adhesion can compromise experimental results and device functionality. While PDMS generally exhibits good biocompatibility, residual uncured oligomers can affect cell cultures. Alternative polymers like COC offer improved chemical resistance but may require surface treatments to achieve optimal biocompatibility.
Scaling production from laboratory prototypes to commercial manufacturing represents perhaps the most significant challenge. The transition from PDMS-based prototyping to thermoplastic mass production requires substantial redesign and optimization. Current industry efforts focus on developing standardized manufacturing processes and materials that maintain the precision of laboratory devices while enabling cost-effective mass production.
Current fabrication technologies for polymer-based microfluidic devices include soft lithography, hot embossing, injection molding, and more recently, 3D printing. While soft lithography offers high resolution and flexibility for prototyping, it faces scalability challenges. Injection molding provides excellent mass production capabilities but requires significant initial investment. 3D printing has emerged as a promising rapid prototyping method but still struggles with resolution limitations and material compatibility issues.
Surface modification represents a critical challenge in polymer microfluidics, particularly for biomedical applications requiring specific surface properties. Techniques such as plasma treatment, chemical functionalization, and layer-by-layer deposition are employed to modify polymer surfaces, though achieving long-term stability of these modifications remains problematic. The hydrophobic recovery of PDMS surfaces, for instance, often limits the shelf life of modified devices.
Integration challenges persist at the interface between polymer microfluidics and other system components. World-to-chip interfaces—connections between the macro world and microfluidic channels—often suffer from leakage, dead volume, and compatibility issues. Additionally, integrating sensors, electrodes, and other functional elements while maintaining the integrity of polymer structures presents significant engineering challenges.
Biocompatibility concerns arise when polymer microfluidic devices interact with biological samples. Material leaching, non-specific protein adsorption, and cellular adhesion can compromise experimental results and device functionality. While PDMS generally exhibits good biocompatibility, residual uncured oligomers can affect cell cultures. Alternative polymers like COC offer improved chemical resistance but may require surface treatments to achieve optimal biocompatibility.
Scaling production from laboratory prototypes to commercial manufacturing represents perhaps the most significant challenge. The transition from PDMS-based prototyping to thermoplastic mass production requires substantial redesign and optimization. Current industry efforts focus on developing standardized manufacturing processes and materials that maintain the precision of laboratory devices while enabling cost-effective mass production.
Contemporary Polymer Solutions for Microfluidic Applications
01 Biomedical polymer applications in drug delivery systems
Biomedical polymers are utilized in various drug delivery systems to facilitate controlled release of therapeutic agents. These polymers can be engineered to respond to specific biological environments, allowing for targeted drug delivery. The polymeric matrices can encapsulate drugs and release them at predetermined rates, improving therapeutic efficacy while reducing side effects. These systems can be designed as implants, microparticles, or nanocarriers depending on the specific medical application.- Biocompatible polymer systems for drug delivery: Biocompatible polymer systems are designed for controlled drug delivery applications in medical treatments. These polymers can be formulated to release therapeutic agents at specific rates and targeted locations within the body. The systems often incorporate biodegradable materials that safely break down over time, eliminating the need for removal after drug depletion. Advanced formulations may include responsive elements that can adjust release rates based on physiological conditions or external stimuli.
- Polymer-based implantable medical devices: Biomedical polymers are utilized in the development of implantable medical devices that provide structural support or functional replacement of biological tissues. These polymers are engineered to have specific mechanical properties, durability, and biocompatibility suitable for long-term implantation. The materials may be designed to integrate with surrounding tissues, resist infection, and minimize inflammatory responses. Applications include orthopedic implants, cardiovascular devices, and tissue engineering scaffolds.
- Smart polymers for biosensing applications: Smart polymers with responsive properties are employed in biosensing technologies to detect biological markers or environmental changes. These materials can undergo conformational or property changes in response to specific stimuli such as pH, temperature, or the presence of target molecules. The responsive nature of these polymers enables real-time monitoring of physiological conditions or disease markers. Integration with electronic components allows for data collection and transmission for medical diagnostics and patient monitoring.
