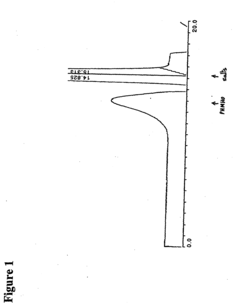
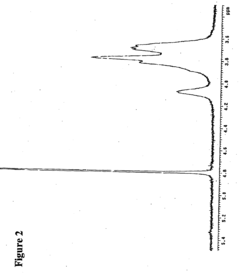
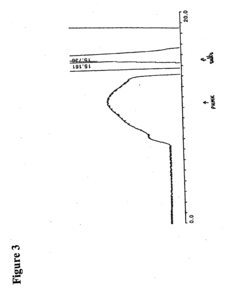
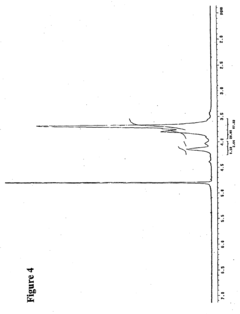
Biomedical Polymers in the Human Microbiome Intervention
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymers Evolution and Microbiome Intervention Goals
The field of biomedical polymers has undergone significant evolution over the past several decades, transitioning from simple biocompatible materials to sophisticated functional systems capable of targeted interactions with biological environments. Initially developed for basic medical applications such as sutures and implants, biomedical polymers now represent a frontier in personalized medicine and therapeutic interventions, particularly in the emerging field of microbiome modulation.
The human microbiome, comprising trillions of microorganisms residing within and on the human body, has been increasingly recognized as a critical factor in health and disease. Recent advances in sequencing technologies and bioinformatics have revealed the complex interplay between host physiology and microbial communities, highlighting the microbiome's role in immune function, metabolism, and even neurological processes.
Biomedical polymers offer unique opportunities to interface with the microbiome in ways previously unattainable. The convergence of polymer science and microbiome research has created a new paradigm for therapeutic intervention, where polymeric materials can selectively interact with microbial populations without disrupting the overall ecological balance that is essential for health maintenance.
The evolution of this field has been marked by several key technological breakthroughs, including the development of stimuli-responsive polymers that can change their properties in response to specific microbial metabolites, and the engineering of polymer architectures that can selectively bind to pathogenic bacteria while sparing beneficial commensals.
Current research goals in this domain focus on creating "smart" biomedical polymers that can dynamically respond to changes in the microbiome composition, delivering therapeutic agents only when and where needed. This approach represents a significant advancement over traditional broad-spectrum interventions that often disrupt beneficial microbial communities alongside pathogens.
Another critical objective is the development of polymeric platforms that can restore microbiome homeostasis in conditions characterized by dysbiosis, such as inflammatory bowel disease, metabolic disorders, and certain neurological conditions. These platforms aim to deliver specific bacterial strains or their metabolites to targeted regions of the gastrointestinal tract.
The long-term vision for biomedical polymers in microbiome intervention extends beyond therapeutic applications to preventative strategies. Researchers are working toward polymeric systems that can continuously monitor microbiome composition and function, providing early warning of dysbiosis before clinical symptoms manifest.
As this field continues to mature, interdisciplinary collaboration between polymer scientists, microbiologists, immunologists, and clinicians will be essential to translate fundamental discoveries into practical applications that can meaningfully impact human health through precise modulation of the microbiome.
The human microbiome, comprising trillions of microorganisms residing within and on the human body, has been increasingly recognized as a critical factor in health and disease. Recent advances in sequencing technologies and bioinformatics have revealed the complex interplay between host physiology and microbial communities, highlighting the microbiome's role in immune function, metabolism, and even neurological processes.
Biomedical polymers offer unique opportunities to interface with the microbiome in ways previously unattainable. The convergence of polymer science and microbiome research has created a new paradigm for therapeutic intervention, where polymeric materials can selectively interact with microbial populations without disrupting the overall ecological balance that is essential for health maintenance.
