Biomedical Polymers for Long-term Drug Delivery Mechanisms
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Evolution and Research Objectives
Biomedical polymers have undergone significant evolution since their initial development in the mid-20th century. The first generation of these materials emerged in the 1960s, primarily focusing on biocompatibility and minimal tissue interaction. These early polymers, such as poly(methyl methacrylate) and polyethylene, served basic medical functions but lacked sophisticated drug delivery capabilities.
The 1970s and 1980s witnessed the development of second-generation biomedical polymers, characterized by biodegradability and improved biocompatibility. Polymers like poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymers gained prominence for their ability to degrade within the body at predictable rates, opening new possibilities for drug delivery applications.
The 1990s marked the emergence of third-generation smart polymers, designed to respond to specific biological stimuli such as pH, temperature, or enzymatic activity. These materials revolutionized controlled drug release by enabling targeted delivery and sustained release profiles, significantly enhancing therapeutic efficacy while reducing systemic side effects.
Current research trends focus on fourth-generation biomedical polymers, incorporating nanotechnology, biomimetic approaches, and molecular engineering to create highly sophisticated drug delivery systems. These advanced materials aim to overcome biological barriers, improve drug stability, and achieve precise spatiotemporal control over drug release kinetics for periods extending from months to years.
The primary objective of this research is to develop novel biomedical polymer platforms capable of maintaining therapeutic drug concentrations within the optimal therapeutic window for extended periods, potentially exceeding one year. This goal addresses critical challenges in treating chronic conditions that require long-term medication adherence, such as psychiatric disorders, hormone replacement therapies, and certain infectious diseases.
Additional research objectives include enhancing polymer biocompatibility to minimize foreign body responses during long-term implantation, developing degradation mechanisms that synchronize with therapeutic timelines, and creating manufacturing processes that ensure consistent quality and scalability for clinical translation.
Furthermore, this research aims to establish predictive models correlating polymer structure with drug release kinetics, enabling rational design of delivery systems tailored to specific therapeutic needs. The ultimate goal is to create platform technologies that can accommodate diverse drug molecules with varying physicochemical properties while maintaining consistent release profiles over extended timeframes.
The 1970s and 1980s witnessed the development of second-generation biomedical polymers, characterized by biodegradability and improved biocompatibility. Polymers like poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymers gained prominence for their ability to degrade within the body at predictable rates, opening new possibilities for drug delivery applications.
The 1990s marked the emergence of third-generation smart polymers, designed to respond to specific biological stimuli such as pH, temperature, or enzymatic activity. These materials revolutionized controlled drug release by enabling targeted delivery and sustained release profiles, significantly enhancing therapeutic efficacy while reducing systemic side effects.
Current research trends focus on fourth-generation biomedical polymers, incorporating nanotechnology, biomimetic approaches, and molecular engineering to create highly sophisticated drug delivery systems. These advanced materials aim to overcome biological barriers, improve drug stability, and achieve precise spatiotemporal control over drug release kinetics for periods extending from months to years.
The primary objective of this research is to develop novel biomedical polymer platforms capable of maintaining therapeutic drug concentrations within the optimal therapeutic window for extended periods, potentially exceeding one year. This goal addresses critical challenges in treating chronic conditions that require long-term medication adherence, such as psychiatric disorders, hormone replacement therapies, and certain infectious diseases.
Additional research objectives include enhancing polymer biocompatibility to minimize foreign body responses during long-term implantation, developing degradation mechanisms that synchronize with therapeutic timelines, and creating manufacturing processes that ensure consistent quality and scalability for clinical translation.
Furthermore, this research aims to establish predictive models correlating polymer structure with drug release kinetics, enabling rational design of delivery systems tailored to specific therapeutic needs. The ultimate goal is to create platform technologies that can accommodate diverse drug molecules with varying physicochemical properties while maintaining consistent release profiles over extended timeframes.
Market Analysis for Sustained-Release Drug Delivery Systems
The global market for sustained-release drug delivery systems has experienced significant growth over the past decade, driven by increasing prevalence of chronic diseases and the need for improved patient compliance. Currently valued at approximately 36.5 billion USD, this market is projected to reach 53.3 billion USD by 2028, representing a compound annual growth rate (CAGR) of 7.9% during the forecast period.
