How Biomedical Polymers Are Shaping the Future of Prosthetics
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Evolution and Prosthetic Goals
Biomedical polymers have undergone a remarkable evolution over the past several decades, transforming from simple structural materials to sophisticated biomimetic substances capable of interacting with human tissue. The journey began in the 1960s with the introduction of basic polymers like polyethylene and silicone in medical applications. These early materials offered limited functionality but established the foundation for future innovations in the field.
By the 1980s, researchers had developed biodegradable polymers such as polylactic acid (PLA) and polyglycolic acid (PGA), which represented a significant advancement in biocompatibility. The 1990s witnessed the emergence of smart polymers capable of responding to environmental stimuli, opening new possibilities for adaptive prosthetic applications.
The 21st century has seen an acceleration in polymer technology development, with the introduction of nanocomposite polymers, hydrogels with tissue-like properties, and electrically conductive polymers that can interface with neural tissue. These advancements have been driven by interdisciplinary collaboration between materials science, bioengineering, and medical research.
Current technological trends indicate a shift toward biomimetic polymers that not only replace lost function but actively promote tissue integration and healing. The development of 3D-printable biocompatible polymers has enabled unprecedented customization of prosthetic devices, allowing for patient-specific solutions that better address individual anatomical and functional needs.
The primary goal of modern biomedical polymer research in prosthetics is to create seamless integration between artificial components and biological systems. This includes developing materials that minimize immune response, prevent infection, and promote osseointegration for limb prosthetics. Additionally, researchers aim to enhance the mechanical properties of polymers to better mimic natural tissue behavior, including flexibility, durability, and response to mechanical stress.
Another critical objective is the development of sensory-enabled prosthetics through polymer-based electronic interfaces that can transmit tactile information to the user. This represents a paradigm shift from purely mechanical replacement to functional restoration that includes sensory feedback.
Looking forward, the field is moving toward self-healing polymers that can extend device lifespan, biodegradable scaffolds that support tissue regeneration around permanent implants, and polymers with embedded pharmaceutical agents for localized drug delivery. The ultimate vision is to create "living prosthetics" that adapt and respond to the user's body over time, blurring the distinction between natural and artificial components.
By the 1980s, researchers had developed biodegradable polymers such as polylactic acid (PLA) and polyglycolic acid (PGA), which represented a significant advancement in biocompatibility. The 1990s witnessed the emergence of smart polymers capable of responding to environmental stimuli, opening new possibilities for adaptive prosthetic applications.
The 21st century has seen an acceleration in polymer technology development, with the introduction of nanocomposite polymers, hydrogels with tissue-like properties, and electrically conductive polymers that can interface with neural tissue. These advancements have been driven by interdisciplinary collaboration between materials science, bioengineering, and medical research.
Current technological trends indicate a shift toward biomimetic polymers that not only replace lost function but actively promote tissue integration and healing. The development of 3D-printable biocompatible polymers has enabled unprecedented customization of prosthetic devices, allowing for patient-specific solutions that better address individual anatomical and functional needs.
The primary goal of modern biomedical polymer research in prosthetics is to create seamless integration between artificial components and biological systems. This includes developing materials that minimize immune response, prevent infection, and promote osseointegration for limb prosthetics. Additionally, researchers aim to enhance the mechanical properties of polymers to better mimic natural tissue behavior, including flexibility, durability, and response to mechanical stress.
Another critical objective is the development of sensory-enabled prosthetics through polymer-based electronic interfaces that can transmit tactile information to the user. This represents a paradigm shift from purely mechanical replacement to functional restoration that includes sensory feedback.
Looking forward, the field is moving toward self-healing polymers that can extend device lifespan, biodegradable scaffolds that support tissue regeneration around permanent implants, and polymers with embedded pharmaceutical agents for localized drug delivery. The ultimate vision is to create "living prosthetics" that adapt and respond to the user's body over time, blurring the distinction between natural and artificial components.