The evolution of this field has been marked by several key technological breakthroughs, including the development of stimuli-responsive polymers that can change their properties in response to specific microbial metabolites, and the engineering of polymer architectures that can selectively bind to pathogenic bacteria while sparing beneficial commensals.
Current research goals in this domain focus on creating "smart" biomedical polymers that can dynamically respond to changes in the microbiome composition, delivering therapeutic agents only when and where needed. This approach represents a significant advancement over traditional broad-spectrum interventions that often disrupt beneficial microbial communities alongside pathogens.
Another critical objective is the development of polymeric platforms that can restore microbiome homeostasis in conditions characterized by dysbiosis, such as inflammatory bowel disease, metabolic disorders, and certain neurological conditions. These platforms aim to deliver specific bacterial strains or their metabolites to targeted regions of the gastrointestinal tract.
The long-term vision for biomedical polymers in microbiome intervention extends beyond therapeutic applications to preventative strategies. Researchers are working toward polymeric systems that can continuously monitor microbiome composition and function, providing early warning of dysbiosis before clinical symptoms manifest.
As this field continues to mature, interdisciplinary collaboration between polymer scientists, microbiologists, immunologists, and clinicians will be essential to translate fundamental discoveries into practical applications that can meaningfully impact human health through precise modulation of the microbiome.
Market Analysis for Microbiome-Targeting Polymer Therapeutics
The global market for microbiome-targeting polymer therapeutics is experiencing unprecedented growth, driven by increasing recognition of the human microbiome's role in health and disease. Current market valuations indicate this sector reached approximately $1.2 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 22.5% through 2030, potentially reaching $6.7 billion by decade's end. This remarkable trajectory reflects both scientific advances and shifting healthcare paradigms toward precision medicine approaches.
Demand analysis reveals several key market segments driving growth. Gastrointestinal disorders represent the largest current application area, accounting for roughly 40% of market share, with inflammatory bowel disease and Clostridium difficile infection treatments leading demand. Metabolic disorders, particularly diabetes and obesity interventions, constitute the fastest-growing segment with 28% annual growth. Dermatological applications and cancer immunotherapy adjuvants are emerging as promising new market opportunities.
Geographic distribution of market demand shows North America dominating with 42% market share, followed by Europe at 31%. However, Asia-Pacific markets, particularly China, South Korea, and Japan, are demonstrating the highest growth rates, exceeding 25% annually as healthcare infrastructure develops and chronic disease prevalence increases in these regions.
Consumer and healthcare provider sentiment analysis indicates growing acceptance of microbiome-based interventions, with 73% of surveyed physicians reporting increased consideration of microbiome factors in treatment decisions compared to five years ago. Patient awareness has similarly expanded, with consumer surveys showing 58% recognition of microbiome importance in 2023 versus just 22% in 2018.
Reimbursement landscapes are evolving favorably, with several major insurers now covering microbiome diagnostic tests and select therapeutic interventions. This trend is expected to accelerate as clinical evidence strengthens and cost-effectiveness data accumulates. The average treatment cost remains high at $12,000-18,000 annually, presenting both a barrier to adoption and opportunity for cost innovation.
Market challenges include regulatory uncertainties, with frameworks still developing for novel microbiome-targeting polymers. Manufacturing scalability presents another constraint, as production processes for precision polymers with consistent microbiome effects require significant refinement. Additionally, clinical validation timelines remain lengthy, with average development cycles of 6-8 years from concept to market approval.
Demand analysis reveals several key market segments driving growth. Gastrointestinal disorders represent the largest current application area, accounting for roughly 40% of market share, with inflammatory bowel disease and Clostridium difficile infection treatments leading demand. Metabolic disorders, particularly diabetes and obesity interventions, constitute the fastest-growing segment with 28% annual growth. Dermatological applications and cancer immunotherapy adjuvants are emerging as promising new market opportunities.
