How Mechanical Properties Are Enhanced in Biomedical Polymers
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Evolution and Enhancement Goals
Biomedical polymers have undergone significant evolution since their initial introduction in the medical field during the mid-20th century. Early applications primarily focused on basic structural components and simple implants, with limited consideration for mechanical performance optimization. The 1960s marked a turning point with the development of the first generation of biocompatible polymers such as polyethylene and polyurethane, which demonstrated acceptable mechanical properties but suffered from long-term stability issues in biological environments.
The progression from first-generation to advanced biomedical polymers has been driven by increasing demands for materials that can withstand complex physiological conditions while maintaining structural integrity. This evolution has been characterized by systematic improvements in tensile strength, fatigue resistance, and elasticity—properties crucial for applications ranging from cardiovascular devices to orthopedic implants and tissue engineering scaffolds.
Current technological goals in biomedical polymer enhancement focus on achieving multifunctional performance profiles that combine superior mechanical properties with bioactivity and controlled degradation characteristics. Researchers aim to develop polymers that can withstand physiological stresses while simultaneously promoting tissue integration and, when appropriate, degrading at rates synchronized with tissue regeneration processes.
A significant objective in contemporary research is the development of biomimetic polymers that replicate the mechanical behavior of natural tissues. This includes creating materials with anisotropic mechanical properties that mirror the directional strength characteristics of tissues like tendons, ligaments, and blood vessels. Such biomimetic approaches represent a paradigm shift from earlier generations of biomedical polymers that primarily sought to maximize absolute strength values without consideration for directional performance.
Another critical goal is the enhancement of interfacial mechanical properties between polymers and biological tissues, addressing the persistent challenge of mechanical mismatch that often leads to implant failure. This includes developing gradient materials that provide transitional mechanical properties between implants and surrounding tissues, thereby reducing stress concentration and improving long-term performance.
The field is increasingly moving toward predictive design methodologies that incorporate computational modeling to forecast mechanical behavior under physiological conditions. This approach aims to accelerate the development cycle by reducing empirical testing requirements and enabling more precise tailoring of mechanical properties to specific biomedical applications.
The progression from first-generation to advanced biomedical polymers has been driven by increasing demands for materials that can withstand complex physiological conditions while maintaining structural integrity. This evolution has been characterized by systematic improvements in tensile strength, fatigue resistance, and elasticity—properties crucial for applications ranging from cardiovascular devices to orthopedic implants and tissue engineering scaffolds.
Current technological goals in biomedical polymer enhancement focus on achieving multifunctional performance profiles that combine superior mechanical properties with bioactivity and controlled degradation characteristics. Researchers aim to develop polymers that can withstand physiological stresses while simultaneously promoting tissue integration and, when appropriate, degrading at rates synchronized with tissue regeneration processes.
A significant objective in contemporary research is the development of biomimetic polymers that replicate the mechanical behavior of natural tissues. This includes creating materials with anisotropic mechanical properties that mirror the directional strength characteristics of tissues like tendons, ligaments, and blood vessels. Such biomimetic approaches represent a paradigm shift from earlier generations of biomedical polymers that primarily sought to maximize absolute strength values without consideration for directional performance.
Another critical goal is the enhancement of interfacial mechanical properties between polymers and biological tissues, addressing the persistent challenge of mechanical mismatch that often leads to implant failure. This includes developing gradient materials that provide transitional mechanical properties between implants and surrounding tissues, thereby reducing stress concentration and improving long-term performance.
The field is increasingly moving toward predictive design methodologies that incorporate computational modeling to forecast mechanical behavior under physiological conditions. This approach aims to accelerate the development cycle by reducing empirical testing requirements and enabling more precise tailoring of mechanical properties to specific biomedical applications.
Market Demand Analysis for High-Performance Biomedical Polymers
The global market for high-performance biomedical polymers has experienced significant growth in recent years, driven by increasing demand for advanced medical devices, implants, and drug delivery systems. The market value for biomedical polymers reached approximately $3.5 billion in 2022 and is projected to grow at a compound annual growth rate of 7.8% through 2028, according to industry analyses.
Healthcare expenditure continues to rise globally, particularly in developed economies where aging populations require more extensive medical interventions. This demographic shift has created substantial demand for durable, biocompatible polymers with enhanced mechanical properties for long-term implantable devices such as orthopedic implants, cardiovascular stents, and dental materials.
The orthopedic segment represents the largest application area for mechanically enhanced biomedical polymers, accounting for roughly 35% of the market share. This is primarily due to the increasing incidence of musculoskeletal disorders and the growing number of joint replacement surgeries worldwide. Ultra-high-molecular-weight polyethylene (UHMWPE) with improved wear resistance and mechanical strength dominates this segment.
Cardiovascular applications follow closely, with a market share of approximately 28%. The demand for polymers with specific mechanical properties such as flexibility, tensile strength, and fatigue resistance is particularly high in this segment for applications including heart valves, vascular grafts, and catheter systems.
Emerging economies in Asia-Pacific, particularly China and India, are showing the fastest growth rates in demand for high-performance biomedical polymers, driven by improving healthcare infrastructure, increasing medical tourism, and growing middle-class populations with access to advanced healthcare services.