- Polymer surface modifications for enhanced biocompatibility: Surface modification techniques are applied to polymers to enhance their biocompatibility and functionality in medical applications. These modifications can alter surface chemistry, topography, or incorporate bioactive molecules to improve cell adhesion, reduce protein adsorption, or prevent bacterial colonization. Methods include plasma treatment, chemical grafting, and coating with bioactive compounds. Such modifications are crucial for improving the performance and integration of polymeric materials in biological environments without altering their bulk properties.
- AI and digital technologies for polymer development: Artificial intelligence and digital technologies are increasingly utilized to facilitate the design, testing, and manufacturing of biomedical polymers. These technologies enable predictive modeling of polymer properties, optimization of formulations, and simulation of in vivo performance. Machine learning algorithms can analyze vast datasets to identify patterns and relationships that inform polymer design decisions. Digital platforms also support supply chain management, quality control, and regulatory compliance in the production of biomedical polymers.
02 Biodegradable polymers for tissue engineering
Biodegradable polymers serve as scaffolds in tissue engineering applications, providing temporary support structures that facilitate cell growth and tissue regeneration. These polymers gradually degrade as new tissue forms, eliminating the need for removal surgeries. The degradation rate can be tailored by modifying the polymer composition and structure. Various natural and synthetic biodegradable polymers are used, including polylactic acid, polyglycolic acid, and their copolymers, which offer biocompatibility and appropriate mechanical properties for different tissue types.Expand Specific Solutions03 Smart polymers for biosensing and diagnostics
Smart polymers that respond to environmental stimuli are increasingly used in biosensing and diagnostic applications. These polymers can change their properties in response to specific biological markers, pH changes, temperature variations, or other stimuli. This responsiveness enables the development of advanced biosensors for continuous monitoring of physiological parameters and early disease detection. The integration of these polymers with electronic components allows for real-time data collection and analysis, enhancing diagnostic capabilities in clinical settings.Expand Specific Solutions04 Polymer-based implantable medical devices
Biomedical polymers are extensively used in the development of implantable medical devices due to their versatility and biocompatibility. These polymers can be processed into various forms including films, fibers, and three-dimensional structures to create devices such as stents, heart valves, and orthopedic implants. The surface properties of these polymers can be modified to enhance biocompatibility and reduce adverse immune responses. Advanced manufacturing techniques like 3D printing allow for the creation of patient-specific implants with complex geometries.Expand Specific Solutions05 AI and digital technologies in polymer development
Artificial intelligence and digital technologies are revolutionizing the development and application of biomedical polymers. Machine learning algorithms can predict polymer properties and behavior in biological environments, accelerating the design process. Digital platforms facilitate the tracking and analysis of polymer performance in clinical applications, enabling continuous improvement. These technologies also support regulatory compliance by maintaining comprehensive documentation of polymer development and testing. The integration of AI with polymer science is creating new opportunities for personalized medicine and advanced therapeutic approaches.Expand Specific Solutions
Leading Companies and Research Institutions in Biomedical Microfluidics
The biomedical polymers in microfluidics market is currently in a growth phase, with increasing adoption across laboratory applications. The global market size is estimated to reach $15-20 billion by 2025, driven by demand for point-of-care diagnostics and lab-on-chip technologies. Technologically, the field is maturing rapidly with key players demonstrating varying levels of innovation. Companies like Corning and HP Development are leading with established polymer-based microfluidic platforms, while Samsung Electronics and Robert Bosch GmbH are advancing integration with electronic systems. Academic institutions including California Institute of Technology, Peking University, and Fudan University are pioneering next-generation materials and fabrication techniques. Research organizations like Agency for Science, Technology & Research and Commonwealth Scientific & Industrial Research Organisation are bridging the gap between fundamental research and commercial applications through collaborative industry partnerships.