Biomedical polymers for long-term drug delivery mechanisms have emerged as a particularly promising segment within this market. These advanced materials enable controlled release of therapeutic agents over extended periods, ranging from days to months or even years, addressing critical challenges in treatment adherence and therapeutic efficacy.
Demand analysis reveals several key factors driving market expansion. The rising global burden of chronic diseases, including diabetes, cardiovascular disorders, and various cancers, necessitates treatment regimens that maintain consistent drug levels over prolonged periods. Additionally, the aging population worldwide has created substantial demand for drug delivery systems that reduce dosing frequency and simplify medication management for elderly patients.
Regional market assessment indicates North America currently holds the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 21%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 9.7% through 2028, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced treatment options.
By application segment, the oncology sector dominates the market with a 31% share, followed by diabetes management at 24% and central nervous system disorders at 18%. The remaining market share is distributed among cardiovascular diseases, pain management, and other therapeutic areas.
Consumer preference analysis highlights strong patient demand for reduced dosing frequency, minimized side effects, and improved quality of life. Healthcare providers increasingly favor sustained-release systems that enhance treatment outcomes while reducing hospital readmissions and overall healthcare costs.
Market challenges include high development and manufacturing costs, complex regulatory approval processes, and technical difficulties in achieving precise release kinetics for certain drug molecules. Despite these challenges, the market presents substantial opportunities for innovation, particularly in biodegradable polymer systems, stimuli-responsive delivery mechanisms, and combination products that integrate multiple therapeutic agents.
Pricing trends indicate premium positioning for novel sustained-release technologies, with gradual price moderation as patents expire and manufacturing processes become optimized. Reimbursement landscapes vary significantly across regions, with favorable coverage in developed markets supporting adoption despite higher initial costs.
Biomedical polymers for long-term drug delivery mechanisms have emerged as a particularly promising segment within this market. These advanced materials enable controlled release of therapeutic agents over extended periods, ranging from days to months or even years, addressing critical challenges in treatment adherence and therapeutic efficacy.
Demand analysis reveals several key factors driving market expansion. The rising global burden of chronic diseases, including diabetes, cardiovascular disorders, and various cancers, necessitates treatment regimens that maintain consistent drug levels over prolonged periods. Additionally, the aging population worldwide has created substantial demand for drug delivery systems that reduce dosing frequency and simplify medication management for elderly patients.
Regional market assessment indicates North America currently holds the largest market share at 42%, followed by Europe at 28% and Asia-Pacific at 21%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 9.7% through 2028, primarily due to improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced treatment options.
By application segment, the oncology sector dominates the market with a 31% share, followed by diabetes management at 24% and central nervous system disorders at 18%. The remaining market share is distributed among cardiovascular diseases, pain management, and other therapeutic areas.
Consumer preference analysis highlights strong patient demand for reduced dosing frequency, minimized side effects, and improved quality of life. Healthcare providers increasingly favor sustained-release systems that enhance treatment outcomes while reducing hospital readmissions and overall healthcare costs.
Market challenges include high development and manufacturing costs, complex regulatory approval processes, and technical difficulties in achieving precise release kinetics for certain drug molecules. Despite these challenges, the market presents substantial opportunities for innovation, particularly in biodegradable polymer systems, stimuli-responsive delivery mechanisms, and combination products that integrate multiple therapeutic agents.
Pricing trends indicate premium positioning for novel sustained-release technologies, with gradual price moderation as patents expire and manufacturing processes become optimized. Reimbursement landscapes vary significantly across regions, with favorable coverage in developed markets supporting adoption despite higher initial costs.
Current Challenges in Long-term Drug Delivery Technologies
Despite significant advancements in drug delivery systems over the past decades, long-term drug delivery technologies continue to face substantial challenges that limit their clinical application and effectiveness. The primary obstacle remains achieving consistent therapeutic drug concentrations over extended periods without requiring frequent administration. Current polymer-based systems often exhibit unpredictable release kinetics, with initial burst releases followed by suboptimal delivery rates that fall below therapeutic thresholds.
