Biomedical Polymers: An Evaluation of Wear Resistance
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Evolution and Research Objectives
Biomedical polymers have undergone significant evolution since their initial introduction in medical applications during the mid-20th century. The journey began with simple polymeric materials like polyethylene and polyvinyl chloride, which offered basic biocompatibility but limited mechanical properties. The 1970s marked a turning point with the development of specialized biomaterials designed specifically for medical implants, including ultra-high-molecular-weight polyethylene (UHMWPE) for orthopedic applications.
The 1980s and 1990s witnessed rapid advancement in polymer science, introducing materials with enhanced wear resistance properties such as cross-linked polyethylene and polymer composites. These innovations addressed critical issues in joint replacement longevity and performance. The early 2000s brought nanotechnology into biomedical polymers, creating nanocomposites with significantly improved wear characteristics while maintaining biocompatibility.
Recent developments have focused on smart polymers capable of responding to biological environments, self-healing polymers that can repair wear damage, and biodegradable polymers with controlled degradation profiles. These advancements represent the convergence of materials science, biology, and engineering in creating next-generation biomaterials.
The wear resistance of biomedical polymers remains a critical challenge in applications such as artificial joints, dental materials, and cardiovascular devices where mechanical stress is constant. Current research objectives center on developing polymers that can withstand millions of cycles of mechanical loading while maintaining structural integrity and minimizing particle generation.
Key research objectives in this field include quantifying and predicting wear mechanisms in physiological environments, understanding the relationship between polymer structure and wear performance, and developing standardized testing protocols that accurately simulate in vivo conditions. Additionally, researchers aim to correlate laboratory wear testing with clinical outcomes to better predict long-term implant performance.
Another significant research direction involves surface modification techniques to enhance wear resistance without compromising bulk material properties. This includes exploring various coating technologies, surface texturing, and gradient materials that provide optimal tribological properties at the interface while maintaining core mechanical strength.
The ultimate goal of biomedical polymer wear resistance research is to develop materials that can function for decades within the human body without significant degradation or the release of wear particles that may trigger adverse biological responses. This requires interdisciplinary collaboration between polymer scientists, tribologists, bioengineers, and clinicians to translate fundamental materials science into practical medical solutions that improve patient outcomes and quality of life.
The 1980s and 1990s witnessed rapid advancement in polymer science, introducing materials with enhanced wear resistance properties such as cross-linked polyethylene and polymer composites. These innovations addressed critical issues in joint replacement longevity and performance. The early 2000s brought nanotechnology into biomedical polymers, creating nanocomposites with significantly improved wear characteristics while maintaining biocompatibility.
Recent developments have focused on smart polymers capable of responding to biological environments, self-healing polymers that can repair wear damage, and biodegradable polymers with controlled degradation profiles. These advancements represent the convergence of materials science, biology, and engineering in creating next-generation biomaterials.
The wear resistance of biomedical polymers remains a critical challenge in applications such as artificial joints, dental materials, and cardiovascular devices where mechanical stress is constant. Current research objectives center on developing polymers that can withstand millions of cycles of mechanical loading while maintaining structural integrity and minimizing particle generation.
Key research objectives in this field include quantifying and predicting wear mechanisms in physiological environments, understanding the relationship between polymer structure and wear performance, and developing standardized testing protocols that accurately simulate in vivo conditions. Additionally, researchers aim to correlate laboratory wear testing with clinical outcomes to better predict long-term implant performance.
Another significant research direction involves surface modification techniques to enhance wear resistance without compromising bulk material properties. This includes exploring various coating technologies, surface texturing, and gradient materials that provide optimal tribological properties at the interface while maintaining core mechanical strength.
The ultimate goal of biomedical polymer wear resistance research is to develop materials that can function for decades within the human body without significant degradation or the release of wear particles that may trigger adverse biological responses. This requires interdisciplinary collaboration between polymer scientists, tribologists, bioengineers, and clinicians to translate fundamental materials science into practical medical solutions that improve patient outcomes and quality of life.