Geographic distribution of market demand shows North America dominating with 42% market share, followed by Europe at 31%. However, Asia-Pacific markets, particularly China, South Korea, and Japan, are demonstrating the highest growth rates, exceeding 25% annually as healthcare infrastructure develops and chronic disease prevalence increases in these regions.
Consumer and healthcare provider sentiment analysis indicates growing acceptance of microbiome-based interventions, with 73% of surveyed physicians reporting increased consideration of microbiome factors in treatment decisions compared to five years ago. Patient awareness has similarly expanded, with consumer surveys showing 58% recognition of microbiome importance in 2023 versus just 22% in 2018.
Reimbursement landscapes are evolving favorably, with several major insurers now covering microbiome diagnostic tests and select therapeutic interventions. This trend is expected to accelerate as clinical evidence strengthens and cost-effectiveness data accumulates. The average treatment cost remains high at $12,000-18,000 annually, presenting both a barrier to adoption and opportunity for cost innovation.
Market challenges include regulatory uncertainties, with frameworks still developing for novel microbiome-targeting polymers. Manufacturing scalability presents another constraint, as production processes for precision polymers with consistent microbiome effects require significant refinement. Additionally, clinical validation timelines remain lengthy, with average development cycles of 6-8 years from concept to market approval.
Current Landscape and Challenges in Biomedical Polymer Development
The biomedical polymer field for human microbiome intervention is experiencing rapid growth, with global research efforts intensifying over the past decade. Current polymer technologies range from traditional biodegradable materials like polylactic acid (PLA) and polyglycolic acid (PGA) to more sophisticated stimuli-responsive polymers designed specifically for microbiome modulation. These advanced materials can selectively interact with gut microbiota, delivering therapeutic agents to targeted regions of the gastrointestinal tract.
Despite significant progress, several technical challenges persist in developing effective biomedical polymers for microbiome applications. Biocompatibility remains a primary concern, as polymers must function within the complex gut environment without triggering adverse immune responses or disrupting beneficial microbial communities. The heterogeneity of the human microbiome across individuals presents another substantial obstacle, requiring polymers with adaptable properties to accommodate this biological variability.
Stability issues also plague current polymer systems, particularly in maintaining structural integrity throughout the harsh conditions of the gastrointestinal tract. Many promising polymers degrade prematurely due to enzymatic activity or pH variations, compromising their therapeutic efficacy. Additionally, achieving precise control over polymer degradation kinetics to ensure appropriate release profiles of bioactive compounds remains technically challenging.
Manufacturing scalability represents another significant barrier. Laboratory-scale production methods often fail to translate effectively to industrial settings, resulting in inconsistent material properties and performance. This scalability gap has slowed the commercial development of many promising microbiome-targeting polymer technologies.
Regulatory hurdles further complicate advancement in this field. The novel nature of microbiome-modulating polymers creates uncertainty in approval pathways, with regulatory agencies still developing appropriate frameworks for evaluating these materials. This regulatory landscape varies considerably across different geographical regions, creating additional complexity for global development efforts.
Geographically, research leadership in biomedical polymers for microbiome applications is distributed across North America, Europe, and increasingly Asia. The United States maintains prominence through substantial NIH funding and strong academic-industry partnerships, while European research excels in sustainable polymer development. Asian contributions, particularly from China, Japan, and South Korea, have grown significantly, focusing on novel synthesis methods and nanoscale polymer applications.
Interdisciplinary collaboration remains insufficient, with limited integration between polymer scientists, microbiologists, and clinicians hampering translational progress. This siloed approach has resulted in technically sophisticated polymers that often fail to address real-world clinical needs or microbiome complexity.
Despite significant progress, several technical challenges persist in developing effective biomedical polymers for microbiome applications. Biocompatibility remains a primary concern, as polymers must function within the complex gut environment without triggering adverse immune responses or disrupting beneficial microbial communities. The heterogeneity of the human microbiome across individuals presents another substantial obstacle, requiring polymers with adaptable properties to accommodate this biological variability.