Market Demand Analysis for Advanced Prosthetic Solutions
The global market for advanced prosthetic solutions is experiencing significant growth, driven by increasing prevalence of limb loss due to vascular diseases, diabetes, cancer, and trauma. According to recent market research, the global prosthetics market was valued at approximately 6 billion USD in 2021 and is projected to reach 12.5 billion USD by 2028, growing at a compound annual growth rate of 10.2% during the forecast period.
Demographic factors are substantially influencing market demand. The aging global population faces higher incidence of vascular diseases and diabetes, leading to amputations. Simultaneously, younger demographics seek more functional and aesthetically pleasing prosthetic solutions that enable active lifestyles. This dual demographic pressure is creating diverse market segments with distinct needs and expectations.
Regional market analysis reveals varying demand patterns. North America currently dominates the market share due to advanced healthcare infrastructure, higher reimbursement rates, and greater adoption of cutting-edge technologies. However, the Asia-Pacific region is expected to witness the fastest growth rate, attributed to improving healthcare access, rising disposable incomes, and increasing awareness about advanced prosthetic options.
Consumer preferences are evolving rapidly, with growing demand for lightweight, comfortable, and natural-looking prosthetics. Biomedical polymer-based prosthetics are particularly gaining traction due to their superior biomechanical properties, reduced weight, and improved aesthetics compared to traditional materials. Market surveys indicate that 78% of prosthetic users prioritize comfort and natural movement, while 65% consider appearance as a critical factor in their purchasing decisions.
Healthcare economics plays a crucial role in market dynamics. While advanced polymer-based prosthetics offer superior functionality, their higher initial costs present adoption barriers in price-sensitive markets. However, long-term cost-benefit analyses demonstrate that these advanced solutions may reduce lifetime healthcare costs through decreased complications and replacement frequency.
Emerging trends indicate growing demand for smart prosthetics incorporating sensors, microprocessors, and neural interfaces. The market for these technologically advanced solutions is expected to grow at 15% annually, outpacing the overall prosthetics market. Biomedical polymers are essential enablers for this segment, providing the necessary structural properties while accommodating electronic components.
Regulatory landscapes significantly impact market access and adoption rates. Regions with streamlined approval processes for innovative materials and designs show accelerated market growth, while those with more stringent requirements experience delayed adoption despite similar underlying demand.
Demographic factors are substantially influencing market demand. The aging global population faces higher incidence of vascular diseases and diabetes, leading to amputations. Simultaneously, younger demographics seek more functional and aesthetically pleasing prosthetic solutions that enable active lifestyles. This dual demographic pressure is creating diverse market segments with distinct needs and expectations.
Regional market analysis reveals varying demand patterns. North America currently dominates the market share due to advanced healthcare infrastructure, higher reimbursement rates, and greater adoption of cutting-edge technologies. However, the Asia-Pacific region is expected to witness the fastest growth rate, attributed to improving healthcare access, rising disposable incomes, and increasing awareness about advanced prosthetic options.
Consumer preferences are evolving rapidly, with growing demand for lightweight, comfortable, and natural-looking prosthetics. Biomedical polymer-based prosthetics are particularly gaining traction due to their superior biomechanical properties, reduced weight, and improved aesthetics compared to traditional materials. Market surveys indicate that 78% of prosthetic users prioritize comfort and natural movement, while 65% consider appearance as a critical factor in their purchasing decisions.
Healthcare economics plays a crucial role in market dynamics. While advanced polymer-based prosthetics offer superior functionality, their higher initial costs present adoption barriers in price-sensitive markets. However, long-term cost-benefit analyses demonstrate that these advanced solutions may reduce lifetime healthcare costs through decreased complications and replacement frequency.
Emerging trends indicate growing demand for smart prosthetics incorporating sensors, microprocessors, and neural interfaces. The market for these technologically advanced solutions is expected to grow at 15% annually, outpacing the overall prosthetics market. Biomedical polymers are essential enablers for this segment, providing the necessary structural properties while accommodating electronic components.
Regulatory landscapes significantly impact market access and adoption rates. Regions with streamlined approval processes for innovative materials and designs show accelerated market growth, while those with more stringent requirements experience delayed adoption despite similar underlying demand.