Corning, Inc.
Technical Solution: Corning has pioneered advanced polymer-based microfluidic solutions through their Corning® Microfluidic Discovery Platform. Their technology utilizes proprietary thermoplastic polymers (primarily cyclic olefin polymers and copolymers) engineered for optimal optical clarity, chemical resistance, and biocompatibility. Corning's approach involves precision injection molding and hot embossing techniques to create complex microfluidic architectures with feature sizes down to 10 micrometers. Their polymer systems incorporate specialized surface treatments that enable controlled wettability and protein adsorption characteristics, critical for biological applications. Corning has developed multi-layer lamination techniques that allow integration of functional elements like valves and pumps directly within the polymer substrate, creating truly integrated lab-on-chip devices for applications ranging from drug discovery to point-of-care diagnostics.
Strengths: Exceptional optical clarity of their polymer formulations enables sensitive fluorescence detection directly on-chip. Their manufacturing processes allow for cost-effective mass production while maintaining tight tolerances. Weaknesses: Some of their thermoplastic systems have temperature limitations that may restrict applications requiring high-temperature reactions or sterilization protocols.
California Institute of Technology
Technical Solution: Caltech has developed groundbreaking polymer-based microfluidic technologies through their Microfluidic Foundry and related research initiatives. Their approach centers on multi-layer soft lithography using polydimethylsiloxane (PDMS), enabling the creation of complex three-dimensional microfluidic architectures with integrated pneumatic valves and pumps. Caltech researchers pioneered the development of "microfluidic large-scale integration" (mLSI), allowing thousands of micromechanical valves and hundreds of individually addressable chambers on a single polymer chip. Their technology utilizes specialized surface modifications of PDMS to control protein adsorption and cell adhesion properties. Recent innovations include thiol-ene based polymer systems that offer improved chemical resistance while maintaining the fabrication advantages of soft lithography. Caltech has also developed novel approaches for integrating sensing elements directly into polymer substrates, enabling real-time monitoring of biological processes within their microfluidic devices.
Strengths: Unparalleled ability to create densely integrated microfluidic circuits with thousands of functional elements on a single chip. Their soft lithography techniques enable rapid prototyping and iteration of complex designs. Weaknesses: PDMS-based systems can suffer from solvent compatibility issues and potential for unwanted absorption of small hydrophobic molecules, which may interfere with certain biochemical assays.
Key Patents and Innovations in Biomedical Polymer Microfluidics
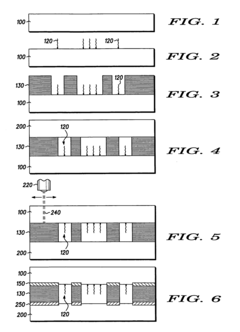
Methods of making microfluidic devices
PatentWO2017089963A1
Innovation
- A non-traditional method using stereolithography with a maskless UV projection system to fabricate microfluidic devices, allowing for rapid fabrication in under 5 minutes using a photocurable resin and a digital light projector, which can cure selected areas between two substrates to form channels.
Microfluidic channels with attached biomolecules
PatentInactiveUS20060105382A1
Innovation
- A method using a thermoplastic mask sandwiched between polymer substrate layers, where the mask absorbs laser radiation to create localized heat for bonding without damaging attached active species, allowing for selective wavelength laser bonding that preserves the functionality of the molecules.
Biocompatibility and Safety Standards for Lab Microfluidics
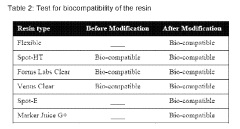
The biocompatibility and safety of microfluidic devices are paramount considerations in laboratory applications, especially when handling biological samples or developing diagnostic tools. Regulatory bodies worldwide have established comprehensive standards to ensure these devices meet stringent safety requirements before implementation in clinical or research settings.