Market Analysis for Wear-Resistant Biomedical Polymers
The global market for wear-resistant biomedical polymers is experiencing robust growth, driven by increasing demand for long-lasting implantable medical devices and prosthetics. Current market valuations indicate that the biomedical polymer sector reached approximately 11 billion USD in 2022, with wear-resistant variants constituting about 3.5 billion USD of this total. Industry analysts project a compound annual growth rate (CAGR) of 7.8% through 2030, significantly outpacing traditional medical materials.
Orthopedic applications represent the largest market segment, accounting for 42% of wear-resistant biomedical polymer usage. This dominance stems from the critical need for joint replacement components that can withstand millions of loading cycles while maintaining biocompatibility. Cardiovascular applications follow at 23%, with dental applications comprising 18% of the market share.
Regionally, North America leads with 38% of global market consumption, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth trajectory at 9.3% CAGR, primarily driven by expanding healthcare infrastructure in China and India, coupled with increasing adoption of advanced medical technologies.
Consumer demand patterns reveal a strong preference for materials offering extended service life, with 78% of surveyed healthcare providers citing durability as a primary selection criterion for implantable devices. This trend is reinforced by healthcare economics, as extended implant longevity significantly reduces revision surgery rates and associated costs, estimated at 75,000 USD per procedure in developed markets.
Regulatory factors are substantially influencing market dynamics. The FDA's recent emphasis on long-term performance data for implantable materials has created higher barriers to entry but also premium positioning opportunities for materials with proven wear resistance. Similarly, the European Medical Device Regulation (MDR) implementation has intensified scrutiny of material performance claims.
Pricing analysis indicates that wear-resistant biomedical polymers command a 30-45% premium over standard medical-grade polymers, with ultra-high-molecular-weight polyethylene (UHMWPE) variants leading the premium segment. This price differential is justified by lifecycle cost analyses demonstrating 22% lower total treatment costs when accounting for reduced revision rates.
Market penetration varies significantly by application, with orthopedic applications showing 67% adoption of advanced wear-resistant polymers, while dental applications lag at 41%, indicating substantial growth potential in underserved segments.
Orthopedic applications represent the largest market segment, accounting for 42% of wear-resistant biomedical polymer usage. This dominance stems from the critical need for joint replacement components that can withstand millions of loading cycles while maintaining biocompatibility. Cardiovascular applications follow at 23%, with dental applications comprising 18% of the market share.
Regionally, North America leads with 38% of global market consumption, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth trajectory at 9.3% CAGR, primarily driven by expanding healthcare infrastructure in China and India, coupled with increasing adoption of advanced medical technologies.
Consumer demand patterns reveal a strong preference for materials offering extended service life, with 78% of surveyed healthcare providers citing durability as a primary selection criterion for implantable devices. This trend is reinforced by healthcare economics, as extended implant longevity significantly reduces revision surgery rates and associated costs, estimated at 75,000 USD per procedure in developed markets.
Regulatory factors are substantially influencing market dynamics. The FDA's recent emphasis on long-term performance data for implantable materials has created higher barriers to entry but also premium positioning opportunities for materials with proven wear resistance. Similarly, the European Medical Device Regulation (MDR) implementation has intensified scrutiny of material performance claims.
Pricing analysis indicates that wear-resistant biomedical polymers command a 30-45% premium over standard medical-grade polymers, with ultra-high-molecular-weight polyethylene (UHMWPE) variants leading the premium segment. This price differential is justified by lifecycle cost analyses demonstrating 22% lower total treatment costs when accounting for reduced revision rates.
Market penetration varies significantly by application, with orthopedic applications showing 67% adoption of advanced wear-resistant polymers, while dental applications lag at 41%, indicating substantial growth potential in underserved segments.
Current Challenges in Biomedical Polymer Wear Resistance
Despite significant advancements in biomedical polymer development, several critical challenges persist in achieving optimal wear resistance for implantable devices and medical components. The primary obstacle remains the complex biological environment in which these polymers must function. Body fluids contain proteins, enzymes, and cells that can accelerate degradation processes through mechanisms not typically encountered in non-biological settings, creating unique wear patterns that are difficult to predict using conventional testing methods.