Material biocompatibility presents another significant hurdle. Many polymers that demonstrate excellent drug encapsulation properties may trigger inflammatory responses or fibrous encapsulation when implanted long-term. This biological reaction not only compromises patient safety but also impedes drug diffusion, rendering the delivery system progressively less effective over time.
Stability issues plague many biomedical polymer formulations, particularly for protein and peptide therapeutics. The microenvironment within polymer matrices can promote protein aggregation, denaturation, or chemical degradation, resulting in loss of therapeutic efficacy. Additionally, polymer degradation products may alter local pH, further compromising drug stability and potentially causing tissue irritation.
The manufacturing scalability of advanced polymer-based delivery systems remains problematic. Complex fabrication processes involving multiple parameters such as solvent selection, temperature control, and precise polymer-drug interactions are difficult to standardize for industrial production while maintaining batch-to-batch consistency.
Regulatory pathways for novel long-term delivery systems are exceptionally demanding, requiring extensive stability data, biocompatibility testing, and long-duration clinical trials. This regulatory burden significantly increases development costs and time-to-market for innovative delivery technologies.
Achieving targeted delivery to specific tissues or cells while maintaining long-term release profiles represents a dual challenge that few current systems can address effectively. Most polymeric systems that excel at sustained release lack sophisticated targeting capabilities, while highly targeted systems typically cannot maintain therapeutic levels for extended periods.
The development of stimuli-responsive polymers that can modulate release rates in response to physiological needs or external triggers remains in early stages. While conceptually promising, these "smart" delivery systems face challenges in reliability, reproducibility, and the integration of sensing mechanisms with controlled release functionality.
Addressing these multifaceted challenges requires interdisciplinary approaches combining polymer chemistry, pharmaceutical sciences, bioengineering, and clinical medicine to develop the next generation of long-term drug delivery technologies.
Material biocompatibility presents another significant hurdle. Many polymers that demonstrate excellent drug encapsulation properties may trigger inflammatory responses or fibrous encapsulation when implanted long-term. This biological reaction not only compromises patient safety but also impedes drug diffusion, rendering the delivery system progressively less effective over time.
Stability issues plague many biomedical polymer formulations, particularly for protein and peptide therapeutics. The microenvironment within polymer matrices can promote protein aggregation, denaturation, or chemical degradation, resulting in loss of therapeutic efficacy. Additionally, polymer degradation products may alter local pH, further compromising drug stability and potentially causing tissue irritation.
The manufacturing scalability of advanced polymer-based delivery systems remains problematic. Complex fabrication processes involving multiple parameters such as solvent selection, temperature control, and precise polymer-drug interactions are difficult to standardize for industrial production while maintaining batch-to-batch consistency.
Regulatory pathways for novel long-term delivery systems are exceptionally demanding, requiring extensive stability data, biocompatibility testing, and long-duration clinical trials. This regulatory burden significantly increases development costs and time-to-market for innovative delivery technologies.
Achieving targeted delivery to specific tissues or cells while maintaining long-term release profiles represents a dual challenge that few current systems can address effectively. Most polymeric systems that excel at sustained release lack sophisticated targeting capabilities, while highly targeted systems typically cannot maintain therapeutic levels for extended periods.
The development of stimuli-responsive polymers that can modulate release rates in response to physiological needs or external triggers remains in early stages. While conceptually promising, these "smart" delivery systems face challenges in reliability, reproducibility, and the integration of sensing mechanisms with controlled release functionality.
Addressing these multifaceted challenges requires interdisciplinary approaches combining polymer chemistry, pharmaceutical sciences, bioengineering, and clinical medicine to develop the next generation of long-term drug delivery technologies.
Current Polymer-Based Drug Release Mechanisms
01 Biodegradable polymer matrices for controlled drug release
Biodegradable polymers can be formulated into matrices that gradually degrade in the body, releasing encapsulated drugs over extended periods. These systems typically utilize polymers such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and other polyesters that break down through hydrolysis. The degradation rate can be tailored by adjusting the polymer composition, molecular weight, and crystallinity, allowing for customized drug release profiles ranging from weeks to months.- Biodegradable polymer matrices for controlled drug release: Biodegradable polymers can be formulated into matrices that allow for the controlled release of drugs over extended periods. These matrices gradually break down in the body, releasing the encapsulated drug at a predetermined rate. The degradation rate can be tailored by modifying the polymer composition, molecular weight, and crystallinity. Common biodegradable polymers used include poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and poly(lactic acid) (PLA). These systems are particularly useful for delivering drugs that require consistent blood levels over weeks or months.