ISO 10993 serves as the cornerstone for biocompatibility evaluation of medical devices, with specific sections applicable to microfluidic systems. This standard outlines protocols for cytotoxicity testing, sensitization assessment, and systemic toxicity evaluation—all critical for devices that contact biological fluids or tissues. For microfluidic applications, ISO 10993-4 specifically addresses interactions with blood, which is essential for hemocompatibility testing of lab-on-chip devices.
The FDA has developed specialized guidance documents for microfluidic technologies, emphasizing material selection criteria and validation methodologies. These guidelines require manufacturers to demonstrate that polymers used in microfluidic channels do not leach harmful substances or alter sample integrity during analysis. The FDA's approach focuses on both the initial safety assessment and long-term stability of polymer-based microfluidic platforms.
European regulations under the Medical Device Regulation (MDR) impose additional requirements for microfluidic devices intended for diagnostic applications. These regulations mandate comprehensive risk management processes and technical documentation that specifically addresses material biocompatibility and potential degradation products.
Biomedical polymers commonly used in microfluidics—such as PDMS, PMMA, and COC—must undergo rigorous testing to verify their compliance with these standards. Testing protocols typically include extraction studies to identify leachables, protein adsorption assessments to evaluate non-specific binding, and endotoxin testing to ensure pyrogen-free surfaces.
The emergence of organ-on-chip technologies has prompted the development of specialized biocompatibility standards that address the unique requirements of these complex systems. These standards focus on maintaining cell viability and functionality within polymer microenvironments over extended periods, necessitating more sophisticated biocompatibility metrics than traditional static culture systems.
International harmonization efforts are underway to standardize biocompatibility testing methodologies specifically for microfluidic applications. The ASTM International Committee F04 has established working groups dedicated to developing consensus standards for evaluating the biological response to microfluidic materials and devices, facilitating global acceptance and implementation of these technologies.
ISO 10993 serves as the cornerstone for biocompatibility evaluation of medical devices, with specific sections applicable to microfluidic systems. This standard outlines protocols for cytotoxicity testing, sensitization assessment, and systemic toxicity evaluation—all critical for devices that contact biological fluids or tissues. For microfluidic applications, ISO 10993-4 specifically addresses interactions with blood, which is essential for hemocompatibility testing of lab-on-chip devices.
The FDA has developed specialized guidance documents for microfluidic technologies, emphasizing material selection criteria and validation methodologies. These guidelines require manufacturers to demonstrate that polymers used in microfluidic channels do not leach harmful substances or alter sample integrity during analysis. The FDA's approach focuses on both the initial safety assessment and long-term stability of polymer-based microfluidic platforms.
European regulations under the Medical Device Regulation (MDR) impose additional requirements for microfluidic devices intended for diagnostic applications. These regulations mandate comprehensive risk management processes and technical documentation that specifically addresses material biocompatibility and potential degradation products.
Biomedical polymers commonly used in microfluidics—such as PDMS, PMMA, and COC—must undergo rigorous testing to verify their compliance with these standards. Testing protocols typically include extraction studies to identify leachables, protein adsorption assessments to evaluate non-specific binding, and endotoxin testing to ensure pyrogen-free surfaces.
The emergence of organ-on-chip technologies has prompted the development of specialized biocompatibility standards that address the unique requirements of these complex systems. These standards focus on maintaining cell viability and functionality within polymer microenvironments over extended periods, necessitating more sophisticated biocompatibility metrics than traditional static culture systems.
International harmonization efforts are underway to standardize biocompatibility testing methodologies specifically for microfluidic applications. The ASTM International Committee F04 has established working groups dedicated to developing consensus standards for evaluating the biological response to microfluidic materials and devices, facilitating global acceptance and implementation of these technologies.
Scalability and Manufacturing Processes for Polymer Microfluidic Devices
The scalability of polymer microfluidic devices represents a critical factor in their widespread adoption across biomedical applications. Traditional manufacturing methods like soft lithography using polydimethylsiloxane (PDMS) excel in prototyping but face significant challenges when transitioning to mass production. These challenges include labor-intensive processes, limited throughput, and difficulties in maintaining consistent quality across large production batches.