Surface oxidation represents another significant challenge, particularly for polyethylene-based components used in joint replacements. When exposed to bodily fluids and mechanical stress simultaneously, these materials undergo accelerated oxidative degradation, leading to embrittlement and subsequent particle generation. These wear particles trigger inflammatory responses that can ultimately lead to implant loosening and failure.
The tribological behavior of biomedical polymers presents additional complications. The boundary lubrication conditions in biological environments fluctuate based on patient activity, hydration status, and disease progression. Current polymer formulations struggle to maintain consistent wear resistance across these variable conditions, resulting in unpredictable clinical outcomes and reduced implant longevity.
Cross-linking techniques, while effective at improving wear resistance, introduce their own set of challenges. Highly cross-linked polymers often exhibit reduced fracture toughness and fatigue resistance, creating a difficult balance between wear performance and mechanical integrity. This trade-off becomes particularly problematic in high-stress applications such as spinal implants or load-bearing joint replacements.
Manufacturing consistency poses another significant hurdle. Small variations in processing parameters can dramatically alter the wear characteristics of biomedical polymers. The industry currently lacks standardized protocols for quality control that specifically address wear resistance parameters, making it difficult to ensure consistent performance across production batches.
The testing methodology itself represents a fundamental challenge. Current accelerated wear testing protocols often fail to accurately replicate in vivo conditions, leading to discrepancies between laboratory predictions and clinical outcomes. The multifactorial nature of wear mechanisms in biological environments makes it exceptionally difficult to develop testing regimens that reliably predict long-term performance.
Regulatory frameworks further complicate innovation in this space. The extended timeline required for approval of novel materials or surface treatments delays the implementation of promising wear-resistant technologies, creating a significant gap between laboratory breakthroughs and clinical application.
Surface oxidation represents another significant challenge, particularly for polyethylene-based components used in joint replacements. When exposed to bodily fluids and mechanical stress simultaneously, these materials undergo accelerated oxidative degradation, leading to embrittlement and subsequent particle generation. These wear particles trigger inflammatory responses that can ultimately lead to implant loosening and failure.
The tribological behavior of biomedical polymers presents additional complications. The boundary lubrication conditions in biological environments fluctuate based on patient activity, hydration status, and disease progression. Current polymer formulations struggle to maintain consistent wear resistance across these variable conditions, resulting in unpredictable clinical outcomes and reduced implant longevity.
Cross-linking techniques, while effective at improving wear resistance, introduce their own set of challenges. Highly cross-linked polymers often exhibit reduced fracture toughness and fatigue resistance, creating a difficult balance between wear performance and mechanical integrity. This trade-off becomes particularly problematic in high-stress applications such as spinal implants or load-bearing joint replacements.
Manufacturing consistency poses another significant hurdle. Small variations in processing parameters can dramatically alter the wear characteristics of biomedical polymers. The industry currently lacks standardized protocols for quality control that specifically address wear resistance parameters, making it difficult to ensure consistent performance across production batches.
The testing methodology itself represents a fundamental challenge. Current accelerated wear testing protocols often fail to accurately replicate in vivo conditions, leading to discrepancies between laboratory predictions and clinical outcomes. The multifactorial nature of wear mechanisms in biological environments makes it exceptionally difficult to develop testing regimens that reliably predict long-term performance.
Regulatory frameworks further complicate innovation in this space. The extended timeline required for approval of novel materials or surface treatments delays the implementation of promising wear-resistant technologies, creating a significant gap between laboratory breakthroughs and clinical application.