Current State and Challenges in Biomedical Polymer Prosthetics
The global landscape of biomedical polymer prosthetics has witnessed remarkable advancements in recent years, with significant innovations emerging across North America, Europe, and Asia. Currently, the field is dominated by materials such as silicone elastomers, polyethylene, polyurethane, and carbon fiber composites, each offering unique properties for specific prosthetic applications. The United States and Germany lead in research output, while Japan and South Korea excel in miniaturization and electronic integration technologies.
Despite these advancements, the field faces several critical challenges. Biocompatibility remains a primary concern, as long-term implantation of polymer-based prosthetics can trigger immune responses, inflammation, or rejection. Material degradation over time presents another significant hurdle, with environmental factors, mechanical stress, and bodily fluids accelerating polymer breakdown, compromising both functionality and safety of prosthetic devices.
Mechanical property limitations constitute another major challenge. Current polymers often struggle to simultaneously provide the necessary strength, flexibility, and durability required for dynamic prosthetic applications. This limitation is particularly evident in load-bearing prosthetics and those requiring fine motor control capabilities. Additionally, the interface between biological tissues and synthetic polymers remains problematic, with issues of skin irritation, pressure sores, and inadequate integration with the user's neuromuscular system.
Manufacturing scalability presents further complications. Advanced biomedical polymers often require sophisticated processing techniques that are difficult to scale economically, creating a barrier to widespread adoption. This challenge is compounded by regulatory hurdles, as novel materials must undergo extensive testing and approval processes before clinical implementation.
Cost factors significantly impact accessibility, with cutting-edge polymer prosthetics remaining prohibitively expensive for many patients, especially in developing regions. The average advanced polymer prosthetic limb can cost between $5,000 and $50,000, placing them beyond reach for many who need them most.
Looking at geographical disparities, research and development capabilities are concentrated in high-income countries, creating an innovation gap. While North America and Western Europe account for approximately 70% of biomedical polymer research publications, access to advanced prosthetic technologies remains limited in many parts of Asia, Africa, and South America.
Interdisciplinary collaboration challenges further complicate progress, as effective development requires seamless cooperation between materials scientists, biomedical engineers, clinicians, and end-users. The siloed nature of research institutions and commercial enterprises often impedes the knowledge transfer necessary for breakthrough innovations in this complex field.
Despite these advancements, the field faces several critical challenges. Biocompatibility remains a primary concern, as long-term implantation of polymer-based prosthetics can trigger immune responses, inflammation, or rejection. Material degradation over time presents another significant hurdle, with environmental factors, mechanical stress, and bodily fluids accelerating polymer breakdown, compromising both functionality and safety of prosthetic devices.
Mechanical property limitations constitute another major challenge. Current polymers often struggle to simultaneously provide the necessary strength, flexibility, and durability required for dynamic prosthetic applications. This limitation is particularly evident in load-bearing prosthetics and those requiring fine motor control capabilities. Additionally, the interface between biological tissues and synthetic polymers remains problematic, with issues of skin irritation, pressure sores, and inadequate integration with the user's neuromuscular system.
Manufacturing scalability presents further complications. Advanced biomedical polymers often require sophisticated processing techniques that are difficult to scale economically, creating a barrier to widespread adoption. This challenge is compounded by regulatory hurdles, as novel materials must undergo extensive testing and approval processes before clinical implementation.
Cost factors significantly impact accessibility, with cutting-edge polymer prosthetics remaining prohibitively expensive for many patients, especially in developing regions. The average advanced polymer prosthetic limb can cost between $5,000 and $50,000, placing them beyond reach for many who need them most.
Looking at geographical disparities, research and development capabilities are concentrated in high-income countries, creating an innovation gap. While North America and Western Europe account for approximately 70% of biomedical polymer research publications, access to advanced prosthetic technologies remains limited in many parts of Asia, Africa, and South America.