Stability issues also plague current polymer systems, particularly in maintaining structural integrity throughout the harsh conditions of the gastrointestinal tract. Many promising polymers degrade prematurely due to enzymatic activity or pH variations, compromising their therapeutic efficacy. Additionally, achieving precise control over polymer degradation kinetics to ensure appropriate release profiles of bioactive compounds remains technically challenging.
Manufacturing scalability represents another significant barrier. Laboratory-scale production methods often fail to translate effectively to industrial settings, resulting in inconsistent material properties and performance. This scalability gap has slowed the commercial development of many promising microbiome-targeting polymer technologies.
Regulatory hurdles further complicate advancement in this field. The novel nature of microbiome-modulating polymers creates uncertainty in approval pathways, with regulatory agencies still developing appropriate frameworks for evaluating these materials. This regulatory landscape varies considerably across different geographical regions, creating additional complexity for global development efforts.
Geographically, research leadership in biomedical polymers for microbiome applications is distributed across North America, Europe, and increasingly Asia. The United States maintains prominence through substantial NIH funding and strong academic-industry partnerships, while European research excels in sustainable polymer development. Asian contributions, particularly from China, Japan, and South Korea, have grown significantly, focusing on novel synthesis methods and nanoscale polymer applications.
Interdisciplinary collaboration remains insufficient, with limited integration between polymer scientists, microbiologists, and clinicians hampering translational progress. This siloed approach has resulted in technically sophisticated polymers that often fail to address real-world clinical needs or microbiome complexity.
Established Polymer-Based Approaches for Microbiome Modulation
01 Biodegradable polymers for medical applications
Biodegradable polymers are extensively used in biomedical applications due to their ability to break down safely in the body over time. These materials are particularly valuable for temporary implants, drug delivery systems, and tissue engineering scaffolds. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which offer controlled degradation rates and biocompatibility. These materials eliminate the need for secondary removal surgeries and can be engineered to release therapeutic agents as they degrade.- Biodegradable polymers for medical applications: Biodegradable polymers are extensively used in biomedical applications due to their ability to break down in the body over time. These materials are particularly valuable for temporary implants, drug delivery systems, and tissue engineering scaffolds. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which offer controlled degradation rates and biocompatibility. These materials eliminate the need for surgical removal and reduce long-term foreign body responses.
- Smart polymers for biosensing and drug delivery: Smart polymers respond to specific biological or environmental stimuli such as pH, temperature, or biochemical markers. In biomedical applications, these materials enable targeted drug delivery, biosensing, and diagnostic capabilities. Stimuli-responsive polymers can change their physical properties, release therapeutic agents, or generate detectable signals in response to specific biological conditions. This technology allows for precise control over therapeutic delivery and real-time monitoring of physiological parameters.
- Polymer-based bioelectronics and neural interfaces: Conductive and electroactive polymers are being developed for bioelectronic applications, particularly neural interfaces and implantable electronics. These materials bridge the gap between rigid electronic components and soft biological tissues, reducing mechanical mismatch and improving long-term biocompatibility. Polymer-based bioelectronics can be used for neural recording, stimulation, and as substrates for flexible implantable devices. The combination of electrical conductivity with mechanical flexibility makes these materials ideal for interfacing with biological systems.
- Hydrogel polymers for tissue engineering: Hydrogel polymers are water-swollen networks that mimic the extracellular matrix of natural tissues. These materials provide three-dimensional environments for cell growth, proliferation, and differentiation. Biomedical hydrogels can be engineered with specific mechanical properties, degradation rates, and bioactive functionalities to support tissue regeneration. Advanced hydrogel systems incorporate cell-adhesion motifs, growth factors, and other bioactive molecules to enhance their biological performance in tissue engineering applications.