Regulatory trends are significantly influencing market demand patterns. Stringent safety and performance standards imposed by regulatory bodies such as the FDA and European Medicines Agency have pushed manufacturers toward developing polymers with better mechanical stability, reduced degradation, and improved biocompatibility profiles.
Sustainability concerns are also reshaping market demands, with increasing interest in biodegradable polymers that maintain necessary mechanical properties during their functional lifetime but degrade safely afterward. This segment is growing at nearly twice the rate of traditional non-degradable polymers.
Customization capabilities represent another significant market driver, with healthcare providers increasingly seeking polymeric materials that can be tailored to specific patient anatomies or clinical requirements while maintaining optimal mechanical performance. This trend is particularly evident in the rapidly expanding 3D printing segment of medical manufacturing.
Healthcare expenditure continues to rise globally, particularly in developed economies where aging populations require more extensive medical interventions. This demographic shift has created substantial demand for durable, biocompatible polymers with enhanced mechanical properties for long-term implantable devices such as orthopedic implants, cardiovascular stents, and dental materials.
The orthopedic segment represents the largest application area for mechanically enhanced biomedical polymers, accounting for roughly 35% of the market share. This is primarily due to the increasing incidence of musculoskeletal disorders and the growing number of joint replacement surgeries worldwide. Ultra-high-molecular-weight polyethylene (UHMWPE) with improved wear resistance and mechanical strength dominates this segment.
Cardiovascular applications follow closely, with a market share of approximately 28%. The demand for polymers with specific mechanical properties such as flexibility, tensile strength, and fatigue resistance is particularly high in this segment for applications including heart valves, vascular grafts, and catheter systems.
Emerging economies in Asia-Pacific, particularly China and India, are showing the fastest growth rates in demand for high-performance biomedical polymers, driven by improving healthcare infrastructure, increasing medical tourism, and growing middle-class populations with access to advanced healthcare services.
Regulatory trends are significantly influencing market demand patterns. Stringent safety and performance standards imposed by regulatory bodies such as the FDA and European Medicines Agency have pushed manufacturers toward developing polymers with better mechanical stability, reduced degradation, and improved biocompatibility profiles.
Sustainability concerns are also reshaping market demands, with increasing interest in biodegradable polymers that maintain necessary mechanical properties during their functional lifetime but degrade safely afterward. This segment is growing at nearly twice the rate of traditional non-degradable polymers.
Customization capabilities represent another significant market driver, with healthcare providers increasingly seeking polymeric materials that can be tailored to specific patient anatomies or clinical requirements while maintaining optimal mechanical performance. This trend is particularly evident in the rapidly expanding 3D printing segment of medical manufacturing.
Current Mechanical Property Challenges in Biomedical Polymers
Biomedical polymers face significant mechanical property challenges that limit their application potential in medical devices and implants. The primary concern is achieving an optimal balance between strength, flexibility, and durability to match the complex mechanical requirements of biological tissues. Many current polymers exhibit either excessive rigidity, leading to stress shielding and tissue damage, or insufficient strength, resulting in premature failure under physiological loads.
Degradation-related mechanical failure represents another critical challenge. When exposed to the aggressive biochemical environment of the human body, many polymers undergo hydrolytic or enzymatic degradation, causing unpredictable changes in mechanical properties over time. This degradation profile often does not align with the tissue healing process, creating a mismatch between implant performance and biological requirements.
Fatigue resistance remains problematic for biomedical polymers subjected to cyclic loading. Applications such as heart valves, vascular grafts, and orthopedic implants must withstand millions of loading cycles without significant mechanical deterioration. Current polymers frequently exhibit microcrack formation and propagation under these conditions, leading to catastrophic failure before the intended service life.
Interface compatibility presents additional challenges, particularly in composite systems where polymers must bond effectively with other materials such as ceramics, metals, or biological tissues. Weak interfacial bonding often results in delamination and mechanical failure at stress concentration points, compromising the overall structural integrity of medical devices.
Processing-induced variability further complicates mechanical property control. Manufacturing techniques like injection molding, extrusion, or 3D printing can introduce inconsistencies in molecular orientation, crystallinity, and internal stress distribution. These variations lead to unpredictable mechanical behavior and quality control issues in final products.
Sterilization processes necessary for medical applications often degrade polymer mechanical properties. Techniques such as gamma irradiation, ethylene oxide treatment, or steam autoclaving can induce chain scission, crosslinking, or oxidation, significantly altering the mechanical performance profile of the material after processing.
The challenge of achieving multifunctional mechanical properties has become increasingly important. Modern biomedical applications demand polymers that simultaneously exhibit seemingly contradictory properties—such as high strength with high elasticity, or controlled degradation with sustained mechanical integrity—creating significant materials engineering challenges that conventional polymer systems struggle to address.
Degradation-related mechanical failure represents another critical challenge. When exposed to the aggressive biochemical environment of the human body, many polymers undergo hydrolytic or enzymatic degradation, causing unpredictable changes in mechanical properties over time. This degradation profile often does not align with the tissue healing process, creating a mismatch between implant performance and biological requirements.