Interdisciplinary collaboration challenges further complicate progress, as effective development requires seamless cooperation between materials scientists, biomedical engineers, clinicians, and end-users. The siloed nature of research institutions and commercial enterprises often impedes the knowledge transfer necessary for breakthrough innovations in this complex field.
Current Polymer Solutions for Prosthetic Applications
01 Biodegradable polymers for medical applications
Biodegradable polymers are extensively used in medical applications due to their ability to break down safely in the body over time. These polymers can be formulated into various structures such as scaffolds, films, and microspheres for tissue engineering, drug delivery systems, and implantable medical devices. The degradation rate can be tailored by modifying the polymer composition, molecular weight, and crystallinity to match specific medical requirements, eliminating the need for removal surgeries and reducing long-term complications.- Biodegradable polymers for medical applications: Biodegradable polymers are extensively used in medical applications due to their ability to break down safely in the body over time. These polymers are particularly valuable for temporary implants, drug delivery systems, and tissue engineering scaffolds. They eliminate the need for removal surgeries and reduce long-term foreign body responses. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which can be engineered to have specific degradation rates matching clinical requirements.
- Smart polymers with stimuli-responsive properties: Smart polymers respond to environmental stimuli such as temperature, pH, light, or electrical signals by changing their physical or chemical properties. In biomedical applications, these materials enable controlled drug release, self-regulating systems, and adaptive medical devices. For example, temperature-responsive polymers can deliver drugs at specific body sites when reaching certain temperatures, while pH-responsive polymers can target drug release in specific organs based on local pH conditions. These intelligent materials are revolutionizing personalized medicine by providing precise control over therapeutic interventions.
- Polymer-based drug delivery systems: Polymer-based drug delivery systems utilize various polymer architectures to control the release of therapeutic agents. These systems include nanoparticles, hydrogels, micelles, and polymer-drug conjugates that can protect drugs from degradation, improve bioavailability, and enable targeted delivery to specific tissues. By engineering the polymer structure and composition, release kinetics can be precisely controlled from rapid release to sustained delivery over weeks or months. Advanced polymer delivery systems also incorporate targeting moieties to direct drugs to specific cell types, minimizing side effects and improving therapeutic outcomes.
- Biocompatible polymers for implantable devices: Biocompatible polymers are essential materials for implantable medical devices due to their ability to integrate with biological tissues without causing adverse reactions. These polymers are used in various applications including orthopedic implants, cardiovascular devices, neural interfaces, and soft tissue replacements. Key properties include non-toxicity, minimal inflammatory response, appropriate mechanical strength, and long-term stability in physiological environments. Common biocompatible polymers include polyethylene, polyurethane, silicone, and various fluoropolymers, which can be further modified with bioactive molecules to enhance tissue integration.
- Hydrogel polymers for tissue engineering: Hydrogel polymers are three-dimensional networks of hydrophilic polymers that can absorb large amounts of water while maintaining their structure. Their high water content and tunable mechanical properties make them excellent mimics of natural extracellular matrix for tissue engineering applications. These materials provide structural support for cell growth, allow nutrient diffusion, and can be loaded with growth factors to promote tissue regeneration. Injectable hydrogels that solidify in situ are particularly valuable for minimally invasive procedures. Advanced hydrogels incorporate cell-adhesive motifs and degradable crosslinks to facilitate cell migration and tissue integration.