Contemporary Wear Resistance Enhancement Techniques
01 Surface modification techniques for enhanced wear resistance
Various surface modification techniques can be applied to biomedical polymers to enhance their wear resistance properties. These techniques include coating, grafting, and chemical treatment of polymer surfaces to create more durable interfaces. Modified surfaces can significantly reduce friction coefficients and improve the longevity of biomedical implants and devices that are subject to mechanical stress and wear during use.- Surface modification techniques for enhanced wear resistance: Various surface modification techniques can be applied to biomedical polymers to enhance their wear resistance properties. These techniques include coating, grafting, and chemical treatment of polymer surfaces to create more durable interfaces. The modified surfaces exhibit reduced friction coefficients and improved hardness, making them suitable for applications where mechanical wear is a concern, such as joint replacements and medical devices with moving parts.
- Composite polymer systems with reinforcing agents: Incorporating reinforcing agents into biomedical polymers creates composite systems with superior wear resistance. These reinforcing agents include nanoparticles, carbon-based materials, ceramic particles, and mineral fillers that enhance the mechanical properties of the base polymer. The resulting composites demonstrate improved hardness, reduced wear rates, and extended service life while maintaining biocompatibility, making them suitable for load-bearing implants and high-friction medical applications.
- Cross-linking and polymer network optimization: Cross-linking techniques and optimization of polymer network structures significantly improve the wear resistance of biomedical polymers. By creating additional bonds between polymer chains, these methods enhance mechanical stability and reduce material loss during friction events. Advanced cross-linking approaches include radiation-induced cross-linking, chemical cross-linking agents, and thermal treatments that can be tailored to specific biomedical applications while maintaining essential biocompatibility properties.
- Self-lubricating and hydrophilic polymer systems: Self-lubricating and hydrophilic polymer systems offer innovative approaches to reducing friction and wear in biomedical applications. These polymers incorporate water-attracting components or release lubricating agents during use, creating a natural boundary layer that minimizes direct contact between surfaces. The hydrophilic nature of these materials helps maintain a fluid film at the interface, reducing the coefficient of friction and subsequent wear while functioning in biological environments.
- Specialized polymer blends for tribological applications: Specialized polymer blends combine the beneficial properties of multiple polymers to create materials with optimized wear resistance for specific biomedical applications. These blends are designed to provide complementary properties such as elasticity, hardness, and surface characteristics that work together to minimize wear. The synergistic effects of carefully selected polymer combinations result in materials that outperform single-polymer systems in tribological performance while maintaining necessary biocompatibility and mechanical properties.
02 Composite polymer systems with reinforcing materials
Incorporating reinforcing materials such as nanoparticles, fibers, or other structural elements into biomedical polymers creates composite systems with superior wear resistance. These composites combine the biocompatibility of polymers with the mechanical strength and durability of reinforcing materials. The resulting materials demonstrate improved resistance to abrasion, reduced wear rates, and enhanced mechanical properties suitable for high-stress biomedical applications.Expand Specific Solutions03 Cross-linking and polymer network optimization
Optimizing the molecular structure of biomedical polymers through cross-linking and network formation significantly improves wear resistance. Controlled cross-linking density and network architecture can enhance mechanical properties while maintaining necessary flexibility. These techniques create more robust polymer structures that can withstand mechanical stresses and resist deformation, making them suitable for applications requiring long-term durability in biological environments.Expand Specific Solutions04 Hydrophilic polymers with self-lubricating properties
Hydrophilic biomedical polymers can be engineered to provide self-lubricating properties that reduce friction and wear. These materials absorb water or biological fluids to create a natural lubricating layer at the interface. The hydration layer acts as a boundary lubricant, minimizing direct contact between surfaces and reducing wear rates. This approach is particularly valuable for articulating surfaces in joint replacements and other moving biomedical components.Expand Specific Solutions05 Polymer blends and interpenetrating networks for wear applications