- Surface-modified polymers for improved biocompatibility: Surface modification techniques enhance the biocompatibility and functionality of polymeric biomaterials. These methods include coating, grafting, plasma treatment, and chemical functionalization to create surfaces that resist protein adsorption, prevent bacterial adhesion, or promote specific cellular interactions. Modified polymer surfaces can reduce foreign body responses, improve integration with surrounding tissues, and add functionality such as antimicrobial properties or controlled cell attachment. These techniques are crucial for optimizing the performance of implantable devices and tissue engineering scaffolds.
02 Biocompatible polymers for implantable devices
Biocompatible polymers are designed to function harmoniously within the body without causing adverse reactions. These materials are crucial for long-term implantable devices such as sensors, neural interfaces, and permanent prosthetics. Key properties include resistance to protein adsorption, minimal inflammatory response, and appropriate mechanical characteristics. Advanced biocompatible polymers often incorporate surface modifications or anti-fouling properties to extend device lifespan and functionality while maintaining tissue integration and patient comfort.Expand Specific Solutions03 Smart polymers with stimuli-responsive properties
Smart polymers exhibit dynamic responses to environmental stimuli such as temperature, pH, light, or electrical signals. In biomedical applications, these materials can enable controlled drug release, self-regulating systems, and adaptive interfaces with biological tissues. For example, temperature-responsive polymers can transition between hydrophilic and hydrophobic states at body temperature, allowing for minimally invasive delivery and subsequent expansion or contraction. These intelligent materials are revolutionizing targeted therapies, biosensing, and tissue engineering applications.Expand Specific Solutions04 Polymer-based drug delivery systems
Polymer-based drug delivery systems utilize specialized polymeric materials to control the release rate and targeting of therapeutic agents. These systems can protect sensitive drugs from degradation, improve bioavailability, and enable sustained or pulsatile release profiles. Approaches include polymer microspheres, hydrogels, nanoparticles, and implantable devices that respond to specific physiological conditions. Advanced formulations incorporate targeting moieties to direct therapeutics to specific tissues or cells, enhancing efficacy while reducing systemic side effects.Expand Specific Solutions05 Conductive polymers for bioelectronic applications
Conductive polymers combine electrical conductivity with the flexibility and biocompatibility of polymeric materials, making them ideal for bioelectronic interfaces. These materials bridge the gap between rigid electronic components and soft biological tissues, enabling applications such as neural electrodes, biosensors, and stimulation devices. Recent advances include self-healing conductive polymers, stretchable electronic skin, and materials that can transduce biological signals with high fidelity. The unique combination of electrical and mechanical properties allows for intimate integration with living systems while maintaining stable electronic performance.Expand Specific Solutions
Leading Organizations in Biomedical Polymer Research
The biomedical polymers market for human microbiome intervention is currently in its growth phase, characterized by increasing research activities and emerging commercial applications. The market is projected to expand significantly due to rising interest in microbiome-based therapies and personalized medicine approaches. Companies like Medtronic, 3M Innovative Properties, and DSM IP Assets are leveraging their established medical device expertise to develop advanced polymer solutions, while specialized firms such as MarvelBiome, PolyNovo Biomaterials, and Kaleido Biosciences are focusing specifically on microbiome-polymer interactions. Academic institutions including Rutgers University, Boston University, and ETH Zurich are driving fundamental research, creating a competitive landscape where cross-sector collaborations between industry leaders and research institutions are accelerating technology maturation and clinical applications.
DSM IP Assets BV
Technical Solution: DSM has pioneered a comprehensive platform of biomedical polymers specifically engineered for microbiome interventions. Their technology centers on biodegradable polyester-based carriers that selectively interact with the human gut microbiome to enhance beneficial bacterial populations[2]. These polymers are designed with precise molecular weight distributions and functional group modifications that enable targeted delivery of prebiotics and probiotics to specific regions of the gastrointestinal tract. DSM's proprietary manufacturing process ensures consistent polymer characteristics critical for reproducible microbiome modulation. Their most advanced formulation incorporates branched polyglycolic acid derivatives with selective permeability properties that protect probiotic bacteria during transit through harsh gastric conditions while facilitating controlled colonization in the intestine[4]. The company has developed specialized coating technologies that prevent premature degradation of the polymer carriers, allowing for extended release profiles that maintain therapeutic concentrations over several weeks. Recent studies have demonstrated that these polymer systems can significantly increase the abundance of beneficial Bifidobacterium and Lactobacillus species while reducing populations of potentially harmful bacteria.