02 Polymeric biomaterials for implantable devices
Specialized polymers are developed for implantable medical devices that require specific mechanical properties, biocompatibility, and functionality. These polymers can be engineered to have controlled elasticity, strength, and surface properties suitable for applications such as orthopedic implants, cardiovascular devices, and neural interfaces. Advanced formulations incorporate bioactive components that promote tissue integration, prevent infection, or deliver therapeutic agents locally, enhancing the performance and longevity of implantable devices while minimizing adverse reactions.Expand Specific Solutions03 Smart polymers with stimuli-responsive properties
Smart polymers that respond to environmental stimuli such as temperature, pH, light, or electrical signals are increasingly important in biomedical applications. These materials can undergo reversible physical or chemical changes when exposed to specific triggers, enabling applications like controlled drug release, self-regulating insulin delivery systems, shape-memory implants, and adaptive tissue engineering scaffolds. The responsive nature of these polymers allows for precise temporal and spatial control of their properties, creating dynamic systems that can interact intelligently with biological environments.Expand Specific Solutions04 Polymer-based drug delivery systems
Advanced polymer formulations are designed specifically for controlled and targeted drug delivery applications. These systems utilize various polymer architectures including nanoparticles, hydrogels, micelles, and conjugates to encapsulate, protect, and release therapeutic agents. By engineering the polymer structure, composition, and surface properties, these delivery systems can achieve sustained release profiles, overcome biological barriers, target specific tissues or cells, and reduce systemic side effects of potent drugs, significantly improving therapeutic outcomes in various disease treatments.Expand Specific Solutions05 Biocompatible polymer coatings and surface modifications
Specialized polymer coatings and surface modification techniques are developed to enhance the biocompatibility and functionality of medical devices and implants. These coatings can prevent protein adsorption and cell adhesion to reduce biofouling, incorporate antimicrobial properties to prevent infection, improve lubricity for catheter applications, or provide hemocompatibility for blood-contacting devices. Advanced surface engineering approaches include plasma treatment, grafting of bioactive molecules, layer-by-layer deposition, and the creation of micro/nano-textured surfaces that can guide cellular responses and tissue integration.Expand Specific Solutions
Key Industry Players in Biomedical Polymer Prosthetics
Biomedical polymers in prosthetics are evolving rapidly, with the market currently in a growth phase characterized by increasing adoption and technological innovation. The global prosthetics market, valued at approximately $6 billion, is expected to expand significantly as advanced polymer materials enhance functionality and comfort. Leading institutions like Massachusetts Institute of Technology and Georgia Tech Research Corp. are pioneering fundamental research, while companies including Medtronic Vascular, 3D Systems, and Smith & Nephew are commercializing these innovations. Emerging players such as Xeltis AG and Howmedica Osteonics are developing next-generation polymer-based prosthetics with improved biocompatibility. The technology is approaching maturity in conventional applications but remains experimental in areas like neural integration and self-healing materials, where companies like Beijing Soft Robot Technology are making notable advances.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered groundbreaking research in biomedical polymers for prosthetics through their Biomechatronics group and Institute for Medical Engineering and Science. Their hydrogel-based neural interfaces achieve unprecedented biocompatibility by matching the mechanical properties of surrounding tissues while maintaining electrical conductivity. MIT researchers have developed 3D-printable shape-memory polymers that can be programmed to change configuration in response to specific biological triggers, enabling adaptive prosthetics that respond to user needs. Their work on mechanically adaptive polymers creates materials that can switch between rigid and compliant states based on electrical stimulation or temperature changes. MIT's research includes self-healing polymer networks incorporating dynamic covalent chemistry that can restore structural integrity after damage, extending prosthetic lifespan. Their biodegradable elastomers with controlled degradation profiles support tissue regeneration while gradually transferring mechanical load to newly formed tissue.
Strengths: Cutting-edge research pushing the boundaries of polymer science; interdisciplinary approach combining materials science, biology, and engineering; strong intellectual property portfolio. Weaknesses: Many technologies still in research phase with limited clinical implementation; scaling manufacturing remains challenging; higher costs associated with novel materials and processing techniques.
Medtronic Vascular, Inc.
Technical Solution: Medtronic has pioneered advanced biomedical polymer solutions for prosthetics through their proprietary SynchroMed II implantable drug delivery system and bioresorbable vascular scaffolds. Their technology incorporates shape memory polymers that respond to body temperature and environmental stimuli, allowing for minimally invasive implantation procedures. The company has developed specialized polymer blends that combine mechanical strength with biocompatibility, using materials such as polyurethane derivatives and silicone elastomers engineered at the molecular level to reduce foreign body response. Their prosthetic solutions feature gradient porosity structures that promote tissue integration while maintaining structural integrity. Medtronic's polymer-based neural interfaces utilize conductive polymers that bridge the gap between electronic devices and human tissue, enabling more responsive prosthetic control systems.