Strategic blending of different polymers or creating interpenetrating polymer networks (IPNs) can produce biomedical materials with enhanced wear resistance. These systems combine the beneficial properties of multiple polymers to achieve superior mechanical performance. The synergistic interaction between different polymer components results in materials with improved hardness, toughness, and resistance to wear while maintaining biocompatibility for medical applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The biomedical polymers market for wear-resistant applications is currently in a growth phase, with increasing demand driven by aging populations and rising orthopedic procedures worldwide. The market is projected to reach significant scale as materials with enhanced durability and biocompatibility become essential in medical devices. Leading companies like DuPont, Medtronic Vascular, and Smith & Nephew are advancing the technological frontier through proprietary polymer formulations with superior wear resistance. Academic institutions including MIT, Tufts University, and USC collaborate with industry leaders to develop next-generation materials. Japanese firms such as NOF Corp., TOTO, and Kuraray are making notable contributions in specialized biocompatible polymers, while European players like Heraeus Medical and Evonik Operations focus on bone cement and high-performance medical-grade polymers.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced UHMWPE (Ultra-High Molecular Weight Polyethylene) formulations specifically engineered for orthopedic implants with enhanced wear resistance. Their proprietary crosslinking technology combined with vitamin E stabilization creates a highly wear-resistant polymer matrix that maintains mechanical properties while reducing oxidative degradation. DuPont's Delrin® acetal homopolymer resins demonstrate exceptional wear resistance in biomedical applications, with documented wear factors as low as 1.5×10^-6 mm³/Nm in dry sliding conditions. Their recent innovations include radiation-crosslinked UHMWPE with antioxidant packages that show up to 95% reduction in wear rates compared to conventional polyethylene in hip simulator testing. DuPont also employs surface modification techniques including ion implantation and plasma treatments to further enhance surface hardness and reduce friction coefficients in their biomedical polymers.
Strengths: Industry-leading expertise in polymer chemistry with extensive material science capabilities; comprehensive testing facilities for wear simulation; established supply chain for medical-grade polymers. Weaknesses: Higher cost compared to standard polymers; some formulations require specialized processing equipment; potential regulatory hurdles for novel polymer modifications.
Howmedica Osteonics Corp.
Technical Solution: Howmedica Osteonics (Stryker) has developed X3™ Advanced Bearing Technology, a highly crosslinked UHMWPE material with exceptional wear resistance for orthopedic implants. Their proprietary sequential annealing process creates a unique molecular structure that maintains mechanical properties while significantly reducing wear rates. In hip simulator testing, X3 material demonstrated up to 97% less wear than conventional polyethylene. Their manufacturing process employs a proprietary irradiation and thermal treatment protocol that optimizes crosslinking while minimizing free radical concentration, resulting in superior oxidation resistance. Howmedica has also developed Tritanium™, a highly porous titanium material with a polymer-friendly interface that enhances fixation while maintaining the wear benefits of their advanced polymers. Their recent innovations include vitamin E-infused UHMWPE that combines the oxidative stability of vitamin E with their established crosslinking technology, resulting in materials with excellent wear resistance even after accelerated aging protocols. Clinical studies have shown their advanced bearing materials can reduce wear rates to less than 0.05 mm/year in total hip replacements.
Strengths: Extensive clinical history and outcomes data; vertically integrated manufacturing from raw material to finished implant; comprehensive testing capabilities for wear simulation. Weaknesses: Technology primarily focused on hip and knee applications; higher manufacturing costs compared to conventional materials; requires specialized processing equipment.
Key Patents and Innovations in Biomedical Polymer Science
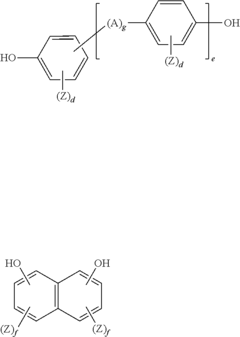
Crosslinking of polyethylene for low wear using radiation and thermal treatments
PatentInactiveUS6800670B2
Innovation
- Crosslinking UHMWPE through gamma irradiation followed by thermal treatment, such as remelting or annealing, and removing the most oxidized surface layer to improve wear resistance, while optimizing radiation doses and thermal processes to balance wear resistance with other physical properties.