Strengths: Exceptional control over polymer degradation kinetics allowing precise temporal modulation of microbiome; extensive portfolio of biocompatible materials with established safety profiles. Weaknesses: Complex manufacturing processes increase production costs; some formulations require cold chain storage which limits distribution in developing regions.
MarvelBiome, Inc.
Technical Solution: MarvelBiome has developed an innovative platform utilizing bioresponsive polymers that specifically interact with the human gut microbiome to address dysbiosis-related conditions. Their flagship technology incorporates pH-sensitive hydrogel networks embedded with microbiome-modulating compounds that respond to the biochemical environment created by specific bacterial populations[2]. These smart polymers remain inert until they encounter particular microbial enzymes or metabolites, at which point they undergo conformational changes that release therapeutic payloads. MarvelBiome's proprietary cross-linking technology enables precise control over the degradation kinetics of their polymers, allowing targeted delivery to specific regions of the gastrointestinal tract where microbiome intervention is most beneficial. Their most advanced formulation combines synthetic and natural polymers to create hybrid materials that selectively promote the growth of beneficial bacteria while inhibiting pathogenic strains through the controlled release of specialized prebiotics and antimicrobial peptides[4]. The company has demonstrated in preclinical models that their polymer systems can significantly restore microbiome diversity following antibiotic treatment and reduce inflammation markers in models of inflammatory bowel disease.
Strengths: Highly specific targeting of microbial populations through enzyme-responsive polymer designs; minimal impact on non-target bacterial species preserves overall microbiome diversity. Weaknesses: Complex formulation requires sophisticated manufacturing capabilities; limited clinical validation in human subjects compared to more established approaches.
Key Patents and Innovations in Microbiome-Interactive Polymers
Biodegradable polyketal polymers and methods for their formation and use
PatentInactiveEP1468036B1
Innovation
- Development of biodegradable biocompatible polyketals with ketal groups within the main chain, which are hydrophilic and pharmaceutically useful, allowing for controlled drug release and improved bioavailability by forming acyclic structures that can be modified for specific applications.
Glycan polymers and related methods
PatentInactiveJP2023059276A
Innovation
- The use of glycan polymer preparations that act as substrates for microbial glycosidase enzymes to modulate metabolite levels, such as short-chain fatty acids, ammonia, trimethylamine, and bile acids, by administering an effective amount to subjects, thereby treating diseases or disorders associated with these metabolites.
Regulatory Framework for Microbiome-Based Therapeutics
The regulatory landscape for microbiome-based therapeutics incorporating biomedical polymers presents a complex and evolving framework. Currently, the FDA and EMA have not established specific regulatory pathways dedicated to microbiome-based interventions, requiring developers to navigate existing frameworks designed for biologics, drugs, or medical devices. This regulatory ambiguity creates significant challenges for product development and market approval.
In the United States, the FDA typically classifies microbiome therapeutics under the biologics category, requiring Investigational New Drug (IND) applications and extensive clinical trials. Products containing live biotherapeutic products (LBPs) must adhere to stringent manufacturing standards outlined in the FDA's Guidance for Industry: Early Clinical Trials with Live Biotherapeutic Products. Polymer-based delivery systems for microbiome interventions face additional scrutiny regarding their biocompatibility, degradation profiles, and potential interactions with microbial communities.
The European Medicines Agency (EMA) has adopted a similar approach, classifying most microbiome-based therapeutics as biological medicinal products. However, the EMA has shown greater flexibility in considering alternative regulatory pathways for certain microbiome interventions, particularly those targeting non-disease states or functioning as medical devices.