Fatigue resistance remains problematic for biomedical polymers subjected to cyclic loading. Applications such as heart valves, vascular grafts, and orthopedic implants must withstand millions of loading cycles without significant mechanical deterioration. Current polymers frequently exhibit microcrack formation and propagation under these conditions, leading to catastrophic failure before the intended service life.
Interface compatibility presents additional challenges, particularly in composite systems where polymers must bond effectively with other materials such as ceramics, metals, or biological tissues. Weak interfacial bonding often results in delamination and mechanical failure at stress concentration points, compromising the overall structural integrity of medical devices.
Processing-induced variability further complicates mechanical property control. Manufacturing techniques like injection molding, extrusion, or 3D printing can introduce inconsistencies in molecular orientation, crystallinity, and internal stress distribution. These variations lead to unpredictable mechanical behavior and quality control issues in final products.
Sterilization processes necessary for medical applications often degrade polymer mechanical properties. Techniques such as gamma irradiation, ethylene oxide treatment, or steam autoclaving can induce chain scission, crosslinking, or oxidation, significantly altering the mechanical performance profile of the material after processing.
The challenge of achieving multifunctional mechanical properties has become increasingly important. Modern biomedical applications demand polymers that simultaneously exhibit seemingly contradictory properties—such as high strength with high elasticity, or controlled degradation with sustained mechanical integrity—creating significant materials engineering challenges that conventional polymer systems struggle to address.
Current Mechanical Enhancement Techniques and Methodologies
01 Biodegradable polymers for tissue engineering
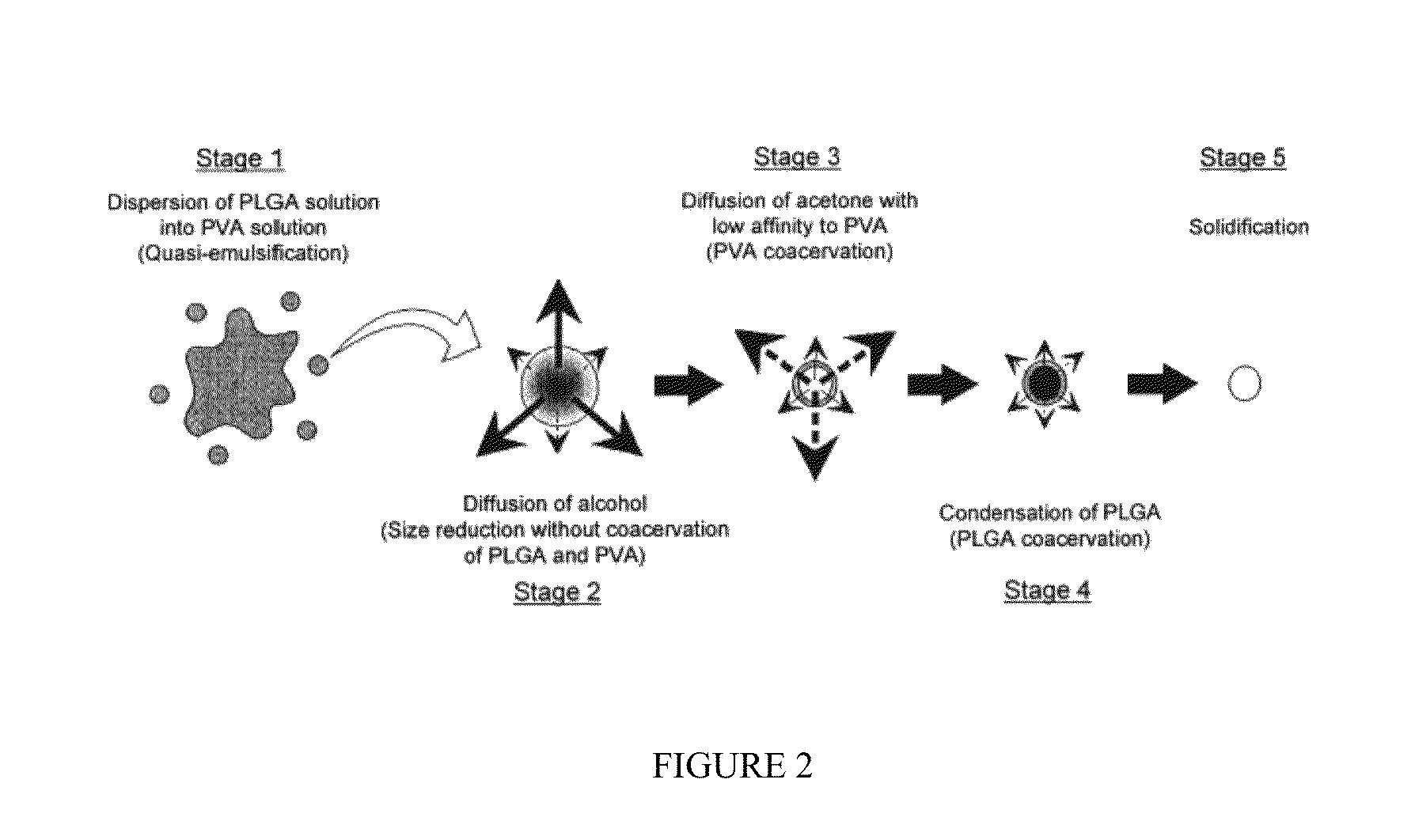
Biodegradable polymers are widely used in biomedical applications, particularly for tissue engineering scaffolds. These polymers provide temporary mechanical support while gradually degrading as new tissue forms. The mechanical properties of these polymers can be tailored by adjusting their composition, molecular weight, and processing conditions to match the requirements of specific tissues. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which offer controllable degradation rates and mechanical strength.- Biodegradable polymers for tissue engineering: Biodegradable polymers are widely used in biomedical applications, particularly for tissue engineering scaffolds. These polymers provide temporary mechanical support while gradually degrading as new tissue forms. The mechanical properties of these polymers can be tailored by adjusting their composition, molecular weight, and processing conditions to match the target tissue requirements. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which offer controllable degradation rates and mechanical strength.