Injection molding has emerged as a promising high-volume manufacturing technique for thermoplastic polymer microfluidic devices. This process offers cycle times of 10-60 seconds per device, enabling production rates of thousands of units daily. Materials such as polycarbonate (PC), polymethyl methacrylate (PMMA), and cyclic olefin copolymer (COC) demonstrate excellent compatibility with this manufacturing approach, providing both cost efficiency and consistent quality.
Hot embossing represents another viable manufacturing route, particularly suitable for medium-volume production scenarios. This technique achieves feature resolutions down to 50 nanometers while maintaining reasonable throughput. The process involves pressing a heated master mold into polymer sheets, creating precise microchannels and structures with high fidelity.
Roll-to-roll manufacturing has revolutionized large-scale production capabilities, enabling continuous fabrication of polymer microfluidic devices on flexible substrates. This approach dramatically increases throughput while reducing per-unit costs, making it particularly attractive for disposable diagnostic applications. However, achieving consistent feature quality across large production runs remains technically challenging.
Bonding technologies constitute a critical aspect of manufacturing scalability. Thermal bonding, solvent bonding, and adhesive bonding each offer distinct advantages depending on the polymer substrate and application requirements. Recent advances in laser welding and ultrasonic welding have significantly improved bonding efficiency and reliability in high-volume production environments.
Quality control systems have evolved to address the unique challenges of microfluidic device manufacturing. Automated optical inspection, in-line testing, and statistical process control methodologies ensure consistent performance across production batches. These systems have become increasingly sophisticated, incorporating machine learning algorithms to detect subtle defects that might affect device functionality.
Cost considerations remain paramount when scaling production. While initial tooling costs for injection molding can exceed $50,000, the per-unit costs drop dramatically at high volumes—often below $1 per device. This economic reality drives strategic decisions regarding manufacturing approach selection, particularly for startups and emerging biomedical applications.
Injection molding has emerged as a promising high-volume manufacturing technique for thermoplastic polymer microfluidic devices. This process offers cycle times of 10-60 seconds per device, enabling production rates of thousands of units daily. Materials such as polycarbonate (PC), polymethyl methacrylate (PMMA), and cyclic olefin copolymer (COC) demonstrate excellent compatibility with this manufacturing approach, providing both cost efficiency and consistent quality.
Hot embossing represents another viable manufacturing route, particularly suitable for medium-volume production scenarios. This technique achieves feature resolutions down to 50 nanometers while maintaining reasonable throughput. The process involves pressing a heated master mold into polymer sheets, creating precise microchannels and structures with high fidelity.
Roll-to-roll manufacturing has revolutionized large-scale production capabilities, enabling continuous fabrication of polymer microfluidic devices on flexible substrates. This approach dramatically increases throughput while reducing per-unit costs, making it particularly attractive for disposable diagnostic applications. However, achieving consistent feature quality across large production runs remains technically challenging.
Bonding technologies constitute a critical aspect of manufacturing scalability. Thermal bonding, solvent bonding, and adhesive bonding each offer distinct advantages depending on the polymer substrate and application requirements. Recent advances in laser welding and ultrasonic welding have significantly improved bonding efficiency and reliability in high-volume production environments.
Quality control systems have evolved to address the unique challenges of microfluidic device manufacturing. Automated optical inspection, in-line testing, and statistical process control methodologies ensure consistent performance across production batches. These systems have become increasingly sophisticated, incorporating machine learning algorithms to detect subtle defects that might affect device functionality.
Cost considerations remain paramount when scaling production. While initial tooling costs for injection molding can exceed $50,000, the per-unit costs drop dramatically at high volumes—often below $1 per device. This economic reality drives strategic decisions regarding manufacturing approach selection, particularly for startups and emerging biomedical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!