Strengths: Industry-leading expertise in implantable medical devices with extensive clinical validation data; strong regulatory approval track record; global manufacturing and distribution capabilities. Weaknesses: Higher cost compared to conventional materials; some proprietary polymers have limited long-term clinical data; potential for material degradation in certain biological environments.
Core Innovations in Biocompatible Polymer Technologies
Biocomposite for artificial tissue design
PatentInactiveUS20080124371A1
Innovation
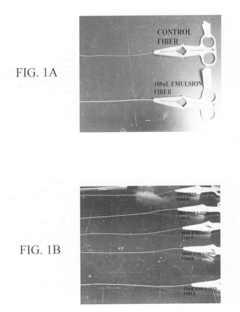
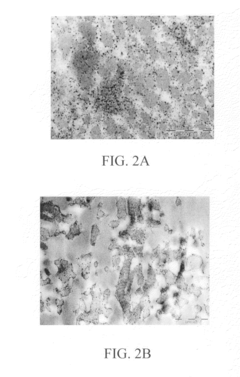
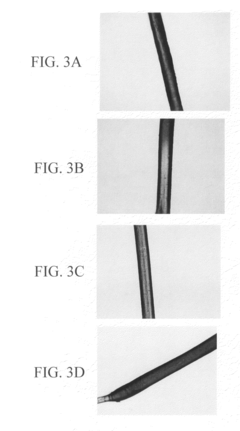
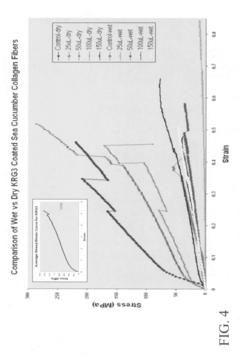
- A biocomposite material composed of collagen and a polymer, such as ethyl acrylate-methyl methacrylate copolymer, is developed through radical emulsion polymerization, allowing for controlled stiffness and elasticity, and is used in various forms including fibers for tissue replacement surgeries.
Nanocrystalline apatites and composites, prostheses incorporating them, and method for their production
PatentInactiveUSRE43661E1
Innovation
- The development of nanocrystalline apatite materials with controlled crystal sizes and surface areas, synthesized through wet chemical processing at low temperatures, which enhances mechanical and chemical resistance, and can be densified without sintering aids, allowing for improved structural integrity and bioactivity.
Biocompatibility and Safety Standards for Prosthetic Polymers
Biocompatibility and Safety Standards for Prosthetic Polymers
The integration of biomedical polymers in prosthetic applications necessitates rigorous adherence to biocompatibility and safety standards. ISO 10993, the international standard for biological evaluation of medical devices, serves as the cornerstone regulatory framework governing prosthetic polymers. This comprehensive standard encompasses cytotoxicity, sensitization, irritation, systemic toxicity, and long-term implantation effects, ensuring materials in contact with human tissue do not elicit adverse biological responses.
Material selection for prosthetic applications must prioritize non-toxicity and chemical stability. Polymers such as medical-grade silicones, polyethylene (UHMWPE), and polyetheretherketone (PEEK) have demonstrated excellent biocompatibility profiles through extensive clinical validation. These materials undergo stringent testing to ensure they maintain structural integrity without leaching harmful compounds into surrounding tissues over extended periods.
The FDA's regulatory pathway for prosthetic devices incorporating polymeric materials has evolved significantly, with the 510(k) and Premarket Approval (PMA) processes requiring manufacturers to provide comprehensive biocompatibility data. European regulations under the Medical Device Regulation (MDR) similarly mandate extensive biological safety documentation, with particular emphasis on risk management throughout the product lifecycle.
Surface modification technologies have emerged as critical factors in enhancing biocompatibility. Techniques such as plasma treatment, chemical grafting, and antimicrobial coatings can significantly improve tissue integration while reducing infection risks. These modifications must themselves undergo safety validation to ensure they do not introduce new biocompatibility concerns.