- Hydrogels with tunable mechanical properties: Hydrogels represent an important class of biomedical polymers with adjustable mechanical properties. These water-swollen polymer networks can be engineered to exhibit a wide range of stiffness, elasticity, and strength by modifying crosslinking density, polymer concentration, and chemical composition. The mechanical properties of hydrogels can be designed to mimic various soft tissues in the body, making them suitable for applications such as drug delivery systems, wound dressings, and artificial cartilage. Smart hydrogels that respond to environmental stimuli like temperature, pH, or electrical signals offer additional functionality for biomedical applications.
- Nanocomposite polymers for enhanced mechanical strength: Incorporating nanoparticles into biomedical polymers creates nanocomposites with significantly enhanced mechanical properties. Materials such as carbon nanotubes, graphene, silica, and hydroxyapatite nanoparticles can be dispersed within polymer matrices to improve tensile strength, modulus, and fracture toughness while maintaining biocompatibility. These nanocomposite systems enable the development of stronger implants and devices that can withstand physiological loads while remaining lightweight. The interface between nanoparticles and the polymer matrix plays a crucial role in determining the overall mechanical performance of these advanced materials.
- Characterization methods for polymer mechanical properties: Various techniques are employed to characterize the mechanical properties of biomedical polymers, including tensile testing, compression testing, dynamic mechanical analysis (DMA), nanoindentation, and atomic force microscopy (AFM). These methods provide critical information about elastic modulus, yield strength, ultimate tensile strength, elongation at break, and viscoelastic behavior. Understanding these properties is essential for predicting polymer performance in biological environments and ensuring their suitability for specific medical applications. Advanced characterization approaches also include in situ testing under physiologically relevant conditions to better simulate the actual performance of materials in the body.
- Shape memory polymers for implantable devices: Shape memory polymers (SMPs) represent an innovative class of biomedical materials that can change their shape in response to external stimuli such as temperature, light, or electrical signals. These polymers can be programmed to transition from a temporary shape to a predetermined permanent shape, making them valuable for minimally invasive implantable devices. The mechanical properties of SMPs, including recovery force, recovery rate, and shape fixity, can be tailored through polymer chemistry and processing. Applications include self-expanding stents, sutures that tighten automatically, and deployable tissue engineering scaffolds that can be inserted through small incisions and then expand to their functional shape.
02 Hydrogels with tunable mechanical properties
Hydrogels represent an important class of biomedical polymers with adjustable mechanical properties. These water-swollen polymer networks can be engineered to exhibit a wide range of stiffness, elasticity, and strength by controlling crosslinking density, polymer concentration, and chemical composition. The mechanical properties of hydrogels can be designed to mimic those of natural tissues, making them suitable for applications such as drug delivery systems, wound dressings, and artificial cartilage. Smart hydrogels that respond to environmental stimuli like temperature, pH, or electrical signals offer additional functionality for biomedical applications.Expand Specific Solutions03 Composite polymers for enhanced mechanical strength
Composite biomedical polymers combine different materials to achieve enhanced mechanical properties that cannot be obtained from single polymers. These composites often incorporate reinforcing elements such as nanoparticles, fibers, or other polymers to improve strength, stiffness, and durability while maintaining biocompatibility. For example, polymer-ceramic composites can provide both the flexibility of polymers and the strength of ceramics, making them suitable for load-bearing implants. The mechanical properties of these composites can be tailored by adjusting the type, amount, and orientation of the reinforcing components.Expand Specific Solutions04 Testing and characterization of polymer mechanical properties
Various methods and techniques are used to test and characterize the mechanical properties of biomedical polymers. These include tensile testing, compression testing, dynamic mechanical analysis, nanoindentation, and rheological measurements. These techniques provide critical information about the strength, elasticity, viscoelasticity, and fatigue resistance of polymers under different conditions. Advanced imaging and spectroscopic methods can also be used to correlate the microstructure of polymers with their mechanical behavior. Standardized testing protocols ensure reproducibility and comparability of mechanical property data across different studies.Expand Specific Solutions05 Stimuli-responsive polymers with adaptive mechanical properties
Stimuli-responsive biomedical polymers can change their mechanical properties in response to external triggers such as temperature, pH, light, or electrical signals. These smart materials can transition between rigid and flexible states, expand or contract, or alter their stiffness based on environmental conditions. This adaptability makes them valuable for applications such as controlled drug delivery, self-adjusting implants, and tissue engineering constructs that can respond to physiological changes. The reversible nature of these mechanical property changes allows for dynamic control over material behavior in biological environments.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Biomedical Polymers
The biomedical polymer enhancement market is currently in a growth phase, with increasing demand driven by aging populations and advanced medical applications. The global market size is expanding rapidly, expected to reach significant valuation as healthcare needs evolve. Technological maturity varies across applications, with companies like Medtronic, Boston Scientific, and Johnson & Johnson (through Ethicon and Synthes) leading commercial innovation. Academic institutions including Northwestern University, Rutgers, and Sichuan University are advancing fundamental research in polymer enhancement techniques. Collaboration between industry leaders and research institutions is accelerating development of stronger, more biocompatible materials. The competitive landscape shows established medical device manufacturers investing heavily in proprietary technologies while academic research provides pipeline innovations for next-generation implantable and biodegradable polymers.