Polymer Composition and Articles For Use in Low Temperature Environments That Are Wear Resistant
PatentInactiveUS20130298427A1
Innovation
- A polymer composition comprising a thermoplastic polymer, thermoplastic elastomer, impact modifier, and ultra-high molecular weight polyethylene, formulated to have a rigidity factor of 2 or less, ensuring stability and abrasion resistance across a broad temperature range, including temperatures below 0°C.
Biocompatibility and Safety Considerations
The biocompatibility of wear-resistant polymers represents a critical consideration in their application within biomedical contexts. When polymers are implanted in the human body, they must not elicit adverse biological responses such as inflammation, toxicity, or immunological reactions. The wear particles generated from polymer degradation pose particular concerns, as these microscopic debris can trigger foreign body reactions and potentially lead to implant failure or tissue damage.
Material safety evaluations for biomedical polymers typically involve a comprehensive battery of tests aligned with ISO 10993 standards. These assessments examine cytotoxicity, sensitization potential, irritation effects, systemic toxicity, and genotoxicity. For wear-resistant polymers specifically, additional testing focuses on the biological response to wear particles, which may differ significantly from the response to the bulk material.
Long-term biocompatibility remains challenging to predict, as some adverse reactions may only manifest after extended exposure periods. Recent studies have demonstrated that ultra-high-molecular-weight polyethylene (UHMWPE), despite its excellent wear resistance, can release particles that activate macrophages and trigger osteolysis around orthopedic implants. This highlights the complex relationship between wear resistance and biocompatibility.
Surface modifications have emerged as promising approaches to enhance both wear resistance and biocompatibility simultaneously. Techniques such as plasma treatment, ion implantation, and bioactive coating applications can create surfaces that resist wear while promoting favorable biological interactions. For instance, phospholipid-based coatings have shown potential in reducing friction and wear while mimicking natural biological interfaces.
Regulatory frameworks for biomedical polymers continue to evolve as understanding of long-term biological responses improves. The FDA and equivalent international bodies now require more rigorous evidence of both mechanical performance and biological safety before approving new polymer-based implants. This includes specific assessments of wear particle characteristics and their biological effects.
Risk management strategies for wear-resistant polymers must balance mechanical performance with biological safety. This often involves trade-offs, as modifications that enhance wear resistance may sometimes compromise biocompatibility. A holistic approach considering the specific application environment, expected service life, and patient factors is essential for optimal material selection and design.
Material safety evaluations for biomedical polymers typically involve a comprehensive battery of tests aligned with ISO 10993 standards. These assessments examine cytotoxicity, sensitization potential, irritation effects, systemic toxicity, and genotoxicity. For wear-resistant polymers specifically, additional testing focuses on the biological response to wear particles, which may differ significantly from the response to the bulk material.
Long-term biocompatibility remains challenging to predict, as some adverse reactions may only manifest after extended exposure periods. Recent studies have demonstrated that ultra-high-molecular-weight polyethylene (UHMWPE), despite its excellent wear resistance, can release particles that activate macrophages and trigger osteolysis around orthopedic implants. This highlights the complex relationship between wear resistance and biocompatibility.
Surface modifications have emerged as promising approaches to enhance both wear resistance and biocompatibility simultaneously. Techniques such as plasma treatment, ion implantation, and bioactive coating applications can create surfaces that resist wear while promoting favorable biological interactions. For instance, phospholipid-based coatings have shown potential in reducing friction and wear while mimicking natural biological interfaces.
Regulatory frameworks for biomedical polymers continue to evolve as understanding of long-term biological responses improves. The FDA and equivalent international bodies now require more rigorous evidence of both mechanical performance and biological safety before approving new polymer-based implants. This includes specific assessments of wear particle characteristics and their biological effects.
Risk management strategies for wear-resistant polymers must balance mechanical performance with biological safety. This often involves trade-offs, as modifications that enhance wear resistance may sometimes compromise biocompatibility. A holistic approach considering the specific application environment, expected service life, and patient factors is essential for optimal material selection and design.