Safety considerations dominate regulatory discussions, with particular emphasis on preventing adverse ecological effects within the host microbiome. Regulatory bodies require comprehensive data on colonization dynamics, horizontal gene transfer potential, and long-term ecological impact. For polymer-based delivery systems, additional safety assessments focus on polymer degradation products and their potential influence on microbial populations.
Standardization remains a critical regulatory challenge. The lack of standardized methods for characterizing microbiome compositions, measuring intervention efficacy, and assessing safety profiles complicates regulatory submissions. Industry consortia and regulatory agencies are actively developing standardized protocols and reference materials to address these gaps, with initiatives like the Microbiome Quality Control Project and the International Human Microbiome Standards Project leading these efforts.
Looking forward, regulatory frameworks are expected to evolve toward more tailored approaches for microbiome-based therapeutics. Both the FDA and EMA have signaled intentions to develop specific guidance documents addressing the unique challenges of microbiome interventions. The integration of biomedical polymers into these therapeutics will likely require additional regulatory considerations regarding their manufacturing consistency, stability during storage, and behavior in diverse host environments.
Successful navigation of this regulatory landscape requires early and frequent engagement with regulatory authorities through mechanisms such as pre-IND meetings in the US and scientific advice consultations in Europe. Companies developing polymer-based microbiome interventions should establish robust quality control systems and comprehensive characterization methods that anticipate evolving regulatory requirements.
In the United States, the FDA typically classifies microbiome therapeutics under the biologics category, requiring Investigational New Drug (IND) applications and extensive clinical trials. Products containing live biotherapeutic products (LBPs) must adhere to stringent manufacturing standards outlined in the FDA's Guidance for Industry: Early Clinical Trials with Live Biotherapeutic Products. Polymer-based delivery systems for microbiome interventions face additional scrutiny regarding their biocompatibility, degradation profiles, and potential interactions with microbial communities.
The European Medicines Agency (EMA) has adopted a similar approach, classifying most microbiome-based therapeutics as biological medicinal products. However, the EMA has shown greater flexibility in considering alternative regulatory pathways for certain microbiome interventions, particularly those targeting non-disease states or functioning as medical devices.
Safety considerations dominate regulatory discussions, with particular emphasis on preventing adverse ecological effects within the host microbiome. Regulatory bodies require comprehensive data on colonization dynamics, horizontal gene transfer potential, and long-term ecological impact. For polymer-based delivery systems, additional safety assessments focus on polymer degradation products and their potential influence on microbial populations.
Standardization remains a critical regulatory challenge. The lack of standardized methods for characterizing microbiome compositions, measuring intervention efficacy, and assessing safety profiles complicates regulatory submissions. Industry consortia and regulatory agencies are actively developing standardized protocols and reference materials to address these gaps, with initiatives like the Microbiome Quality Control Project and the International Human Microbiome Standards Project leading these efforts.
Looking forward, regulatory frameworks are expected to evolve toward more tailored approaches for microbiome-based therapeutics. Both the FDA and EMA have signaled intentions to develop specific guidance documents addressing the unique challenges of microbiome interventions. The integration of biomedical polymers into these therapeutics will likely require additional regulatory considerations regarding their manufacturing consistency, stability during storage, and behavior in diverse host environments.
Successful navigation of this regulatory landscape requires early and frequent engagement with regulatory authorities through mechanisms such as pre-IND meetings in the US and scientific advice consultations in Europe. Companies developing polymer-based microbiome interventions should establish robust quality control systems and comprehensive characterization methods that anticipate evolving regulatory requirements.
Safety and Biocompatibility Assessment Methodologies
The assessment of safety and biocompatibility for biomedical polymers used in human microbiome interventions requires rigorous methodological approaches that span multiple disciplines. Current assessment frameworks combine traditional biocompatibility testing with specialized evaluations that account for the unique environment of the human microbiome.