Medtronic, Inc.
Technical Solution: Medtronic has developed proprietary polymer modification techniques for their implantable medical devices, focusing on enhancing mechanical properties through cross-linking methodologies and surface treatments. Their approach involves the incorporation of nanoparticles into polymer matrices to create composite materials with superior tensile strength and flexibility. The company utilizes a multi-phase polymer blending process that creates interpenetrating networks, significantly improving wear resistance while maintaining biocompatibility. Their Shape Memory Polymer (SMP) technology allows for devices that can change shape upon implantation, providing both structural support and conformability to anatomical structures. Medtronic has also pioneered techniques for controlling crystallinity in semi-crystalline polymers used in cardiovascular applications, resulting in materials with optimized mechanical properties that can withstand physiological stresses while maintaining long-term stability in vivo.
Strengths: Exceptional balance between flexibility and durability in their polymer systems; proprietary cross-linking technology that enhances fatigue resistance without compromising biocompatibility. Weaknesses: Higher manufacturing costs associated with their advanced polymer processing techniques; some enhanced polymers may have limited shelf life compared to conventional materials.
Boston Scientific Scimed, Inc.
Technical Solution: Boston Scientific has developed innovative polymer enhancement technologies focused on vascular and interventional medical devices. Their approach centers on multi-layer extrusion techniques that create gradient materials with optimized mechanical properties throughout the structure. The company employs radiation cross-linking methods that significantly improve the tensile strength and burst pressure resistance of thin-walled components while maintaining flexibility. Their proprietary polymer blending technology incorporates nanoscale reinforcements that enhance mechanical properties without compromising processability or biocompatibility. Boston Scientific has pioneered techniques for controlling crystallinity in semi-crystalline polymers used in balloon catheters, resulting in materials with exceptional strength-to-weight ratios and controlled expansion characteristics. Their recent innovations include stimulus-responsive polymers that can change mechanical properties in response to specific physiological triggers, enabling adaptive performance in complex anatomical environments.
Strengths: Superior balance of flexibility and strength in thin-walled components; excellent control over expansion properties in balloon materials through proprietary processing techniques. Weaknesses: Some enhanced polymers require specialized processing equipment; potential for batch-to-batch variability in certain high-performance formulations.