Hemocompatibility represents a particular challenge for vascular prosthetics, where polymers must resist thrombosis and hemolysis. Advanced testing protocols including platelet adhesion assays and complement activation studies have become standard requirements for blood-contacting polymeric devices, with heparin-modified surfaces showing promising clinical outcomes.
Immunological considerations have gained prominence in safety standards, with increasing focus on preventing foreign body responses. Chronic inflammation can lead to fibrous encapsulation and device failure, necessitating polymers that minimize immune system activation. Recent advances in immunomodulatory polymers represent a significant step toward addressing these challenges.
Degradation behavior and the toxicological profile of degradation products constitute critical safety parameters for biodegradable prosthetic polymers. Standards now require comprehensive characterization of degradation kinetics and metabolic pathways of breakdown products, ensuring safe elimination from the body without accumulation of potentially harmful intermediates.
The emergence of 3D-printed prosthetics has introduced new regulatory challenges, with standards bodies developing specific guidelines for additively manufactured implants. These address unique considerations including material purity, process validation, and the potential for unexpected structural variations that could impact biocompatibility.
The integration of biomedical polymers in prosthetic applications necessitates rigorous adherence to biocompatibility and safety standards. ISO 10993, the international standard for biological evaluation of medical devices, serves as the cornerstone regulatory framework governing prosthetic polymers. This comprehensive standard encompasses cytotoxicity, sensitization, irritation, systemic toxicity, and long-term implantation effects, ensuring materials in contact with human tissue do not elicit adverse biological responses.
Material selection for prosthetic applications must prioritize non-toxicity and chemical stability. Polymers such as medical-grade silicones, polyethylene (UHMWPE), and polyetheretherketone (PEEK) have demonstrated excellent biocompatibility profiles through extensive clinical validation. These materials undergo stringent testing to ensure they maintain structural integrity without leaching harmful compounds into surrounding tissues over extended periods.
The FDA's regulatory pathway for prosthetic devices incorporating polymeric materials has evolved significantly, with the 510(k) and Premarket Approval (PMA) processes requiring manufacturers to provide comprehensive biocompatibility data. European regulations under the Medical Device Regulation (MDR) similarly mandate extensive biological safety documentation, with particular emphasis on risk management throughout the product lifecycle.
Surface modification technologies have emerged as critical factors in enhancing biocompatibility. Techniques such as plasma treatment, chemical grafting, and antimicrobial coatings can significantly improve tissue integration while reducing infection risks. These modifications must themselves undergo safety validation to ensure they do not introduce new biocompatibility concerns.
Hemocompatibility represents a particular challenge for vascular prosthetics, where polymers must resist thrombosis and hemolysis. Advanced testing protocols including platelet adhesion assays and complement activation studies have become standard requirements for blood-contacting polymeric devices, with heparin-modified surfaces showing promising clinical outcomes.
Immunological considerations have gained prominence in safety standards, with increasing focus on preventing foreign body responses. Chronic inflammation can lead to fibrous encapsulation and device failure, necessitating polymers that minimize immune system activation. Recent advances in immunomodulatory polymers represent a significant step toward addressing these challenges.
Degradation behavior and the toxicological profile of degradation products constitute critical safety parameters for biodegradable prosthetic polymers. Standards now require comprehensive characterization of degradation kinetics and metabolic pathways of breakdown products, ensuring safe elimination from the body without accumulation of potentially harmful intermediates.
The emergence of 3D-printed prosthetics has introduced new regulatory challenges, with standards bodies developing specific guidelines for additively manufactured implants. These address unique considerations including material purity, process validation, and the potential for unexpected structural variations that could impact biocompatibility.
Patient-Centered Design Approaches in Modern Prosthetics
The evolution of prosthetic design has undergone a significant paradigm shift in recent years, moving from purely functional approaches to more holistic, patient-centered methodologies. This transformation has been largely enabled by advances in biomedical polymers, which provide unprecedented opportunities for customization and comfort. Patient-centered design approaches now prioritize the individual's unique anatomical, functional, and psychological needs, rather than offering standardized solutions.