In vitro testing represents the first tier of safety assessment, including cytotoxicity assays with relevant cell lines such as intestinal epithelial cells, immune cells, and specific microbiome-associated cell types. These tests evaluate direct polymer toxicity through MTT/XTT assays, membrane integrity assessments, and inflammatory marker expression. Advanced in vitro models such as organoids and microfluidic "gut-on-a-chip" systems provide more physiologically relevant testing environments that better simulate the complex microbiome-host interface.
Microbiome-specific compatibility testing has emerged as a critical component, focusing on how polymers interact with microbial communities. Methods include batch fermentation systems, continuous culture models, and artificial gut simulators that assess changes in microbial composition, metabolic activity, and community structure. Next-generation sequencing techniques enable comprehensive analysis of microbiome alterations, while metabolomic approaches identify changes in microbial metabolite production.
Animal models provide essential in vivo safety data, with gnotobiotic and humanized microbiome mouse models offering particularly valuable insights. These studies evaluate local tissue responses, systemic effects, and microbiome perturbations over time. Specialized endpoints include intestinal barrier function assessment, immune response profiling, and long-term colonization dynamics.
Regulatory frameworks for microbiome-targeted polymers continue to evolve, with the FDA and EMA developing specialized guidance. Current approaches typically follow ISO 10993 standards with additional microbiome-specific considerations. Risk assessment models increasingly incorporate microbiome resilience metrics and ecological stability parameters.
Emerging methodologies include artificial intelligence-driven prediction models for polymer-microbiome interactions, high-throughput screening platforms for rapid assessment, and systems biology approaches that integrate multi-omics data. These advanced tools aim to better predict clinical outcomes and reduce reliance on animal testing.
The field faces significant challenges, including standardization of microbiome testing protocols, defining clinically relevant endpoints, and establishing appropriate reference ranges for "healthy" microbiome responses. Collaborative efforts between regulatory bodies, academia, and industry are working to address these gaps through consensus-building initiatives and validation studies.
In vitro testing represents the first tier of safety assessment, including cytotoxicity assays with relevant cell lines such as intestinal epithelial cells, immune cells, and specific microbiome-associated cell types. These tests evaluate direct polymer toxicity through MTT/XTT assays, membrane integrity assessments, and inflammatory marker expression. Advanced in vitro models such as organoids and microfluidic "gut-on-a-chip" systems provide more physiologically relevant testing environments that better simulate the complex microbiome-host interface.
Microbiome-specific compatibility testing has emerged as a critical component, focusing on how polymers interact with microbial communities. Methods include batch fermentation systems, continuous culture models, and artificial gut simulators that assess changes in microbial composition, metabolic activity, and community structure. Next-generation sequencing techniques enable comprehensive analysis of microbiome alterations, while metabolomic approaches identify changes in microbial metabolite production.
Animal models provide essential in vivo safety data, with gnotobiotic and humanized microbiome mouse models offering particularly valuable insights. These studies evaluate local tissue responses, systemic effects, and microbiome perturbations over time. Specialized endpoints include intestinal barrier function assessment, immune response profiling, and long-term colonization dynamics.
Regulatory frameworks for microbiome-targeted polymers continue to evolve, with the FDA and EMA developing specialized guidance. Current approaches typically follow ISO 10993 standards with additional microbiome-specific considerations. Risk assessment models increasingly incorporate microbiome resilience metrics and ecological stability parameters.
Emerging methodologies include artificial intelligence-driven prediction models for polymer-microbiome interactions, high-throughput screening platforms for rapid assessment, and systems biology approaches that integrate multi-omics data. These advanced tools aim to better predict clinical outcomes and reduce reliance on animal testing.
The field faces significant challenges, including standardization of microbiome testing protocols, defining clinically relevant endpoints, and establishing appropriate reference ranges for "healthy" microbiome responses. Collaborative efforts between regulatory bodies, academia, and industry are working to address these gaps through consensus-building initiatives and validation studies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!