Key Patents and Innovations in Polymer Mechanical Reinforcement
Resorbable polymeric medical goods with improved mechanical properties and method for producing same
PatentInactiveEP1744792A1
Innovation
- A method involving the use of specific plasticizing liquids or solids to enhance chain orientation in bioresorbable polymers during the drawing process, promoting transient chain mobility and alignment through a forming device with a conical cross-section, which results in higher tensile and flexural strength and moduli.
Biodegradable nanocomposites with enhanced mechanical properties for soft tissue engineering
PatentActiveUS20140135407A1
Innovation
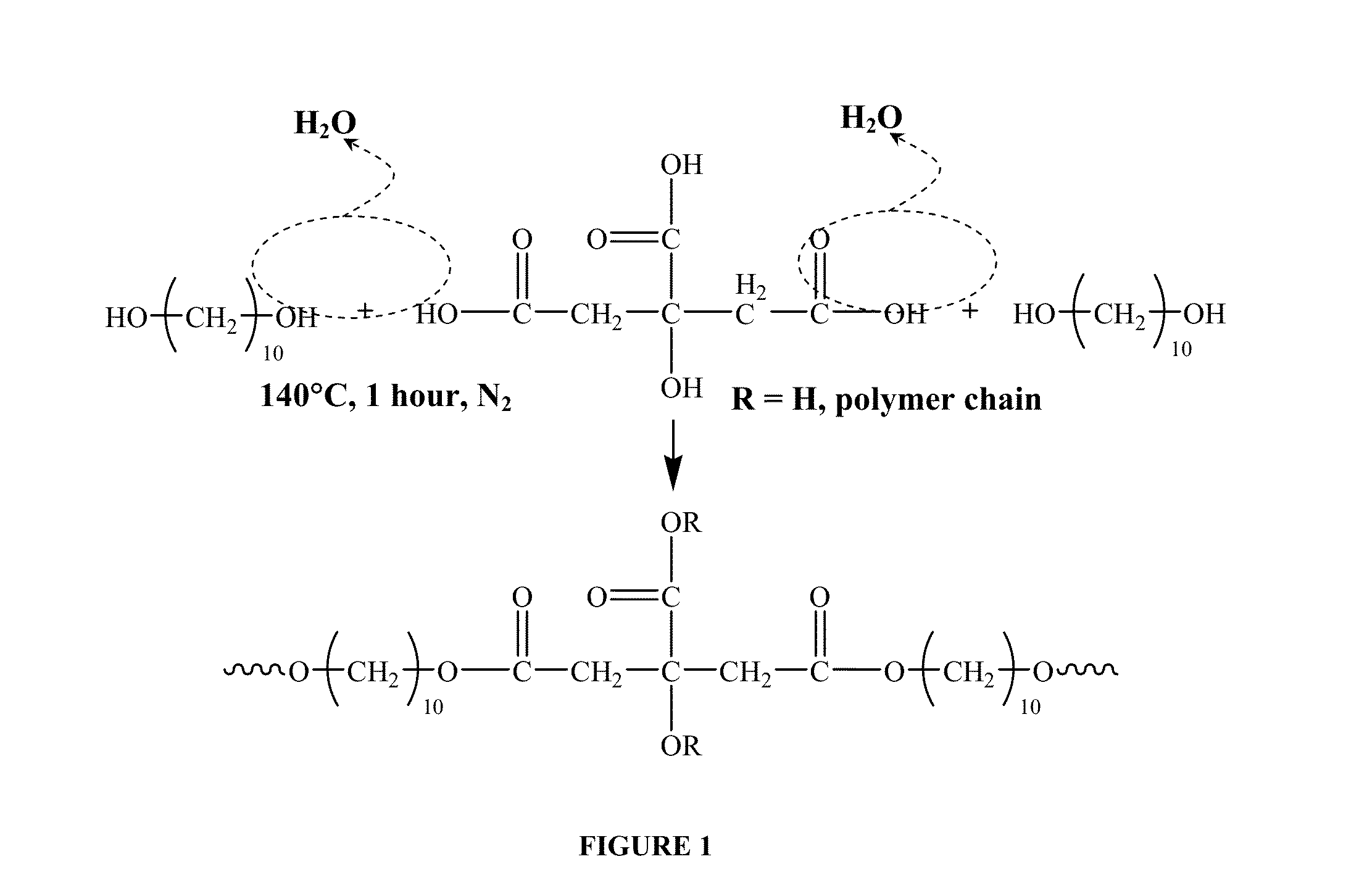
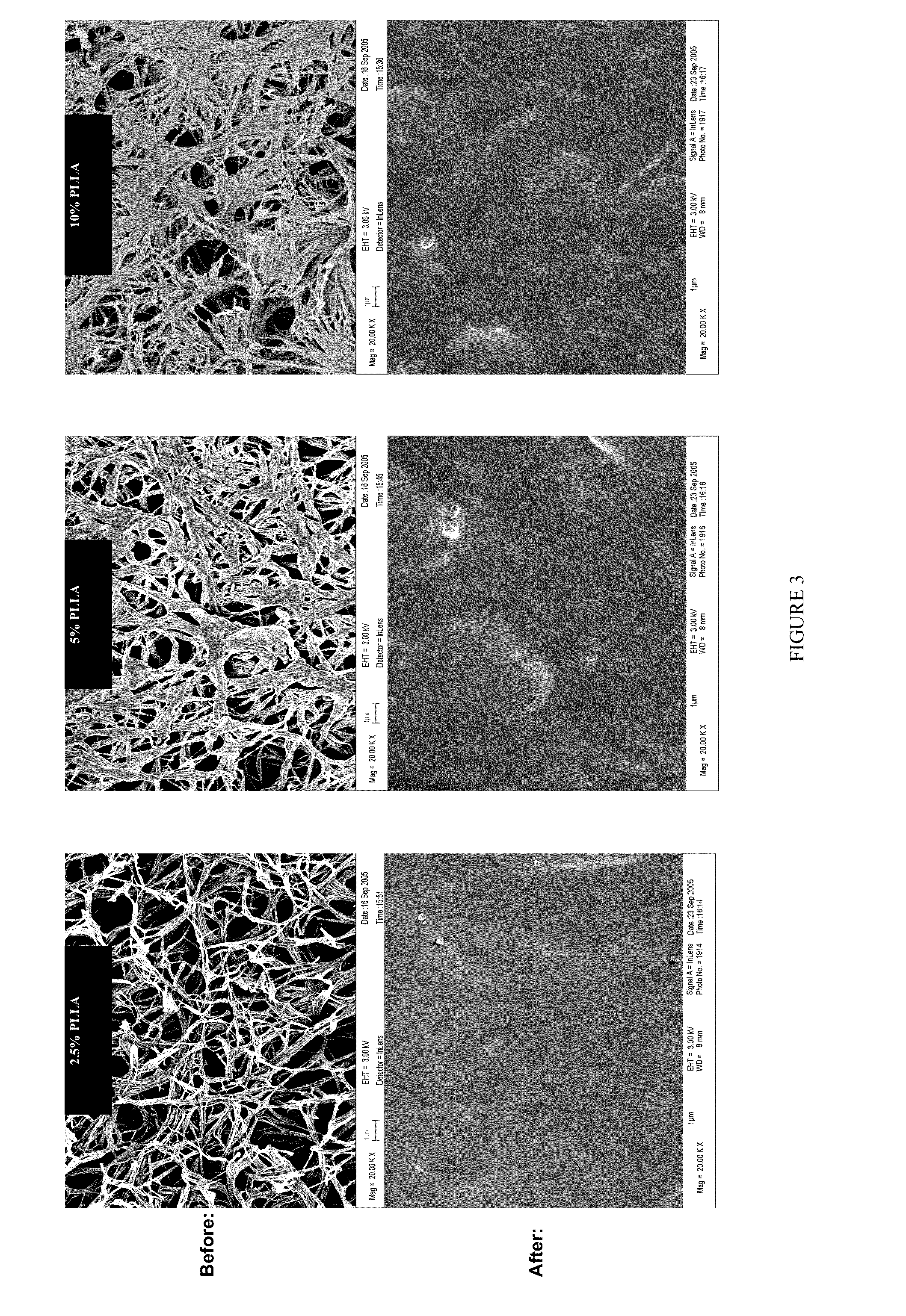
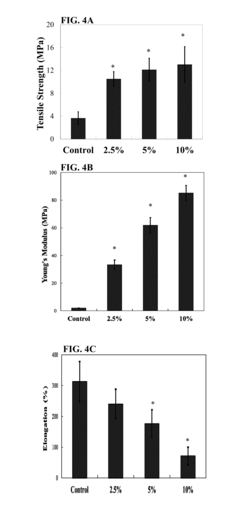
- A biodegradable elastomeric composite is developed using poly(diol citrate) reinforced with biodegradable poly(L-lactic acid) (PLLA) nanoscaffolds or PLGA nanoparticles, which provide enhanced mechanical properties while maintaining biocompatibility and the ability to be elongated multiple times before rupture.
Biocompatibility and Safety Considerations
When enhancing mechanical properties of biomedical polymers, biocompatibility and safety considerations must remain paramount. The interaction between modified polymers and biological systems requires rigorous evaluation to ensure patient safety. Enhanced mechanical properties often result from structural modifications, additives, or processing techniques that may introduce new biological responses when implanted or used in medical applications.
Material cytotoxicity represents a critical concern, as reinforcing agents or cross-linking chemicals might leach from the polymer matrix over time. For instance, carbon nanotubes used to enhance tensile strength have demonstrated potential cytotoxicity in certain formulations, necessitating careful selection and surface functionalization to mitigate these effects. Similarly, metal-based nanoparticles incorporated for improved mechanical performance must be thoroughly assessed for potential inflammatory responses.
Immune system reactions present another significant challenge when enhancing polymer mechanical properties. Surface modifications that alter topography or chemistry may trigger foreign body responses, potentially leading to fibrous encapsulation that compromises device functionality. Recent advances in immunomodulatory surface treatments have shown promise in reducing these adverse reactions while maintaining mechanical enhancements.
Degradation profiles of mechanically enhanced polymers require particular attention, especially for temporary implants. The breakdown products must remain non-toxic throughout the material's lifecycle. Mechanical enhancement techniques like orientation strengthening or crystallinity modification can significantly alter degradation kinetics, potentially creating unexpected metabolites or accelerating material breakdown in vivo.