- Implantable polymer devices for long-term drug delivery: Implantable polymer devices represent a significant advancement in long-term drug delivery. These devices can be surgically placed in specific body locations to provide localized drug release over extended periods, ranging from months to years. The polymer composition can be engineered to control drug diffusion rates and device degradation. Some implants are designed to be non-degradable for very long-term therapy, while others gradually dissolve after drug depletion. These systems are particularly valuable for treating chronic conditions requiring consistent medication levels while minimizing systemic side effects.
- Hydrogel-based systems for sustained drug release: Hydrogels are three-dimensional networks of hydrophilic polymers that can absorb significant amounts of water while maintaining their structure. These biomedical polymers can be loaded with therapeutic agents for sustained release applications. The drug release rate is controlled by factors such as crosslinking density, polymer composition, and environmental responsiveness (pH, temperature, or enzyme sensitivity). Injectable hydrogels that form in situ provide minimally invasive delivery options. These systems are particularly effective for delivering proteins, peptides, and other large molecular weight drugs that require protection from degradation.
- Nanoparticle polymer systems for targeted drug delivery: Polymer-based nanoparticles offer sophisticated approaches to long-term drug delivery with targeting capabilities. These systems typically consist of drug-loaded polymeric nanoparticles ranging from 10-1000 nm in size. The polymer composition can be modified to control drug release kinetics and circulation time in the bloodstream. Surface modifications with ligands enable targeting to specific tissues or cells. These nanoparticle systems can cross biological barriers that larger delivery systems cannot penetrate, making them valuable for delivering drugs to challenging sites such as the brain or intracellular targets.
- Stimuli-responsive polymer systems for on-demand drug release: Stimuli-responsive polymer systems represent an advanced approach to long-term drug delivery where release is triggered by specific stimuli. These smart polymers can respond to environmental changes such as pH, temperature, light, ultrasound, or biochemical signals. The polymer structure undergoes conformational changes upon exposure to the stimulus, allowing for on-demand drug release. This approach enables precise temporal control over drug delivery, which is particularly valuable for therapies requiring intermittent dosing or release in response to physiological changes. These systems can maintain drug stability during storage and release it only when therapeutically needed.
02 Implantable drug delivery devices using biomedical polymers
Implantable devices made from biomedical polymers offer sustained drug delivery directly at target sites. These devices include polymer-based implants, films, and scaffolds that can be placed subcutaneously or at specific anatomical locations. The polymer composition provides mechanical support while controlling drug diffusion and release kinetics. Such implants minimize systemic exposure to drugs while maintaining therapeutic concentrations at the target site for extended periods, potentially lasting several months to years.Expand Specific Solutions03 Hydrogel-based systems for prolonged drug delivery
Hydrogels composed of crosslinked hydrophilic polymers can absorb large amounts of water while maintaining their structure, making them excellent candidates for long-term drug delivery. These systems can respond to environmental stimuli such as pH, temperature, or specific biomolecules to trigger drug release. The highly hydrated polymer network allows for controlled diffusion of drug molecules, while the crosslinking density can be modified to adjust release rates. Injectable hydrogels can form in situ, providing minimally invasive delivery options.Expand Specific Solutions04 Nanoparticle drug delivery systems using biocompatible polymers
Polymeric nanoparticles offer versatile platforms for long-term drug delivery with enhanced pharmacokinetics. These systems typically consist of drug molecules encapsulated within or conjugated to biocompatible polymers formed into nanoscale particles. The small size allows for targeted delivery to specific tissues, while the polymer composition controls drug release through mechanisms such as diffusion, polymer degradation, or responsive behavior. Surface modifications can further enhance circulation time and targeting efficiency.Expand Specific Solutions05 Composite polymer systems for sequential and multi-drug delivery
Advanced composite polymer systems combine different polymeric materials to achieve complex drug release profiles or deliver multiple therapeutic agents. These systems may incorporate layered structures, interpenetrating networks, or polymer blends to create sequential release patterns or deliver combination therapies. By integrating polymers with different properties, these composites can overcome limitations of single-polymer systems and provide precisely controlled release of multiple drugs over extended timeframes, improving treatment efficacy for complex conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Drug Delivery
The biomedical polymers for long-term drug delivery market is currently in a growth phase, with an expanding market size driven by increasing demand for sustained-release therapeutics. The competitive landscape features a mix of established pharmaceutical companies (Pfizer, IBM), specialized biotech firms (Mersana Therapeutics, Braeburn), and academic research institutions (Johns Hopkins University, California Institute of Technology). Technical maturity varies across applications, with leading players like The General Hospital Corporation, Boston University, and Agency for Science, Technology & Research demonstrating advanced polymer delivery systems. Pharmaceutical companies including Omeros Corp. and Alligator Bioscience are commercializing innovative formulations, while academic-industry partnerships are accelerating development. The field is characterized by continuous innovation in biodegradable polymers, implantable devices, and targeted delivery mechanisms, with competition intensifying around patent portfolios and clinical validation.