Modern prosthetic development begins with comprehensive patient assessment, including detailed anatomical measurements, lifestyle requirements, and psychological considerations. This multi-dimensional understanding allows designers to create solutions that address both physical functionality and emotional well-being. Digital technologies such as 3D scanning and modeling have revolutionized this process, enabling precise mapping of residual limbs and creation of perfectly fitted interfaces.
Biomedical polymers have been instrumental in facilitating iterative design processes that involve patients as active participants. These materials allow for rapid prototyping and modification based on real-time feedback, ensuring that the final prosthetic meets the user's specific needs. This collaborative approach has significantly improved adoption rates and reduced abandonment of prosthetic devices, a common issue with traditional design methodologies.
User experience research has become central to prosthetic development, with designers employing ethnographic methods, usability testing, and longitudinal studies to understand how patients interact with their devices in daily life. These insights drive continuous refinement of both materials and designs. Biomedical polymers with customizable properties enable designers to respond precisely to user feedback, adjusting factors like flexibility, weight, and texture.
The psychological aspects of prosthetic use are now recognized as equally important as physical functionality. Designers are incorporating aesthetic considerations and personalization options that allow users to express their identity through their prosthetics. Some biomedical polymers can be colored, textured, or even embedded with personal designs, transforming prosthetics from medical devices into extensions of self.
Interdisciplinary collaboration has become a hallmark of patient-centered prosthetic design. Teams now typically include not only engineers and medical professionals but also psychologists, occupational therapists, and industrial designers. This diverse expertise ensures that all aspects of the patient experience are considered. The versatility of biomedical polymers provides these multidisciplinary teams with the material capabilities to implement their holistic solutions.
As wearable technology advances, patient-centered design is increasingly incorporating biofeedback mechanisms and adaptive systems that learn from user behavior. These smart prosthetics, enabled by polymer-based sensors and flexible electronics, represent the cutting edge of personalized medical devices that truly respond to individual needs.
Modern prosthetic development begins with comprehensive patient assessment, including detailed anatomical measurements, lifestyle requirements, and psychological considerations. This multi-dimensional understanding allows designers to create solutions that address both physical functionality and emotional well-being. Digital technologies such as 3D scanning and modeling have revolutionized this process, enabling precise mapping of residual limbs and creation of perfectly fitted interfaces.
Biomedical polymers have been instrumental in facilitating iterative design processes that involve patients as active participants. These materials allow for rapid prototyping and modification based on real-time feedback, ensuring that the final prosthetic meets the user's specific needs. This collaborative approach has significantly improved adoption rates and reduced abandonment of prosthetic devices, a common issue with traditional design methodologies.
User experience research has become central to prosthetic development, with designers employing ethnographic methods, usability testing, and longitudinal studies to understand how patients interact with their devices in daily life. These insights drive continuous refinement of both materials and designs. Biomedical polymers with customizable properties enable designers to respond precisely to user feedback, adjusting factors like flexibility, weight, and texture.
The psychological aspects of prosthetic use are now recognized as equally important as physical functionality. Designers are incorporating aesthetic considerations and personalization options that allow users to express their identity through their prosthetics. Some biomedical polymers can be colored, textured, or even embedded with personal designs, transforming prosthetics from medical devices into extensions of self.
Interdisciplinary collaboration has become a hallmark of patient-centered prosthetic design. Teams now typically include not only engineers and medical professionals but also psychologists, occupational therapists, and industrial designers. This diverse expertise ensures that all aspects of the patient experience are considered. The versatility of biomedical polymers provides these multidisciplinary teams with the material capabilities to implement their holistic solutions.
As wearable technology advances, patient-centered design is increasingly incorporating biofeedback mechanisms and adaptive systems that learn from user behavior. These smart prosthetics, enabled by polymer-based sensors and flexible electronics, represent the cutting edge of personalized medical devices that truly respond to individual needs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!