Sterilization compatibility represents a frequently overlooked aspect of mechanically enhanced polymers. Common sterilization methods such as gamma irradiation, ethylene oxide treatment, or autoclaving can compromise carefully engineered mechanical properties. For example, radiation cross-linking may initially enhance mechanical strength but potentially introduce long-term embrittlement or degradation acceleration.
Regulatory considerations have evolved significantly, with authorities increasingly requiring comprehensive biocompatibility testing specific to mechanically enhanced materials. The FDA and European regulatory bodies now mandate specialized testing protocols for novel polymer formulations, particularly those incorporating nanomaterials or novel cross-linking agents for mechanical enhancement.
Long-term safety monitoring has revealed that mechanical property stability over the implantation period directly impacts biocompatibility. Materials engineered for enhanced initial mechanical properties that subsequently deteriorate may release particles or create stress concentrations that lead to inflammation or tissue damage. This understanding has driven development of more sophisticated in vitro testing regimes that better predict in vivo performance of mechanically enhanced polymers.
Material cytotoxicity represents a critical concern, as reinforcing agents or cross-linking chemicals might leach from the polymer matrix over time. For instance, carbon nanotubes used to enhance tensile strength have demonstrated potential cytotoxicity in certain formulations, necessitating careful selection and surface functionalization to mitigate these effects. Similarly, metal-based nanoparticles incorporated for improved mechanical performance must be thoroughly assessed for potential inflammatory responses.
Immune system reactions present another significant challenge when enhancing polymer mechanical properties. Surface modifications that alter topography or chemistry may trigger foreign body responses, potentially leading to fibrous encapsulation that compromises device functionality. Recent advances in immunomodulatory surface treatments have shown promise in reducing these adverse reactions while maintaining mechanical enhancements.
Degradation profiles of mechanically enhanced polymers require particular attention, especially for temporary implants. The breakdown products must remain non-toxic throughout the material's lifecycle. Mechanical enhancement techniques like orientation strengthening or crystallinity modification can significantly alter degradation kinetics, potentially creating unexpected metabolites or accelerating material breakdown in vivo.