Pfizer Inc.
Technical Solution: Pfizer has developed advanced biodegradable polymer-based drug delivery systems focusing on sustained release formulations. Their technology utilizes poly(lactic-co-glycolic acid) (PLGA) microspheres and implants that provide controlled release of therapeutic agents over periods ranging from weeks to months. Pfizer's approach incorporates precise polymer molecular weight distribution control and specialized manufacturing processes to achieve predictable degradation kinetics. Their systems include injectable in-situ forming implants that transition from liquid to solid state upon administration, creating a depot for sustained drug release[1]. Additionally, Pfizer has pioneered smart polymer technologies responsive to physiological triggers such as pH, temperature, and enzymatic activity, allowing for targeted drug release at specific disease sites[3].
Strengths: Extensive manufacturing infrastructure and quality control systems ensure consistent product performance across batches. Global regulatory expertise facilitates faster market access. Weaknesses: Higher production costs compared to conventional formulations may limit application in cost-sensitive markets. Some polymer-based systems require specialized administration techniques.
Tolmar International Ltd.
Technical Solution: Tolmar has developed advanced polymer-based drug delivery platforms focusing on long-acting injectable (LAI) technologies. Their proprietary Atrigel® delivery system utilizes biodegradable polymers (primarily PLGA and PLA) dissolved in biocompatible solvents that solidify upon contact with aqueous environments, forming a solid implant in situ. This technology enables sustained release of pharmaceuticals over periods ranging from one to six months. Tolmar has refined polymer selection and processing to achieve precise control over degradation rates and drug release profiles[7]. Their manufacturing process incorporates specialized techniques for polymer purification and characterization to ensure batch-to-batch consistency. The company has successfully commercialized several products using this technology, particularly in the fields of oncology and endocrinology, demonstrating the platform's versatility across different therapeutic molecules and release durations[8].
Strengths: Injectable system eliminates need for surgical implantation and removal procedures. Versatile technology applicable to small molecules, peptides, and proteins. Weaknesses: Initial burst release can be challenging to control with some drug compounds. Limited application for drugs requiring very precise daily dosing schedules.
Key Patents and Innovations in Controlled Release Technology
Long term drug delivery devices with polyurethane based polymers and their manufacture
PatentInactiveUS20150174301A1
Innovation
- Development of polyurethane-based drug delivery devices with a cylindrically shaped reservoir surrounded by a polyurethane polymer, which allows for controlled release of drugs without a liquid medium, using thermoplastic or thermoset polyurethane polymers with specific functional groups and manufacturing processes like precision extrusion or reaction injection molding to achieve desired delivery rates.
Biodegradable polyketal polymers and methods for their formation and use
PatentInactiveEP1468036B1
Innovation
- Development of biodegradable biocompatible polyketals with ketal groups within the main chain, which are hydrophilic and pharmaceutically useful, allowing for controlled drug release and improved bioavailability by forming acyclic structures that can be modified for specific applications.
Biocompatibility and Degradation Kinetics Assessment
Biocompatibility and degradation kinetics represent critical parameters in the development of polymeric drug delivery systems intended for long-term therapeutic applications. The interface between synthetic materials and biological systems demands meticulous evaluation to ensure patient safety while maintaining therapeutic efficacy over extended periods.