Regulatory Framework for Medical-Grade Polymers
The regulatory landscape for medical-grade polymers is complex and multifaceted, with stringent requirements designed to ensure patient safety and product efficacy. For biomedical polymers with enhanced wear resistance properties, compliance with these regulations is particularly critical due to their application in load-bearing implants and high-friction medical devices.
The U.S. Food and Drug Administration (FDA) maintains the most comprehensive regulatory framework through its 510(k) clearance and Premarket Approval (PMA) pathways. Wear-resistant polymers typically require extensive testing under ISO 10993 standards for biocompatibility, with specific attention to ISO 10993-13 for degradation products and ISO 10993-5 for cytotoxicity evaluations. Additionally, ASTM F732 provides standardized methods for wear testing of polymeric materials used in total joint prostheses.
In Europe, the Medical Device Regulation (MDR 2017/745) has significantly increased requirements for clinical evidence and post-market surveillance of implantable devices, including those utilizing wear-resistant polymers. The classification system places most wear-resistant polymer applications in Class IIb or III, necessitating conformity assessment by notified bodies and comprehensive technical documentation.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements similar requirements through its own regulatory framework, with particular emphasis on long-term stability testing for polymeric materials intended for extended use in the human body. The Japanese Industrial Standards (JIS) complement these regulations with specific testing protocols for wear resistance.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established common reporting formats for adverse events and technical documentation, streamlining the global approval process for novel wear-resistant polymers. However, significant regional variations persist, particularly in emerging markets where regulatory frameworks are still evolving.
Material master files (MAF) and drug master files (DMF) systems in various jurisdictions allow polymer manufacturers to protect proprietary information while providing necessary safety data to regulatory authorities. For wear-resistant applications, these files typically include comprehensive wear particle analysis and biocompatibility assessments of wear debris.
Recent regulatory trends indicate increasing scrutiny of microparticle generation from wear processes, with particular concern for potential systemic effects. The FDA's guidance on "Characterization of Ultrahigh Molecular Weight Polyethylene (UHMWPE)" specifically addresses wear resistance testing methodologies and reporting requirements for orthopedic applications, establishing a precedent likely to extend to other wear-resistant polymer technologies.
The U.S. Food and Drug Administration (FDA) maintains the most comprehensive regulatory framework through its 510(k) clearance and Premarket Approval (PMA) pathways. Wear-resistant polymers typically require extensive testing under ISO 10993 standards for biocompatibility, with specific attention to ISO 10993-13 for degradation products and ISO 10993-5 for cytotoxicity evaluations. Additionally, ASTM F732 provides standardized methods for wear testing of polymeric materials used in total joint prostheses.
In Europe, the Medical Device Regulation (MDR 2017/745) has significantly increased requirements for clinical evidence and post-market surveillance of implantable devices, including those utilizing wear-resistant polymers. The classification system places most wear-resistant polymer applications in Class IIb or III, necessitating conformity assessment by notified bodies and comprehensive technical documentation.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) implements similar requirements through its own regulatory framework, with particular emphasis on long-term stability testing for polymeric materials intended for extended use in the human body. The Japanese Industrial Standards (JIS) complement these regulations with specific testing protocols for wear resistance.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) have established common reporting formats for adverse events and technical documentation, streamlining the global approval process for novel wear-resistant polymers. However, significant regional variations persist, particularly in emerging markets where regulatory frameworks are still evolving.
Material master files (MAF) and drug master files (DMF) systems in various jurisdictions allow polymer manufacturers to protect proprietary information while providing necessary safety data to regulatory authorities. For wear-resistant applications, these files typically include comprehensive wear particle analysis and biocompatibility assessments of wear debris.
Recent regulatory trends indicate increasing scrutiny of microparticle generation from wear processes, with particular concern for potential systemic effects. The FDA's guidance on "Characterization of Ultrahigh Molecular Weight Polyethylene (UHMWPE)" specifically addresses wear resistance testing methodologies and reporting requirements for orthopedic applications, establishing a precedent likely to extend to other wear-resistant polymer technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!