Sterilization compatibility represents a frequently overlooked aspect of mechanically enhanced polymers. Common sterilization methods such as gamma irradiation, ethylene oxide treatment, or autoclaving can compromise carefully engineered mechanical properties. For example, radiation cross-linking may initially enhance mechanical strength but potentially introduce long-term embrittlement or degradation acceleration.
Regulatory considerations have evolved significantly, with authorities increasingly requiring comprehensive biocompatibility testing specific to mechanically enhanced materials. The FDA and European regulatory bodies now mandate specialized testing protocols for novel polymer formulations, particularly those incorporating nanomaterials or novel cross-linking agents for mechanical enhancement.
Long-term safety monitoring has revealed that mechanical property stability over the implantation period directly impacts biocompatibility. Materials engineered for enhanced initial mechanical properties that subsequently deteriorate may release particles or create stress concentrations that lead to inflammation or tissue damage. This understanding has driven development of more sophisticated in vitro testing regimes that better predict in vivo performance of mechanically enhanced polymers.
Regulatory Framework for Enhanced Biomedical Polymers
The regulatory landscape governing enhanced biomedical polymers is complex and multifaceted, requiring manufacturers to navigate various approval pathways depending on the intended application and jurisdiction. In the United States, the Food and Drug Administration (FDA) classifies medical devices containing enhanced polymers into three categories based on risk level, with Class III devices requiring the most rigorous premarket approval process including extensive clinical trials to demonstrate safety and efficacy.
The European Union's regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stricter requirements for clinical evidence and post-market surveillance. These regulations specifically address mechanically enhanced polymers through more stringent testing protocols for mechanical properties such as tensile strength, elongation at break, and fatigue resistance.
International Organization for Standardization (ISO) standards play a crucial role in establishing globally recognized testing methodologies for biomedical polymers. ISO 10993 series addresses biocompatibility evaluation, while ISO 527 and ISO 178 provide standardized methods for determining tensile and flexural properties respectively. Compliance with these standards is often a prerequisite for regulatory approval in most markets.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has implemented specific guidelines for polymeric materials with enhanced mechanical properties, particularly focusing on long-term stability and performance under physiological conditions. These guidelines require accelerated aging studies to predict the mechanical behavior of polymers throughout their intended service life.
Regulatory bodies increasingly require manufacturers to demonstrate that mechanical enhancement techniques do not compromise biocompatibility or introduce leachable compounds. This has led to the development of specialized testing protocols that simultaneously evaluate mechanical performance and biological safety, particularly for load-bearing implants and devices subject to cyclic mechanical stress.
Recent regulatory trends indicate a shift toward harmonization of international standards, with initiatives like the Medical Device Single Audit Program (MDSAP) allowing manufacturers to undergo a single regulatory audit acceptable in multiple jurisdictions. Additionally, regulatory frameworks are evolving to accommodate innovative manufacturing techniques such as 3D printing of mechanically enhanced polymers, with the FDA issuing guidance documents specifically addressing additive manufacturing technologies.
Compliance with these regulatory requirements necessitates comprehensive documentation of manufacturing processes, quality control measures, and validation protocols specific to the mechanical enhancement techniques employed. This documentation burden represents a significant challenge for manufacturers but ultimately ensures the safety and efficacy of enhanced biomedical polymers in clinical applications.
The European Union's regulatory framework has undergone significant transformation with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stricter requirements for clinical evidence and post-market surveillance. These regulations specifically address mechanically enhanced polymers through more stringent testing protocols for mechanical properties such as tensile strength, elongation at break, and fatigue resistance.
International Organization for Standardization (ISO) standards play a crucial role in establishing globally recognized testing methodologies for biomedical polymers. ISO 10993 series addresses biocompatibility evaluation, while ISO 527 and ISO 178 provide standardized methods for determining tensile and flexural properties respectively. Compliance with these standards is often a prerequisite for regulatory approval in most markets.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has implemented specific guidelines for polymeric materials with enhanced mechanical properties, particularly focusing on long-term stability and performance under physiological conditions. These guidelines require accelerated aging studies to predict the mechanical behavior of polymers throughout their intended service life.
Regulatory bodies increasingly require manufacturers to demonstrate that mechanical enhancement techniques do not compromise biocompatibility or introduce leachable compounds. This has led to the development of specialized testing protocols that simultaneously evaluate mechanical performance and biological safety, particularly for load-bearing implants and devices subject to cyclic mechanical stress.
Recent regulatory trends indicate a shift toward harmonization of international standards, with initiatives like the Medical Device Single Audit Program (MDSAP) allowing manufacturers to undergo a single regulatory audit acceptable in multiple jurisdictions. Additionally, regulatory frameworks are evolving to accommodate innovative manufacturing techniques such as 3D printing of mechanically enhanced polymers, with the FDA issuing guidance documents specifically addressing additive manufacturing technologies.
Compliance with these regulatory requirements necessitates comprehensive documentation of manufacturing processes, quality control measures, and validation protocols specific to the mechanical enhancement techniques employed. This documentation burden represents a significant challenge for manufacturers but ultimately ensures the safety and efficacy of enhanced biomedical polymers in clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!