The biocompatibility assessment of polymeric delivery systems encompasses multiple levels of biological interaction. At the cellular level, cytotoxicity testing using standardized protocols such as MTT assays and live/dead cell staining provides fundamental insights into material safety. Recent advancements in 3D cell culture models have significantly enhanced the predictive value of in vitro biocompatibility assessments by better mimicking the complex tissue architecture encountered in vivo.
Inflammatory response evaluation constitutes another crucial dimension of biocompatibility testing. Polymeric materials intended for long-term implantation must demonstrate minimal activation of pro-inflammatory pathways. Current methodologies incorporate analysis of cytokine profiles, macrophage polarization states, and complement activation to comprehensively characterize the host response to implanted materials.
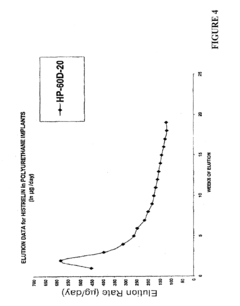
Degradation kinetics of biomedical polymers directly influence drug release profiles and material longevity within biological environments. Hydrolytic degradation mechanisms predominate in polyesters such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), while enzymatic processes significantly impact natural polymers including collagen and hyaluronic acid derivatives. The interplay between these degradation pathways creates complex release profiles that can be engineered for specific therapeutic applications.
Advanced analytical techniques including gel permeation chromatography, differential scanning calorimetry, and mass spectrometry enable precise characterization of degradation products and kinetics. In situ monitoring technologies utilizing spectroscopic methods have emerged as valuable tools for real-time assessment of material degradation in physiologically relevant conditions.
The correlation between degradation kinetics and drug release profiles presents both challenges and opportunities in delivery system design. Mathematical modeling approaches incorporating Monte Carlo simulations and finite element analysis have demonstrated increasing accuracy in predicting long-term degradation behavior, enabling rational design of release kinetics tailored to specific therapeutic requirements.
Recent innovations in this field include stimuli-responsive degradation mechanisms that respond to specific biological triggers such as pH fluctuations, enzymatic activity, or redox conditions. These smart materials offer unprecedented control over spatial and temporal aspects of drug delivery, potentially revolutionizing treatment paradigms for chronic conditions requiring precise therapeutic management over extended timeframes.
The biocompatibility assessment of polymeric delivery systems encompasses multiple levels of biological interaction. At the cellular level, cytotoxicity testing using standardized protocols such as MTT assays and live/dead cell staining provides fundamental insights into material safety. Recent advancements in 3D cell culture models have significantly enhanced the predictive value of in vitro biocompatibility assessments by better mimicking the complex tissue architecture encountered in vivo.
Inflammatory response evaluation constitutes another crucial dimension of biocompatibility testing. Polymeric materials intended for long-term implantation must demonstrate minimal activation of pro-inflammatory pathways. Current methodologies incorporate analysis of cytokine profiles, macrophage polarization states, and complement activation to comprehensively characterize the host response to implanted materials.
Degradation kinetics of biomedical polymers directly influence drug release profiles and material longevity within biological environments. Hydrolytic degradation mechanisms predominate in polyesters such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), while enzymatic processes significantly impact natural polymers including collagen and hyaluronic acid derivatives. The interplay between these degradation pathways creates complex release profiles that can be engineered for specific therapeutic applications.
Advanced analytical techniques including gel permeation chromatography, differential scanning calorimetry, and mass spectrometry enable precise characterization of degradation products and kinetics. In situ monitoring technologies utilizing spectroscopic methods have emerged as valuable tools for real-time assessment of material degradation in physiologically relevant conditions.
The correlation between degradation kinetics and drug release profiles presents both challenges and opportunities in delivery system design. Mathematical modeling approaches incorporating Monte Carlo simulations and finite element analysis have demonstrated increasing accuracy in predicting long-term degradation behavior, enabling rational design of release kinetics tailored to specific therapeutic requirements.
Recent innovations in this field include stimuli-responsive degradation mechanisms that respond to specific biological triggers such as pH fluctuations, enzymatic activity, or redox conditions. These smart materials offer unprecedented control over spatial and temporal aspects of drug delivery, potentially revolutionizing treatment paradigms for chronic conditions requiring precise therapeutic management over extended timeframes.
Regulatory Pathway for Novel Drug Delivery Systems
The regulatory landscape for biomedical polymers in long-term drug delivery systems presents a complex pathway that developers must navigate carefully. In the United States, the FDA evaluates these systems through either the Center for Drug Evaluation and Research (CDER) or the Center for Devices and Radiological Health (CDRH), depending on whether the primary mode of action is pharmacological or mechanical. Novel drug delivery systems utilizing biomedical polymers typically fall under combination product regulations, requiring comprehensive documentation addressing both the drug and device components.
For initial regulatory submission, developers must conduct extensive preclinical testing focusing on polymer biocompatibility, degradation profiles, and potential toxicity of degradation products. ISO 10993 standards provide the framework for biological evaluation, with particular emphasis on long-term implantation studies for sustained-release systems. Leachables and extractables testing becomes especially critical for polymeric delivery systems due to the extended contact duration with biological tissues.
Clinical trial design for long-term delivery systems presents unique challenges, requiring extended monitoring periods to match the intended duration of drug release. The FDA's guidance on controlled release dosage forms recommends specialized pharmacokinetic studies demonstrating consistent drug release profiles throughout the intended therapeutic period. Accelerated stability testing protocols must be developed and validated to predict real-time performance over extended timeframes.
International regulatory pathways show notable variations. The European Medicines Agency (EMA) applies a more centralized approach through the Medical Device Regulation (MDR) for drug-device combinations, with specific requirements for novel polymeric materials. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation specifically for innovative delivery technologies, potentially expediting approval for qualifying polymer-based systems.
Post-market surveillance requirements are particularly stringent for long-term delivery systems, with regulatory bodies mandating comprehensive risk management plans and long-term patient registries. The FDA's Sentinel Initiative and the EMA's EUDAMED database serve as platforms for monitoring real-world performance and adverse events related to novel delivery systems.
Recent regulatory trends indicate movement toward adaptive licensing pathways for innovative delivery technologies, allowing staged approvals with ongoing evidence generation. The FDA's Breakthrough Devices Program and the EMA's PRIME (PRIority MEdicines) scheme offer accelerated pathways for transformative technologies addressing unmet medical needs, potentially benefiting novel polymer-based delivery systems with significant therapeutic advantages over conventional approaches.
For initial regulatory submission, developers must conduct extensive preclinical testing focusing on polymer biocompatibility, degradation profiles, and potential toxicity of degradation products. ISO 10993 standards provide the framework for biological evaluation, with particular emphasis on long-term implantation studies for sustained-release systems. Leachables and extractables testing becomes especially critical for polymeric delivery systems due to the extended contact duration with biological tissues.
Clinical trial design for long-term delivery systems presents unique challenges, requiring extended monitoring periods to match the intended duration of drug release. The FDA's guidance on controlled release dosage forms recommends specialized pharmacokinetic studies demonstrating consistent drug release profiles throughout the intended therapeutic period. Accelerated stability testing protocols must be developed and validated to predict real-time performance over extended timeframes.
International regulatory pathways show notable variations. The European Medicines Agency (EMA) applies a more centralized approach through the Medical Device Regulation (MDR) for drug-device combinations, with specific requirements for novel polymeric materials. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation specifically for innovative delivery technologies, potentially expediting approval for qualifying polymer-based systems.
Post-market surveillance requirements are particularly stringent for long-term delivery systems, with regulatory bodies mandating comprehensive risk management plans and long-term patient registries. The FDA's Sentinel Initiative and the EMA's EUDAMED database serve as platforms for monitoring real-world performance and adverse events related to novel delivery systems.
Recent regulatory trends indicate movement toward adaptive licensing pathways for innovative delivery technologies, allowing staged approvals with ongoing evidence generation. The FDA's Breakthrough Devices Program and the EMA's PRIME (PRIority MEdicines) scheme offer accelerated pathways for transformative technologies addressing unmet medical needs, potentially benefiting novel polymer-based delivery systems with significant therapeutic advantages over conventional approaches.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!